ABSTRACT
Optimal dosing of gentamicin in neonates is still a matter of debate despite its common use. We identified gentamicin dosing regimens from eight international guidelines and seven Swiss neonatal intensive care units. The dose per administration, the dosing interval, the total daily dose, and the demographic characteristics between guidelines were compared. There was considerable variability with respect to dose (4 to 6 mg/kg), dosing interval (24 h to 48 h), total daily dose (2.5 to 6 mg/kg/day), and patient demographic characteristics that were used to calculate individualized dosing regimens. A model-based simulation study in 1071 neonates was performed to determine the achievement of efficacious peak gentamicin concentrations according to predefined MICs (Cmax/MIC ≥ 10) and safe trough concentrations (Cmin ≤ 2 mg/liter) with recommended dosing regimens. MIC targets of 0.5 and 1 mg/liter were used. Dosing optimization was performed giving priority to the first day of treatment and with the goal of simplifying dosing. Current gentamicin neonatal guidelines allow to achieve effective peak concentrations for MICs ≤ 0.5 mg/liter but not higher. Model-based simulations indicate that to attain peak gentamicin concentrations of ≥10 mg/liter, a dose of 7.5 mg/kg should be administered using an extended dosing interval regimen. Trough concentrations of ≤2 mg/liter can be maintained with a dosing interval of 36 to 48 h in neonates according to gestational and postnatal age. For treatment beyond 3 days, therapeutic drug monitoring is advised to maintain adequate serum concentrations.
KEYWORDS: aminoglycosides, pediatrics, pharmacokinetics, MIC
INTRODUCTION
In 2015, about 1.4 million children died worldwide of infections such as pneumonia or sepsis/meningitis in the first 5 years of life, most often during the neonatal period (1). The most common cause of Gram-negative early-onset neonatal sepsis is Escherichia coli (2). Other Gram-negative and Gram-positive microorganisms are involved in early or late neonatal sepsis, including Klebsiella spp. and Pseudomonas aeruginosa (3, 4). By virtue of their bactericidal activity and their low costs, aminoglycosides, such as gentamicin, remain the first-line therapy in combination with a β-lactam antibiotic for confirmed or suspected neonatal sepsis (5, 6). However, gentamicin has a narrow therapeutic index, and optimal, personalized dosing in neonates is still debated (7). Based on in vitro studies, optimal gentamicin efficacy is associated with a plasma peak concentration over MIC ratio (Cmax/MIC) that ranges from 8 to 10 (8–10). It has been suggested that the area-under-the-curve/MIC ratio (AUC/MIC) could represent another pharmacodynamic predictor of the efficacy of aminoglycosides (11). Achieving this optimal efficacy in vivo needs to be balanced against nephrotoxicity and ototoxicity associated with high trough concentrations of gentamicin. The nephrotoxicity of aminoglycosides affects both glomerular and tubular functions (12). Although nephrotoxicity is generally temporary and reversible upon treatment discontinuation, ototoxicity might be permanent (13, 14). It has been suggested that trough plasma concentrations of gentamicin should not exceed 1 to 2 mg/liter to minimize potential toxic effects (15–17). Further, it has been reported that multiple daily dosing and a long duration of treatment are more likely to increase the risk of toxicity (18).
In the neonatal population, development and organ maturation is a dynamic process that influences gentamicin pharmacokinetics (PK). Variability in kidney function and body composition in particular is responsible for the large interpatient variability in clearance and volume of distribution of gentamicin in this population. The clearance of gentamicin is indeed almost entirely dependent on glomerular filtration (19). Nephrogenesis is completed after 34 to 35 weeks of gestation, and preterm neonates present a lower glomerular filtration rate (GFR) compared to late-preterm and term neonates (20). Birth is marked by major hemodynamic changes that are responsible for a rapid postnatal increase in GFR in all neonates (21–24). Gentamicin distribution is mostly limited to the extracellular fluid compartment. Neonates have a body water content that is proportionally larger compared to adults and older children. Therefore, an increased gentamicin volume of distribution is often observed and explains why a relatively higher dose per kilogram in neonatal dosing is recommended in order to achieve an effective peak concentration (25).
Our pharmacokinetic understanding of gentamicin in neonates has increased throughout the years. However, this newly acquired knowledge has resulted in many different gentamicin dosing regimens rather than one consistent, optimal dosing regimen for use in daily clinical care (26). Pharmacometric analyses, including PK/pharmacodynamic (PD) modeling and simulation, facilitate evaluation of existing dosing regimens with respect to target attainment and can provide a quantitative rationale for optimizing and personalizing dosing approaches in neonates (27, 28).
The key objectives of this study were to (i) assess the variability in dosing of gentamicin in international guidelines and Swiss neonatal intensive care units (NICUs), (ii) evaluate and compare target achievement of current dosing recommendations with respect to efficacy and safety, and (iii) provide a quantitative rationale for an optimal, personalized gentamicin dosing approach to be implemented in a high resource setting such as Swiss NICUs in light of currently relevant MIC breakpoints.
RESULTS
Variability in national and international guidelines.
Considerable variability in dosing regimen recommendations provided by international guidelines and in Swiss NICUs was observed with respect to dose (4 to 6 mg/kg), dosing interval (24 h to 48 h), total daily dose (2.5 to 6 mg/kg/day), and patient characteristics (qualitative and quantitative) that are used to individualize dosing regimens (Table 1). Although two Swiss NICUs did not use any demographic characteristics for a priori selection of dosing regimens, most guidelines suggested individualized dosing regimens based on a single patient demographic characteristic or a combination of patient demographic characteristics. Gestational age (GA) combined with postnatal age (PNA) was the most frequently observed regimen. Three different dosing intervals were observed (24, 36, and 48 h), with the longest interval used in the youngest preterm neonates. Although the same demographic characteristics were mostly used, the cutoff values to define the patient subgroups varied between recommendations. The variability in gentamicin dosing used in Swiss NICUs and proposed in international guidelines is illustrated for two typical patients (preterm and term neonates) at different postnatal ages in Table 2.
TABLE 1.
