ABSTRACT
Recent studies and experience suggest that cefazolin might be equally as effective as antistaphylococcal penicillins for methicillin-susceptible Staphylococcus aureus (MSSA), with a better safety profile and lower cost. The objective of these meta-analyses was to compare the safeties of antistaphylococcal penicillins and cefazolin. The PubMed, Embase, and International Pharmaceutical Abstracts databases and websites for clinical trial registries through 23 June 2017 were searched. In addition, recent abstracts from infectious disease and pharmacy conferences were reviewed. We estimated Peto odds ratios (ORs) with 95% confidence intervals (CIs) using random-effects models. One analysis focused on hospitalized patients, and the other focused on outpatients. Eleven retrospective studies of hospitalized patients and three retrospective studies of outpatients were included. In hospitalized patients, lower rates of nephrotoxicity (Peto OR, 0.225; 95% CI, 0.127 to 0.513), acute interstitial nephritis (Peto OR, 0.189; 95% CI, 0.053 to 0.675), hepatotoxicity (Peto OR, 0.160; 95% CI, 0.066 to 0.387), and drug discontinuation due to adverse reactions (Peto OR, 0.192; 95% CI, 0.089 to 0.414) were found with cefazolin. In outpatients, lower rates of nephrotoxicity (Peto OR, 0.372; 95% CI, 0.192 to 0.722), hepatotoxicity (Peto OR, 0.313; 95% CI, 0.156 to 0.627), and hypersensitivity reactions (Peto OR, 0.372; 95% CI, 0.201 to 0.687) were observed with cefazolin. Compared to antistaphylococcal penicillins, cefazolin was associated with significant reductions in nephrotoxicity and hepatotoxicity in hospitalized patients and outpatients. Additionally, cefazolin was associated with lower likelihoods of discontinuation due to side effects in hospitalized patients and hypersensitivity reactions in outpatients. Cefazolin should be considered a first-line option for patients with MSSA infections for which efficacy is presumed to be similar to that of antistaphylococcal penicillin therapy.
KEYWORDS: cefazolin, nafcillin, oxacillin, antistaphylococcal penicillins, MSSA
INTRODUCTION
The β-lactams are preferred over other classes of antibiotics for the treatment of infections caused by methicillin-susceptible Staphylococcus aureus (MSSA) (1–3). Typically, antistaphylococcal penicillins are recommended as first-line agents, while cefazolin is recommended as a second-line alternative (1–3). Concerns that have been raised with cefazolin include the limited studies of and experience with its use for serious MSSA infections, its unnecessarily broad spectrum of activity due to additional Gram-negative coverage, the possibility of greater susceptibility to hydrolysis by some β-lactamases (especially type A), and an inoculum effect resulting in potentially higher rates of treatment failure in deep-seated MSSA infections (4–10).
In recent years, there have been increases in both clinical experience and the number of reported studies involving cefazolin treatment for MSSA infections, leading to switching to cefazolin as a first-line agent in many institutions. Cefazolin contains less sodium, requires lower volumes of diluent, and is available for use as a rapid bolus injection, while antistaphylococcal penicillins need to be administered by slow intravenous infusion because of the risk of extravasation (11). Also, cefazolin can be dosed less frequently than antistaphylococcal penicillins (every 8 h [q8h] versus q4h to q6h, respectively) in patients with normal kidney function, it can be dosed much less frequently in patients on hemodialysis (due to adjustment for renal dysfunction), and it is much less expensive than antistaphylococcal penicillins.
Despite these potential advantages of cefazolin, most previous studies involving cefazolin and antistaphylococcal penicillins had relatively small sample sizes. Therefore, we conducted meta-analyses to compare the safety of cefazolin to that of antistaphylococcal penicillins in hospitalized patients and outpatients.
RESULTS
Search results.