Probability of target attainment for guidelines and Swiss centers for effective peak concentrations (≥5 or ≥10 mg/liter) and trough concentrations (>2 mg/liter)a
| Guidelineb | Demographic characteristics | Dose (mg/kg) | Interval (h) | No. of subgroupsc | Targets (mg/liter) |
||
|---|---|---|---|---|---|---|---|
| Peak (%) |
Trough (%) (>2) | ||||||
| ≥5 | ≥10 | ||||||
| No demographic variable | |||||||
| Center 5 | 4 | 24 | 1 | 96 | 26 | 4 | |
| Center 7c | |||||||
| 1 | 5 | 24 | 1 | 99 | 54 | 12 | |
| 2 | 5 | 36 | 1 | 99 | 54 | <0.5 | |
| 3 | 5 | 48 | 1 | 99 | 54 | <0.5 | |
| One demographic variable | |||||||
| BNFc | PNA | 5 | 24/36 | 2 | 99 | 54 | 3 |
| Blue Book (min) | GA | 4 | 24/36 | 2 | 96 | 26 | 1 |
| Blue Book (max) | GA | 5 | 24/36 | 2 | 99 | 54 | 4 |
| Two demographic variables | |||||||
| Center 1 | PNA, wt | 4/5 | 24–48 | 5 | 97 | 39 | 2 |
| Center 2 | PNA, wt | 5/6 | 24–48 | 7 | 99 | 58 | 4 |
| Center 3 (min) | PNA, GA | 4/5 | 24–48 | 6 | 96 | 28 | 1 |
| Center 3 (max) | PNA, GA | 5 | 24–48 | 6 | 99 | 54 | 4 |
| Center 4 | PNA, PMA | 4/4.5/5 | 24–48 | 6 | 96 | 30 | 2 |
| Center 6 | PNA, GA | 4/4.5/5 | 24–48 | 6 | 96 | 30 | 1 |
| Nelson | PNA, GA | 4/4.5/5 | 24–48 | 7 | 97 | 37 | 1 |
| NNF7 | PNA, GA | 5 | 24–48 | 4 | 99 | 55 | 5 |
| Lexicomp | PNA, GA | 4/4.5/5 | 24–48 | 6 | 98 | 43 | 1 |
| Red Book (min) | PNA, wt | 4/5 | 24–48 | 6 | 97 | 39 | 2 |
| Red Book (max) | PNA, wt | 4/5 | 24–48 | 6 | 98 | 44 | 2 |
| Neofax | PNA, PMA | 4/4.5/5 | 24–48 | 5 | 96 | 30 | 1 |
| Shann | PNA, wt | 5/6 | 24–48 | 7 | 99 | 58 | 4 |
Abbreviations: BNFc, British National Formulary for Children; Blue Book, Manual of Childhood Infections Blue Book; Nelson, Nelson Textbook of Pediatrics; NNF7, Neonatal Formulary, 7th ed.; Lexicomp, Lexicomp Pediatric and Neonatal Dosage Handbook; Red Book, The Red Book Report of the Committee on Infectious Diseases; Shann, Drug Doses (Frank Shann) PNA, postnatal age; GA, gestational age; wt; weight; PMA, postmenstrual age. Slashes (/) between numbers indicate “or.”
The guidelines suggested a dosing interval range of 24 to 48 h, and therapeutic drug monitoring was recommended after the first dose.
Subgroups are based on demographic characteristics, as indicated in the guidelines.
TABLE 2.
Variability in gentamicin dosing recommendations for two typical patientsa
| Guideline | Dosing regimen (mg/kg; h)b |
|||
|---|---|---|---|---|
| Patient 1 (GA = 30 wks) |
Patient 2 (GA = 38 wks) |
|||
| PNA, 2 days; wt, 1.3 kg | PNA, 15 days; wt, 1.5 kg | PNA, 2 days; wt, 3.0 kg | PNA, 15 days; wt, 3.3 kg | |
| Nelson | 5; 48 | 4; 24 | 4; 24 | 4; 24 |
| BNFc | 5; 36 | 5; 24 | 5; 36 | 5; 24 |
| Shann | 5; 36 | 5; 24 | 5; 24 | 6; 24 |
| Lexicomp | 4.5; 36 | 5; 36 | 4; 24 | 5; 24 |
| Center 1 | 5; 48 | 5; 36 | 4; 24 | 4; 24 |
| Center 3 | 4–5; 36 | 4–5; 24 | 4–5; 24 | 4–5; 24 |
| Center 6 | 4.5; 36 | 4; 24 | 4; 24 | 4; 24 |
| Center 7 | 5; 24–48 | 5; 24–48 | 5; 24–48 | 5; 24–48 |
See Table 1, footnote a, for abbreviation definitions.
Dosing regimens are expressed as follows: amount (mg/kg); interval (h). Hence, “5; 48” indicates “5 mg/kg administered every 48 h.”
Achievement of efficacious and safe gentamicin exposure.
Considering achieving target gentamicin exposure in at least 90% of neonatal patients (90% probability of target attainment [PTA]) as an appropriate outcome, simulations suggested that all recommendations were adequate in terms of efficacy for pathogens with an MIC of 0.5 mg/liter but appeared to be inadequate for pathogens with an MIC of 1.0 mg/liter (Table 1). Gentamicin peak concentrations of ≥5 mg/liter were achieved in >96% of neonates, whereas a peak concentration of ≥10 mg/liter was found in <60% of neonates. Recommendations were successful in maintaining trough concentrations of <2 mg/liter in >95% of the patients, with one exception (Center 7).
Dosing optimization. (i) Efficacy target attainment.
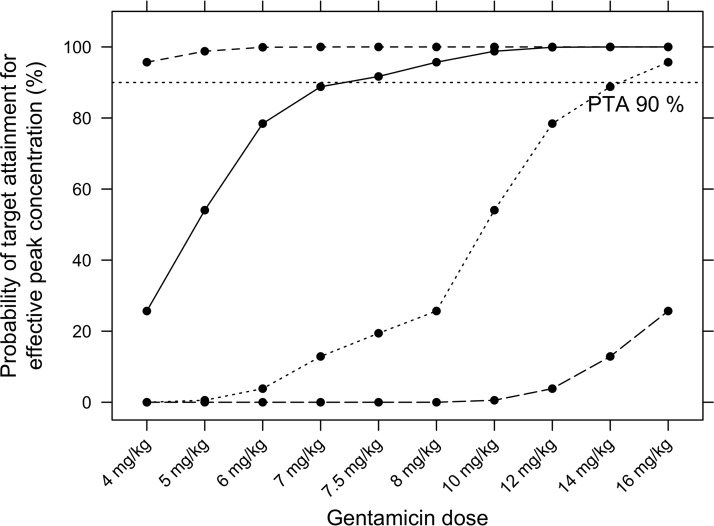
None of the reviewed guidelines was an obvious candidate for optimal and simplified gentamicin dosing. Therefore, dosing optimization was undertaken for MICs of 0.5 and 1 mg/liter (Cmax ≥ 5 mg/liter and Cmax ≥ 10 mg/liter, respectively). A dose per administration of 4 mg/kg appeared sufficient to achieve a Cmax concentration of at least 5 mg/liter with a PTA of ≥96%. Simulations suggest that the dose needs to be increased to 7.5 mg/kg to achieve target peak concentrations of ≥10 mg/liter in ≥90% neonates (Fig. 1).
FIG 1.
Percentage of neonates with target peak concentrations for various gentamicin doses per kg of body weight after the first dose for the entire neonatal population. The tested target peak concentrations were ≥5, ≥10, ≥20, and ≥40 mg/liter corresponding to MICs of 0.5, 1.0, 2.0, or 4.0 mg/liter, respectively.
(ii) Safety target attainment: first dose.
For a dosing regimen of 7.5 mg/kg, only 6% of the patients would present trough concentrations of ≥2 mg/liter after the dosing interval has been increased to 36 h for all neonates (see Table S1 in the supplemental material). However, it was observed that neonates with PNA < 7 days showed more frequently high trough concentrations (9%) than neonates with PNA ≥ 7 days (4%) (data not shown). If neonates with PNA < 7 days were dosed every 48 h, only 1% reached these high concentrations (Table 3).
TABLE 3.
Probability of target attainment for predefined peak and trough concentration targets following an optimal dosing regimen (i.e., the administration of 7.5 mg/kg over different dosing intervals according to patient characteristics)a
| Dosing regimen (mg/kg; h)b | Demographic characteristics | First dose |
After 1 wk of treatment |
||||
|---|---|---|---|---|---|---|---|
| % neonates with ratio peak/MIC of >10 | % neonates with trough of: |
% neonates with ratio peak/MIC of >10 | % neonates with trough of: |
||||
| <1 mg/liter | <2 mg/liter | <1 mg/liter | <2 mg/liter | ||||
| MIC = 0.5 mg/liter | |||||||
| 4; 36 | PNA < 7, GA ≤ 28 | 98 | 62 | 100 | 98 | 43 | 94 |
| 4; 36 | PNA < 7, GA > 28 | 97 | 91 | 100 | 97 | 86 | 99 |
| 4; 36 | PNA ≥ 7, GA ≤ 28 | 96 | 88 | 100 | 97 | 70 | 96 |
| 4; 24 | PNA ≥ 7, GA > 28 | 94 | 80 | 99 | 95 | 65 | 93 |
| MIC = 1.0 mg/liter | |||||||
| 7.5; 48 | PNA < 7, GA ≤ 28 | 98 | 40 | 98 | 98 | 38 | 87 |
| 7.5; 48 | PNA < 7, GA > 28 | 91 | 85 | 99 | 92 | 84 | 97 |
| 7.5; 48 | PNA ≥ 7, GA ≤ 28 | 95 | 81 | 99 | 95 | 67 | 92 |
| 7.5; 36 | PNA ≥ 7, GA > 28 | 90 | 83 | 99 | 91 | 74 | 95 |
Abbreviations: PNA, postnatal age (days); GA, gestational age (weeks). To achieve a probability of target attainment (PTA) of 90% would require a dosing interval of 60 h (PTA = 97%). After a second dose with a dosing interval of 48 h (96 h after the start of treatment), the PTA would still be 93%.
Dosing regimens are expressed as follows: amount (mg/kg); interval (h). Hence, “4; 36” indicates “4 mg/kg administered every 36 h.”
(iii) Safety target attainment: after 1 week of treatment.
A dosing regimen of 7.5 mg/kg every 36 h for neonates with PNA ≥ 7 days and every 48 h for those with PNA < 7 days would result in some accumulation after 1 week of treatment in the oldest subgroup (PNA ≥ 7 days), with 13% of them reaching trough concentrations of ≥2 mg/liter (data not shown). Additional subgroup stratification for patients in the oldest subgroup (PNA ≥ 7 days) who received 7.5 mg/kg every 36 h if their GA ≥ 28 weeks and every 48 h if their GA ≤ 28 weeks would allow target achievements of trough concentrations below the predefined safety threshold in both groups in >90% of neonates (Table 3). Neonates with PNA < 7 days and GA ≤ 28 weeks would require a dosing interval of 60 h. However, with a 48-h dosing interval, 93% would show trough concentrations of <2 mg/liter after the second dose (96 h after the start of treatment, whereas most treatment courses are discontinued at 72 h) (data not shown). Similar subgroup stratification, based on the PNA and GA, were required for a dose of 4 mg/kg (Table 3).
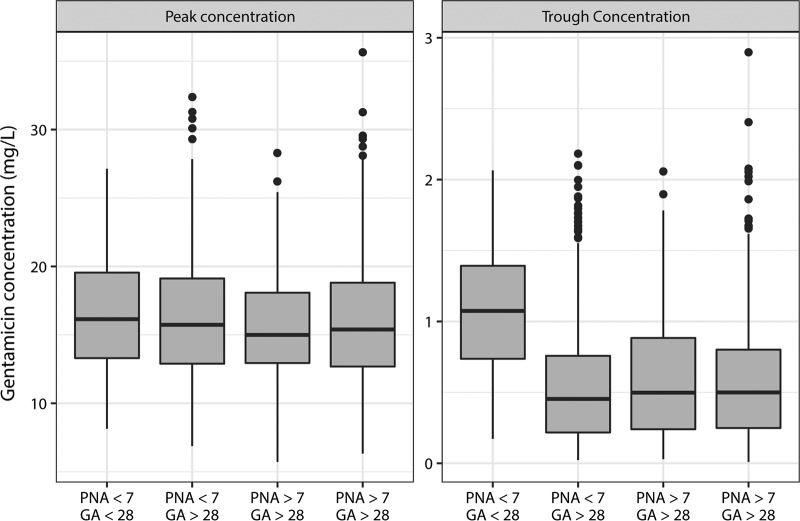
In the scope of simplifying the dosing regimen of gentamicin in high resource settings, a standard dose of 7.5 mg/kg to achieve an effective exposure (Cmax/MIC ≥ 10) is favored from the first dose, irrespective of any demographic factors, when an MIC of 1 mg/liter is considered. Individual dosing intervals for the following doses from 36 to 48 h are suggested according to PNA and GA (Table 3 and Fig. 2). Therapeutic drug monitoring (TDM) should be considered for treatment periods beyond 3 days to fine-tune dosing intervals at the individual level, particularly in the youngest preterm neonates.
FIG 2.
Distribution of peak and trough concentration after the administration of a single 7.5-mg/kg gentamicin dose for four subgroups over a 48-h interval (PNA < 7 days or PNA ≥ 7 days and GA ≤ 28 weeks) or a 36-h interval (PNA ≥ 7 days and GA > 28 weeks). PNA, postnatal age; GA, gestational age. Boxes represent the interquartile ranges (IQR), solid lines indicate the medians and the 25th and 75th quartiles, and the whiskers indicate the 25th quantile minus 1.5 IQR or the 75th quantile plus 1.5 IQR.
Sensitivity analysis.
The proposed dosing regimen would not suffice in ascertaining a trough concentration of <1 mg/liter in ≥90% of the patients (Table 3). For an initial gentamicin dose of 7.5 mg/kg, 90% of the patients would achieve a trough concentration of <1 mg/liter after 1 week of treatment by increasing the dosing interval by 72 h (or more) for patients with GA ≤ 28 weeks and by 48 h or 60 h for patients with a GA > 28 and PNA < 7 days or a PNA ≥ 7 days, respectively. After an initial dose of 4 mg/kg, the dosing intervals should be increased by 12 h for each subgroup except for patients with a PNA < 7 days and a GA ≤ 28 weeks, which would require a dosing interval of 60 h (data not shown). Predicted concentrations and AUC distributions are provided (see Table S2 and Fig. S1 to S3 in the supplemental material).