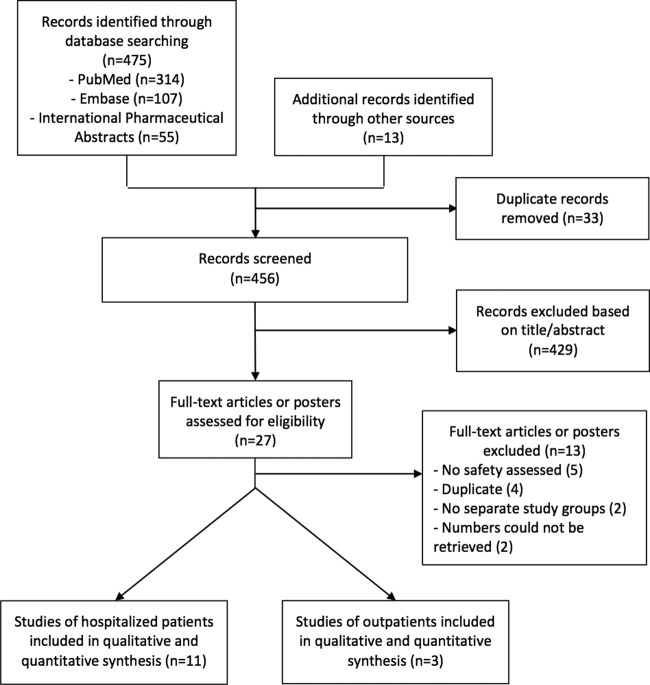
The database search process identified 475 articles; 11 (12–22) were included in the analysis of hospitalized patients, and 3 (23–25) were included in the analysis of outpatients (Fig. 1).
FIG 1.
Flowchart of the processes of the literature search and extraction of data from studies meeting the inclusion criteria.
Study characteristics.
The characteristics of the included studies are summarized in Table 1. The earliest study included was reported in 2011 (17); all studies were of a retrospective cohort design (12–16, 18–22), except for two case-control studies (17, 23). None of the studies were funded by manufacturing companies; seven were published (14, 17–19, 21, 23–25), and six were unpublished except as conference abstracts (12, 13, 15, 16, 20, 22). All studies were conducted in the United States (12–16, 18–22), except for two performed in South Korea (17, 23); all studies were single-center investigations (12, 13, 15–17, 19, 20, 22, 23), except for three that were multicenter investigations (14, 18, 21, 24); and all studies were in English. The average age of subjects ranged from 49 to 65 years. Most studies included patients with MSSA bloodstream infections (12–19, 21, 22). Six of the studies did not report the doses of antistaphylococcal penicillins or cefazolin (12, 13, 17, 19, 20, 24). Nafcillin was used in 11 studies (12–17, 19, 20, 24), while oxacillin was used in 5 studies (18, 21–23, 25). Five studies defined nephrotoxicity as an increase in the serum creatinine level of ≥0.5 mg/dl or 50% (12, 15, 17, 22–24). The exact definitions of nephrotoxicity in all included studies are provided in Table 1. The assessments of bias risk are summarized in Table 2.
TABLE 1.