DISCUSSION
Considerable variability in gentamicin dosing recommendations is apparent in current international guidelines, as well as in Swiss NICUs, in agreement with other studies (29). According to simulations of neonatal exposure, results suggest that a dose of 4 mg/kg, as frequently used in current recommendations, would be sufficient when an MIC breakpoint of 0.5 mg/liter is considered. A higher MIC breakpoint of 1 mg/liter requires a dose of 7.5 mg/kg to achieve efficacious gentamicin exposures in at least 90% of treated neonates. Maintaining trough concentrations of ≤2 mg/liter requires a dosing interval of 36 to 48 h in neonates according to postnatal age and gestational age.
Observed sources of variation in Swiss and international guidelines include differences in dose per administration, dosing interval, total daily dose, and/or patient characteristics used for dose individualization. Complex dosing recommendations for personalized treatment increase the risk of prescription errors and are factors triggering suboptimal patient management (30, 31), highlighting the potential benefit of using dosing harmonization and simplification for a large number of patients. Variation between recommendations did not result in improved efficacy and/or safety of gentamicin use. All recommendations managed to achieve gentamicin peak concentrations of ≥5 mg/liter (MIC of 0.5 mg/liter) but failed to achieve peak concentrations of ≥10 m/liter (MIC of 1 mg/liter) in a high proportion of neonates. Except for one recommendation, all lead to a relatively small proportion of neonates (<5%) with potentially unsafe trough levels of ≤2 mg/liter.
It is likely that guidelines were established considering lower MICs and therefore lower peak concentrations. Dosing strategies should ideally rely on individual MICs, but in NICUs the majority of neonates are treated empirically at the stage when infection cannot yet be definitively confirmed and, in many cases, cannot be identified at all. Treatment should therefore target the most likely and the most virulent pathogens involved in neonatal infections, and MIC targets are based upon standard MIC breakpoints from antimicrobial susceptibility testing databases (32, 33). With this approach, it is possible that the MIC breakpoint used is higher than the observed gentamicin MIC in individual patient isolates (34). In the present study, MICs up to 1 mg/liter are addressed. Although MICs for many Enterobacteriaceae were historically 0.5 mg/liter, MICs of 1 mg/liter have been increasingly observed, especially for the spectrum of pathogens encountered in late neonatal onset sepsis (Pseudomonas and Klebsiella spp.) (2). The EUCAST sensitivity breakpoint for Escherichia coli is currently 2 mg/liter, although this is rather rarely observed in Switzerland (36). In addition, the rates of multidrug resistance of Gram-negative infections to empirical treatment are increasing, especially in resource-limited settings where MICs of up to 4 mg/liter are now encountered (see Table S3 in the supplemental material) (32). Accordingly, peak concentrations of 20 to 40 mg/liter would be required but are very challenging to achieve (Fig. 1) and could result in inacceptable toxicity.
The predefined exposure target for efficacy was set to Cmax/MIC ≥ 10. This is more conservative compared to a ratio of 8 (9, 37) but was preferred since Cmax/MIC ratio of 10 was associated with peak efficacy according to a pooled analysis of the 1980 data reported by Turnidge (38), and a Cmax/MIC ≥ 10 ratio has been shown to be necessary if deep tissue penetration for infections is required (39–41). It has also been reported that attainment of a PD target (Cmax/MIC > 10) within 48 h of therapy is associated with an early therapeutic response (38). In addition, the impact of the immature neonatal immune system on the appropriate efficacy target is unknown, and this slightly higher target might be more suitable in this population (42).
Finally, although a PTA ≥ 90% was considered an appropriate outcome, the acceptable level of PTA is still under debate, with values from ranging 90 to 99% (43). However, the definition of a target PTA has not been applied in a majority of previous gentamicin studies, and dosing recommendations from previous analysis are based on much lower proportions of infants achieving target exposure (44–52).
As for many drugs, solid trial data supporting the use of specific doses associated with good clinical outcome in vivo in this vulnerable population are lacking. As a result, current dosing recommendations for gentamicin are variable and often complex. More evidence-based dosing recommendations are required (26). However, trials for (suspected) infections are difficult to design due to endpoint definitions, the low number of actual confirmed infections in the neonatal population, and obvious ethical reasons. Dosing optimization and possibly simplification can benefit from pharmacometric modeling and simulations techniques. We have used exposure simulations in 1,071 neonatal patients, leveraging an existing neonatal gentamicin PK model to identify dosing regimens with a high probability of reaching predefined efficacy and safety targets in a high proportion of patients. Priority was given to optimizing and simplifying the first dose of gentamicin in order to maximize the microorganism clearance as early as possible during infection (a “hit hard and hit fast” paradigm) (53).
The combination of higher-efficacy criteria and a higher PTA set in this study might appear conservative compared to previous studies but is in line with the current methodology used in simulation and dosing optimization for other antibiotics and with MICs encountered in NICUs (54–56). Presumably, this explains why our simulations suggest a higher dose (7.5 mg/kg) compared to current international and local guidelines. It is acknowledged that a large number of patients are exposed to gentamicin while not having a true infection, putting them at risk of adverse events with no benefits. However, effective initial therapy to cover pathogens which are difficult to treat is essential for infants with a true infection to minimize an adverse outcome due to the infection (2).
Nephro- and ototoxicity do not seem to be associated with peak concentrations (57) but rather with drug accumulation and prolonged treatment (58). However, the safety consequences of higher peak concentration to target higher MICs are unknown. Nevertheless, the toxicity incidence remains low in the pediatric population and is lower than the rates reported in adults, in particular when extended dosing intervals are used (18). To maintain trough concentrations of ≤2 mg/liter with a dose of 7.5 mg/kg, the dosing interval should be extended to 36 to 48 h. This dosing regimen would also ensure trough concentrations of <1 mg/liter in the majority of patients (>82%), a target sometimes used as a more stringent surrogate for safety. Thomson et al. investigated the daily intramuscular administration of an 8-mg/kg gentamicin dose, and trough concentrations of <2 mg/liter were observed (59). Lopez et al. investigated extended intervals (24 and 36 h) after high gentamicin doses (8 mg/kg), and no nephrotoxicity was observed in that study, although gentamicin was not administered for prolonged periods (no longer than 5 days) (57). In addition, it was found that a gentamicin dose of 8 mg/kg provided a nearly 100% probability of achieving adequate peak concentrations of >16 mg/liter (for a population that included children up to 4 years old) (57). In a study involving newborns receiving a 6-mg/kg gentamicin dose over various intervals ranging from 24 to 48 h, trough concentrations of ≥2 mg/liter were observed in only 6% of all treatment episodes. No evidence for ototoxicity was observed, and potential nephrotoxicity was not assessed in any detail (21).
Since the first hours of infection are crucial, the administration of antibiotics within 1 h of the identification of sepsis is recommended (60). Therapeutic drug monitoring is recommended for longer courses to evaluate the necessity of adjusting dosing interval on any individual basis (61). Considering that trough gentamicin TDM is cumbersome in neonates and that steady-state definition in neonates is not applicable, a Bayesian-based TDM approach allowing opportunistic TDM at the time of routine blood tests based on one concentration measurement would present numerous advantages (19). For a large proportion of patients, treatment will be discontinued after 48 to 72 h, and most of them would receive only one to two doses and therefore would not require TDM, limiting the burden of blood sampling.
Another important constraint concerns the selection of the model used to investigate gentamicin drug exposure in neonatal patients in this simulation study. The choice of the most robust model (the Germovsek et al. model) was evaluated with respect to the population on which the model was built, the data used for model development (the number of centers, prospective collection, the number of subjects, and concentration measurements), the relevance of covariate effects included in the model, and the assessment of the predictive performance of the model. Simulation results were also compared to those obtained with the two other published models to avoid any systematic bias in the prediction. This sensitivity test yielded results similar to those shown in Table S4 and Fig. S6 in the supplemental material.
Conclusion.
A simulation study in 1,071 neonatal patients suggests that a gentamicin dose per administration of 7.5 mg/kg is optimal to achieve an efficacious peak concentration corresponding to an MIC of 1.0 mg/liter in 90% of neonates. To ensure a trough concentration associated with less toxicity during the first 60 days of life, dosing intervals of 36 to 48 h are recommended, depending on the PNA and GA. Therapeutic drug monitoring should be considered for treatment longer than 3 days to adjust and individualize dosing intervals and to avoid potentially harming trough concentrations of gentamicin. The results here also highlight the lack of consensus on the magnitude of the targeted PK/PD index, the desirable PTA to achieve, and the need for models to address the immaturity of the immune system of neonates. Our findings stress the urgent need for prospective clinical evaluations of efficacy and safety outcomes with gentamicin.
MATERIALS AND METHODS
Data collection dosing regimens.
Gentamicin dosing regimens were collected from eight international guidelines (Frank Shann's Drug Doses, British National Formulary for Children, Nelson Textbook of Pediatrics, Neonatal Formulary [7th edition], Manual of Childhood Infections Blue Book, Lexicomp Pediatric and Neonatal Dosage Handbook, The Red Book, and Neofax) (17, 62–68) and seven Swiss NICUs (located in Aarau, Bern, Chur, Geneva, Lausanne, St. Gallen, and Zurich). The variables used for the selection of a priori dosing regimens were compared, i.e., dose per administration, dosing interval, total daily dose, and demographic characteristics.
Simulation of gentamicin exposure. (i) Demographic data.
Simulation of individual gentamicin exposure used real demographic data from the Antibiotic Resistance and Prescribing in European Children (ARPEC) (69, 70) point prevalence study and included only European neonates with a complete set of the following characteristics: gestational age, birth weight, current weight, and postnatal age. Since all data were for neonates and infants treated for suspected infection, the skewed distribution of demographic characteristics in this population likely reflects the epidemiology of suspected sepsis at birth (Table 4). The postmenstrual age was computed as the sum of gestational age and postnatal age. The final data set included 1,071 patients with real-life demographic data and their correlations.
TABLE 4.
Demographic characteristics from the Antibiotic Resistance and Prescribing in European Children data subset used for exposure simulation
| Parameter | Result |
|---|---|
| Total population, no. (%)a | 1,071 (100) |
| Preterm (GA < 37 weeks) | 654 (58) |
| Preterm (GA < 28 weeks) | 201 (18) |
| Demographic characteristicsb | |
| Median gestational age (wks) | 34 (22–44) |
| Median birth wt (kg) | 2.1 (0.4–4.8) |
| Median postnatal age (days) | 7 (0–60) |
| % patients aged ≤7 days | 54 |
| Median current wt (kg) | 2.2 (0.48–4.86) |
| Median postmenstrual age (wks) | 35.7 (23.7–47.6) |
GA, gestational age.
Ranges are indicated in parentheses where applicable.
(ii) Model selection.
Multiple population PK models for gentamicin in neonates have been published and were recently reviewed (28). The search strategy provided by Wilbaux et al. was applied and extended until February 2017. Criteria for model selection consisted of (i) data on which the model was developed including, the population of interest (i.e., term and preterm neonates aged up to at least 60 days); (ii) the robustness of data used for model development (the number of centers, prospective collection, the number of subjects, and concentration measurements); (iii) the relevance of covariate effects included in the model with respect to developmental and maturational changes in neonates; and (iv) assessment and documentation of the predictive performance of the model.
The population PK model of Germovsek et al. (19) was preferred over others (44–52, 57, 59, 71–77) for the following reasons. (i) This model was developed with rich data collected prospectively in three large previously conducted studies (44, 74). (ii) The analysis data set consisted of data from 205 neonates providing 1,325 gentamicin serum concentrations. (iii) The model provided for appropriate representation of the target population with respect to gestational age, postnatal age, and weight ranging from 23.3 to 42.3 weeks, 1 to 78 days, and 2.03 to 5.05 kg, respectively. In this analysis, data were best described by a three-compartmental model with linear elimination. Clearance and the volume of distribution were scaled allometrically to body weight. A maturation function incorporating PMA (78), in addition to PNA and serum creatinine concentration (SCr), influenced drug clearance.
Since there were no SCr values available in the ARPEC data set used for simulations, the SCr was set to typical values in this neonatal population, as proposed by Germovsek et al. (19), i.e., the “measured SCr/typical SCr ratio” was set to 1. A deviation of SCr concentration to 60 μmol/liter from a typical SCr concentration of 70 μmol/liter has only a marginal effect on drug clearance in the applied model (clearance 2% lower). Linear PK were assumed for the total range of doses tested, and the weight remained constant during the first week of treatment. Gentamicin exposures associated with dosing regimens of interest were simulated in all neonatal patients in the available data set (n = 1,071). Each patient was simulated once, and peak concentrations were retrieved at 1 h postdose, i.e., 0.5 h after the end of infusion.
(iii) Evaluation steps.
Germovsek et al. evaluated their model by bootstrap and visual predictive checks, as well as against by using an external data set (163 neonates, prospective collection from five hospitals). Model trough concentrations predicted from their model and from the literature (44, 45, 48, 49, 57, 71–75) were compared using their external evaluation data set. The predicted trough concentrations were the least biased for their model (19). We also compared predicted gentamicin exposure with the applied model to two other published models (44, 61) using our final dosing recommendation.
Model-based simulations for gentamicin dosing up to 7 days were performed with the software package NONMEM (v7.3.0; ICON Development Solutions, Ellicott City, MD). Data evaluation and visual representations were performed with R (v3.1.2; R Development Core Team, Vienna, Austria [http://www.r-project.org]).
(iv) Pharmacodynamic surrogates.
Cmax/MIC ratio of >10 was chosen as the PD surrogate. Gentamicin concentrations of ≥5 and ≥10 mg/liter, corresponding to MIC breakpoints of 0.5 and 1.0 mg/liter, respectively, were set as peak targets. A trough concentration of ≤2 mg/liter was set as an appropriate target to minimize toxic effects. The proportion of patients reaching the target for efficacy and safety surrogates was computed after the first dose (first dose on study day 1) and after 1 week of treatment (last dose on study day 7) and is defined as the probability of target achievement (PTA). The aim was to select a dosing regimen leading to a PTA ≥ 90% within the predefined targets for efficacy and safety (43).
Gentamicin dosing optimization.
A stepwise approach was applied to identify an optimal dosing regimen. As a first step, the minimal dose per administration (mg/kg) that achieved target peak concentrations was selected (target attainment with respect to efficacy). The following escalating single doses per body weight were simulated: 4, 5, 6, 7, 7.5, 8, 10, 12, 14, and 16 mg/kg. As a second step, adequate dosing intervals were evaluated for the selected dose to avoid accumulation and maintain target trough concentrations of ≤2 mg/liter (target attainment with respect to safety). The following dosing intervals were evaluated in the simulation study: 24, 36, 48, and 72 h. As a third step, neonatal patients were categorized into subgroups to test whether dosing could be further optimized and personalized in neonates with dose adjustments based on patient characteristics (e.g., various doses based on PNA categories). A sensitivity analysis was performed for a trough concentration of ≤1 mg/liter.
The results were retrieved after the first dose and after 1 week of treatment, but priority was given to achieving efficacious and safe exposure after the first dose, considering that (i) accurate treatment within the first hours of infection is crucial (53), (ii) treatment will be discontinued within 72 h in most neonatal patients for nonconfirmed infection or switched to a more targeted therapy for confirmed infection, and (iii) a large proportion of treated neonatal patients are expected to undergo therapeutic drug monitoring to ensure efficacious and safe exposures beyond the first 2 to 3 days of treatment in high-income countries.
Supplementary Material
ACKNOWLEDGMENTS
This project is part of the national SwissPedDose project for harmonizing dosing in neonates, infants, and children supported by the Swiss Federal Public Health Office (BAG), which supports the research group of Pediatric Pharmacology and Pharmacometrics at University Children’s Hospital Basel.
We thank all physicians, nurses, and medical personnel from the seven hospitals that helped us and allowed us the data acquisition about drug dosing regimens. We thank the following members of the expert committee of the SwissPedDose project, in addition to those listed as authors: Eric Giannoni, (Clinic of Neonatology, Department Woman-Mother-Child, Lausanne University Hospital, Lausanne, Switzerland), Thomas M. Berger (Board Member, Swiss Society of Neonatology, Lucerne, Switzerland), and René Glanzmann (Division of Neonatology, University of Basel Children's Hospital, Basel, Switzerland). We also thank Herman Goossens and Ann Versporten, University of Antwerp and ARPEC project group, for providing the ARPEC data set.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02004-17.
REFERENCES
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. 2016. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet (London, England) 388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kent A, Kortsalioudaki C, Monahan IM, Bielicki J, Planche TD, Heath PT, Sharland M. 2016. Neonatal gram-negative infections, antibiotic susceptibility and clinical outcome: an observational study. Arch Dis Child Fetal Neonatal Ed 101:F507. doi: 10.1136/archdischild-2015-309554. [DOI] [PubMed] [Google Scholar]
- 3.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. 2014. Early-onset neonatal sepsis. Clin Microbiol Rev 27:21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, Laforgia N, Ciccone MM. 2016. Early and late infections in newborns: where do we stand? A review. Pediatr Neonatol 57:265–273. doi: 10.1016/j.pedneo.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Cantey JB, Wozniak PS, Sanchez PJ. 2015. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J 34:267–272. doi: 10.1097/INF.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2013. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 7.Chattopadhyay B. 2002. Newborns and gentamicin: how much and how often? J Antimicrob Chemother 49:13–16. doi: 10.1093/jac/49.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Allegaert K, Veerle C, van den Anker JN. 2015. Dosing guidelines of aminoglycosides in neonates: a balance between physiology and feasibility. Curr Pharmaceut Design 21:5699–5704. doi: 10.2174/1381612821666150901110659. [DOI] [PubMed] [Google Scholar]
- 9.Kirby WM, Standiford HC. 1969. Gentamicin: in vitro studies. J Infect Dis 119:361–363. [DOI] [PubMed] [Google Scholar]
- 10.Lacy MK, Nicolau DP, Nightingale CH, Quintiliani R. 1998. The pharmacodynamics of aminoglycosides. Clin Infect Dis 27:23–27. doi: 10.1086/514620. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen EI, Cars O, Friberg LE. 2011. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother 55:4619–4630. doi: 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samiee-Zafarghandy S, van den Anker JN. 2013. Nephrotoxic effects of aminoglycosides on the developing kidney. J Pediatr Neonatal Individualized Med 2:e020227. [Google Scholar]
- 13.Germovsek E, Barker CI, Sharland M. 2017. What do I need to know about aminoglycoside antibiotics? Arch Dis Child Educ Pract Ed 102:89–93. doi: 10.1136/archdischild-2015-309069. [DOI] [PubMed] [Google Scholar]
- 14.Etienne I, Joannides R, Dhib M, Fillastre JP. 1992. Drug-induced nephropathies. Rev Prat 42:2210–2216. (In French.) [PubMed] [Google Scholar]
- 15.Young TE. 2002. Aminoglycoside therapy in neonates with particular reference to gentamicin. Neoreviews 3:e243–e248. http://neoreviews.aappublications.org/content/3/12/e243. [Google Scholar]
- 16.Rao SC, Srinivasjois R, Hagan R, Ahmed M. 2011. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 11:CD005091. [DOI] [PubMed] [Google Scholar]
- 17.Joint Formulary Committee. 2015. Infection: blood infection, bacterial, p 274 In British national formulary for children. BMJ Group/Pharmaceutical Press, London, United Kingdom. [Google Scholar]
- 18.Kent A, Turner MA, Sharland M, Heath PT. 2014. Aminoglycoside toxicity in neonates: something to worry about? Expert Rev Anti-Infect Ther 12:319–331. doi: 10.1586/14787210.2014.878648. [DOI] [PubMed] [Google Scholar]
- 19.Germovsek E, Kent A, Metsvaht T, Lutsar I, Klein N, Turner MA, Sharland M, Nielsen EI, Heath PT, Standing JF. 2016. Development and evaluation of a gentamicin pharmacokinetic model that facilitates opportunistic gentamicin therapeutic drug monitoring in neonates and infants. Antimicrob Agents Chemother 60:4869–4877. doi: 10.1128/AAC.00577-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abitbol CL, DeFreitas MJ, Strauss J. 2016. Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol 31:2213–2222. doi: 10.1007/s00467-016-3320-x. [DOI] [PubMed] [Google Scholar]
- 21.Fjalstad JW, Laukli E, van den Anker JN, Klingenberg C. 2013. High-dose gentamicin in newborn infants: is it safe? Eur J Pediatr 173:489–495. doi: 10.1007/s00431-013-2194-1. [DOI] [PubMed] [Google Scholar]
- 22.Allegaert K, van den Anker J. 2015. Neonatal drug therapy: the first frontier of therapeutics for children. Clin Pharmacol Ther 98:288–297. doi: 10.1002/cpt.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillman N, Kallapur SG, Jobe A. 2012. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 39:769–783. doi: 10.1016/j.clp.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford S, Calvert J. 2008. Adaptation for life: a review of neonatal physiology. Anaesth Intensive Care Med 9:93–98. [Google Scholar]
- 25.Hartnoll G, Betremieux P, Modi N. 2000. Body water content of extremely preterm infants at birth. Arch Dis Child Educ Pract Ed 83:F56–F59. doi: 10.1136/fn.83.1.F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metsvaht T, Nellis G, Varendi H, Nunn AJ, Graham S, Rieutord A, Storme T, McElnay J, Mulla H, Turner MA, Lutsar I. 2015. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr 15:41. doi: 10.1186/s12887-015-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samardzic J, Allegaert K, Wilbaux M, Pfister M, van den Anker JN. 2016. Quantitative clinical pharmacology practice for optimal use of antibiotics during the neonatal period. Expert Opin Drug Metab Toxicol 12:367–375. doi: 10.1517/17425255.2016.1147559. [DOI] [PubMed] [Google Scholar]
- 28.Wilbaux M, Fuchs A, Samardzic J, Rodieux F, Csajka C, Allegaert K, van den Anker JN, Pfister M. 2016. Pharmacometric approaches to personalize use of primarily renally eliminated antibiotics in preterm and term neonates. J Clin Pharmacol 56:909–935. doi: 10.1002/jcph.705. [DOI] [PubMed] [Google Scholar]
- 29.Spyridis N, Syridou G, Goossens H, Versporten A, Kopsidas J, Kourlaba G, Bielicki J, Drapier N, Zaoutis T, Tsolia M, Sharland M, Members APG. 2016. Variation in paediatric hospital antibiotic guidelines in Europe. Arch Dis Child 101:72–76. doi: 10.1136/archdischild-2015-308707. [DOI] [PubMed] [Google Scholar]
- 30.Pawluk S, Jaam M, Hazi F, Al Hail MS, El Kassem W, Khalifa H, Thomas B, Abdul Rouf P. 2017. A description of medication errors reported by pharmacists in a neonatal intensive care unit. Int J Clin Pharm 39:88–94. doi: 10.1007/s11096-016-0399-x. [DOI] [PubMed] [Google Scholar]
- 31.Koumpagioti D, Varounis C, Kletsiou E, Nteli C, Matziou V. 2014. Evaluation of the medication process in pediatric patients: a meta-analysis. J Pediatr 90:344–355. doi: 10.1016/j.jped.2014.01.008 (In Italian.) [DOI] [PubMed] [Google Scholar]
- 32.EUCAST. 2017. Breakpoint tables for interpretation of MICs and zone diameters, version 7.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf.
- 33.CLSI. 2009. Performance standards for antimicrobial disk diffusion susceptibility tests, 19th ed; approved standard. CLSI document M100-S19 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 34.Woksepp H, Hällgren A, Borgström S, Kullberg F, Wimmerstedt A, Oscarsson A, Nordlund P, Lindholm ML, Bonnedahl J, Brudin L, Carlsson B, Schön T. 2017. High target attainment for β-lactam antibiotics in intensive care unit patients when actual minimum inhibitory concentrations are applied. Eur J Clin Microbiol Infect Dis 36:553–563. doi: 10.1007/s10096-016-2832-4. [DOI] [PubMed] [Google Scholar]
- 35.Reference deleted.
- 36.Federal Office of Public Health and Federal Food Safety and Veterinary Office. 2016. Swiss Antibiotic Resistance Report 2016: usage of antibiotics and occurrence of antibiotic resistance in bacteria from humans and animals in Switzerland. Publication 2016-OEG-30. Federal Office of Public Health and Federal Food Safety and Veterinary Office, Bern, Switzerland. [Google Scholar]
- 37.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 38.Turnidge J. 2003. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 17:503–528. doi: 10.1016/S0891-5520(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 39.Scheetz MH, Hurt KM, Noskin GA, Oliphant CM. 2006. Applying antimicrobial pharmacodynamics to resistant gram-negative pathogens. Am J Health-System Pharmacy 63:1346–1360. doi: 10.2146/ajhp050403. [DOI] [PubMed] [Google Scholar]
- 40.Eliopoulos GM, Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 41.Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother 43:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strunk T, Richmond P, Simmer K, Currie A, Levy O, Burgner D. 2007. Neonatal immune responses to coagulase-negative staphylococci. Curr Opin Infect Dis 20:370–375. doi: 10.1097/QCO.0b013e3281a7ec98. [DOI] [PubMed] [Google Scholar]
- 43.Mouton JW, Brown DFJ, Apfalter P, Cantón R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen EI, Sandström M, Honoré PH, Ewald U, Friberg LE. 2009. Developmental pharmacokinetics of gentamicin in preterm and term neonates. Clin Pharmacokinet 48:253–263. doi: 10.2165/00003088-200948040-00003. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs A, Guidi M, Giannoni E, Werner D, Buclin T, Widmer N, Csajka C. 2014. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol 78:1090–1101. doi: 10.1111/bcp.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiCenzo R, Forrest A, Slish JC, Cole C, Guillet R. 2003. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy 23:585–591. doi: 10.1592/phco.23.5.585.32196. [DOI] [PubMed] [Google Scholar]
- 47.Frymoyer A, Meng L, Bonifacio SL, Verotta D, Guglielmo BJ. 2013. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 33:718–726. doi: 10.1002/phar.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelman AW, Thomson AH, Whiting B, Bryson SM, Steedman DA, Mawer GE, Samba-Donga LA. 1984. Estimation of gentamicin clearance and volume of distribution in neonates and young children. Br J Clin Pharmacol 18:685–692. doi: 10.1111/j.1365-2125.1984.tb02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanao JM, Calvo MV, Mesa JA, Martin-Suarez A, Carbajosa MT, Miguelez F, Dominguez-Gil A. 2004. Pharmacokinetic basis for the use of extended interval dosage regimens of gentamicin in neonates. J Antimicrob Chemother 54:193–198. doi: 10.1093/jac/dkh261. [DOI] [PubMed] [Google Scholar]
- 50.Stolk LML, Degraeuwe PLJ, Nieman FHM, de Wolf MC, de Boer A. 2002. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of gentamicin in neonates. Ther Drug Monit 24:527–531. doi: 10.1097/00007691-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 51.García B, Barcia E, Pérez F, Molina IT. 2006. Population pharmacokinetics of gentamicin in premature newborns. J Antimicrob Chemother 58:372–379. doi: 10.1093/jac/dkl244. [DOI] [PubMed] [Google Scholar]
- 52.Bijleveld YA, de Haan TR, van der Lee HJH, Groenendaal F, Dijk PH, van Heijst A, de Jonge RCJ, Dijkman KP, van Straaten HLM, Rijken M, Zonnenberg IA, Cools F, Zecic A, Nuytemans DHGM, van Kaam AH, Mathot RAA, PharmaCool Study Group. 2016. Altered gentamicin pharmacokinetics in term neonates undergoing controlled hypothermia. Br J Clin Pharmacol 81:1067–1077. doi: 10.1111/bcp.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar A. 2014. An alternate pathophysiologic paradigm of sepsis and septic shock: Implications for optimizing antimicrobial therapy. Virulence 5:80–97. doi: 10.4161/viru.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshizawa K, Ikawa K, Ikeda K, Ohge H, Morikawa N. 2013. Population pharmacokinetic-pharmacodynamic target attainment analysis of imipenem plasma and urine data in neonates and children. Pediatr Infect Dis J 32:1208–1216. doi: 10.1097/INF.0b013e31829b5880. [DOI] [PubMed] [Google Scholar]
- 55.Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. 2008. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J 27:794–799. doi: 10.1097/INF.0b013e318170f8d2. [DOI] [PubMed] [Google Scholar]
- 56.Tremoulet A, Le J, Poindexter B, Sullivan JE, Laughon M, Delmore P, Salgado A, Ian-U Chong S, Melloni C, Gao J, Benjamin DK, Capparelli EV, Cohen-Wolkowiez M. 2014. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother 58:3013–3020. doi: 10.1128/AAC.02374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez SA, Mulla H, Durward A, Tibby SM. 2010. Extended-interval gentamicin: population pharmacokinetics in pediatric critical illness. Pediatr Crit Care Med 11:267–274. doi: 10.1097/PCC.0b013e3181b80693. [DOI] [PubMed] [Google Scholar]
- 58.Quiros Y, Vicente-Vicente L, Morales AI, López-Novoa JM, López-Hernández FJ. 2011. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci 119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 59.Thomson AH, Kokwaro GO, Muchohi SN, English M, Mohammed S, Edwards G. 2003. Population pharmacokinetics of intramuscular gentamicin administered to young infants with suspected severe sepsis in Kenya. Br J Clin Pharmacol 56:25–31. doi: 10.1046/j.1365-2125.2003.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs A, Zimmermann L, Bickle Graz M, Cherpillod J, Tolsa J-F, Buclin T, Giannoni E. 2016. Gentamicin exposure and sensorineural hearing loss in preterm infants. PLoS One 11:e0158806. doi: 10.1371/journal.pone.0158806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley JS, Kimberlin DW (ed). 2015. Nelson's pediatric antimicrobial therapy, 21st ed American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 63.Shann F. 2014. Drug doses, 16th ed Intensive Care Unit Royal Children’s Hospital, Parkville, Victoria, Australia. [Google Scholar]
- 64.Ainsworth SB. 2014. Neonatal formulary: drug use in pregnancy and the first year of life. John Wiley & Sons, New York, NY. [Google Scholar]
- 65.Sharland M, Butler K, Cant A, Dagan R, Davies G, de Groot R, Elliman D, Esposito S, Finn A, Galanakis M. 2016. Manual of childhood infections: the blue book. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 66.Taketomo CK, Hodding JH, Kraus DM. 2013. Pediatric and neonatal dosage handbook: a comprehensive resource for all clinicians treating pediatric and neonatal patients. Lexi-Comp, Inc, Hudson, OH. [Google Scholar]
- 67.Kimberlin DW, Bradley MT, Jackson MA, et al. 2015. Red book: Report of the Committee on Infectious Diseases, 30th ed American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 68.Thomas EY, Mangum B. 2010. Neofax 2010. Thomson Reuters, New York, NY. [Google Scholar]
- 69.Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, Calle GM, Garrahan JP, Clark J, Cooper C, Blyth CC, Francis JR, Alsalman J, Jansens H, Mahieu L, Van Rossom P, Vandewal W, Lepage P, Blumental S, Briquet C, de Louvain C, Robbrecht D, Maton P, Gabriels P, Rubic Z, Kovacevic T, Nielsen JP, Petersen JR, Poorisrisak P, Jensen LH, Laan M, Tamm E, Matsinen M, Rummukainen M-L, Gajdos V, Olivier R, Le Maréchal F, Martinot A, Prot-Labarthe S, Lorrot M, Orbach D, Pagava K, Hufnagel M, Knuf M, Schlag SAA, Liese J, Renner L, Enimil A, Awunyo M, Syridou G, Spyridis N, et al. 2016. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 71:1106–1117. doi: 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 70.Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H. 2013. The Antibiotic Resistance and Prescribing in European Children Project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 Hospitals worldwide. Pediatr Infect Dis J 32:e242–e253. doi: 10.1097/INF.0b013e318286c612. [DOI] [PubMed] [Google Scholar]
- 71.Lingvall M, Reith D, Broadbent R. 2005. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br J Clin Pharmacol 59:54–61. doi: 10.1111/j.1365-2125.2005.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Botha JH, du Preez MJ, Adhikari M. 2003. Population pharmacokinetics of gentamicin in South African newborns. Eur J Clin Pharmacol 59:755–759. doi: 10.1007/s00228-003-0663-6. [DOI] [PubMed] [Google Scholar]
- 73.Weber W, Kewitz G, Rost KL, Looby M, Nitz M, Harnisch L. 1993. Population kinetics of gentamicin in neonates. Eur J Clin Pharmacol 44:S23–S25. doi: 10.1007/BF01428387. [DOI] [PubMed] [Google Scholar]
- 74.Thomson AH, Way S, Bryson SM, McGovern EM, Kelman AW, Whiting B. 1988. Population pharmacokinetics of gentamicin in neonates. Dev Pharmacol Ther 11:173–179. doi: 10.1159/000457685. [DOI] [PubMed] [Google Scholar]
- 75.Jensen PD, Edgren BE, Brundage RC. 1992. Population pharmacokinetics of gentamicin in neonates using a nonlinear, mixed-effects model. Pharmacotherapy 12:178–182. [PubMed] [Google Scholar]
- 76.Sherwin CM, Kostan E, Broadbent RS, Medlicott NJ, Reith DM. 2009. Evaluation of the effect of intravenous volume expanders upon the volume of distribution of gentamicin in septic neonates. Biopharm Drug Dispos 30:276–280. doi: 10.1002/bdd.666. [DOI] [PubMed] [Google Scholar]
- 77.Bijleveld YA, van den Heuvel ME, Hodiamont CJ, Mathôt RAA, de Haan TR. 2017. Population pharmacokinetics and dosing considerations for gentamicin in newborns with suspected or proven sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother 61:e01304–e01316. doi: 10.1128/AAC.01304-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NHG. 2008. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.