Characteristics of included studiesa
| Reference | Study design | Study period (yr) | Location | No. of patients in cefazolin arm vs no. of patients in antistaphylococcal penicillin arm | Avg age of patients in cefazolin arm vs age of patients in antistaphylococcal penicillin arm (yr) | Patient characteristic(s) | Antistaphylococcal penicillin(s) | Nephrotoxicity definition | Hepatotoxicity definition | Neutropenia definition | No. of days of therapy in cefazolin arm vs no. of days of therapy in antistaphylococcal penicillin arm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | Retrospective cohort | 2011–2015 | 1 site in U.S. | 27 vs 73 | 56 vs 58 | Inpatients with MSSA BSI; renal dysfunction in 41% vs 7%; hepatic dysfunction in 11% vs 8%; ICU admission in 44% vs 23%; APACHE II score of 17 vs 10 | Nafcillin | Increase in SCr level of ≥0.5 mg/dl or 50% | ALT level >3× the upper normal limit | NA | |
| 13 | Retrospective cohort | 2013–2015 | 1 site in U.S. | 50 vs 46 | NA | Inpatients with MSSA BSI | Nafcillin | Increases in GFR of ≥25%, ≥50%, and ≥75% | Increase in AST or ALT level | NA | |
| 14 | Retrospective cohort | 2013–2015 | 4 sites in U.S. | 68 vs 81 | 65 vs 54 | Inpatients with MSSA BSI; those with renal dysfunction were excluded | Nafcillin at 12 g/day | Increase in SCr level of ≥0.3 mg/dl or 50% | LFTs >3× the upper normal limit | ANC of <1,000 | 9 vs 8 |
| 15 | Retrospective cohort | 2011–2015 | 1 site in U.S. | 30 vs 50 | 56 vs 52 | Inpatients with MSSA BSI; those with ESRD were excluded | Nafcillin at 10–12 g/day | ANC of 1,000–1,500, 500–999, and <500 | 23 vs 31 | ||
| 16 | Retrospective cohort | 2011–2014 | 1 site in U.S. | 71 vs 71 | 50 vs 53 | Inpatients with MSSA BSI; ESRD in 31% vs 14%; liver cirrhosis in 7% vs 11%; ICU admission in 11% vs 31% | Nafcillin at 12 g/day | Increase in SCr level of ≥0.5 mg/dl or 50% | ANC of <1,000 | 14 vs 10 | |
| 17 | Case-control | 2004–2009 | 1 site in South Korea | 41 vs 41 | 53 vs 54 | Propensity score-matched inpatients with MSSA BSI; ESRD in 5% vs 15%; liver cirrhosis in 17% vs 12% | Nafcillin | 17 vs 15 | |||
| 18 | Retrospective cohort | 2008–2012 | 2 sites in U.S. | 59 vs 34 | 51 vs 51 | Inpatients with MSSA BSI; ESRD in 25% vs 0%; ICU admission in 7% vs 18% | Oxacillin at 12 g/day | Increase in SCr level of ≥0.5 mg/dl or 50% | ALT or AST level >5× the upper normal limit | 39 vs 31 | |
| 19 | Retrospective cohort | 2000–2009 | 1 site in U.S. | 26 vs 13 | NA | Inpatients with MSSA BSI | Nafcillin | 36 | |||
| 20 | Retrospective cohort | 2007–2015 | 1 site in U.S. | 518 vs 518 | 49 vs 50 | Propensity score-matched inpatients with any infection; those with renal dysfunction were excluded; ICU admission in 11% vs 31% | Nafcillin | Increases in GFR of ≥25%, ≥50%, and ≥75% | NA | ||
| 21 | Retrospective cohort | 2010–2013 | 2 sites in U.S. | 103 vs 58 | 53 vs 54 | MSSA BSI; renal dysfunction in 50% vs 29%; ICU admission in 42% vs 33%; APACHE II scores of 13 vs 10.3 | Oxacillin at 12 g/day | Not defined | Not defined | 29 vs 33 | |
| 22 | Retrospective cohort | 2011–2013 | 1 site in U.S. | 54 vs 29 | NA | Inpatients with MSSA BSI | Nafcillin and oxacillin at 12 g/day | Increase in SCr level of ≥0.5 mg/dl or 50% | ALT or AST level >5× the upper normal limit | ANC of <1,500 | 9 vs 7 |
| 23 | Case-control | 2009–2013 | 1 site in South Korea | 38 vs 130 | 61 vs 56 | Propensity score-matched outpatients (OPAT in-home and rehabilitation facility) with any infection; chronic kidney disease in 42% vs 15%; liver dysfunction in 24% vs 11% | Oxacillin at 8–12 g/day | Increase in SCr level of ≥0.5 mg/dl or 50% from baseline | ALT level of >42 U/liter or AST level of >54 U/liter | ANC of <1,500 | NA |
| 24 | Retrospective cohort | 2007–2011 | 1 site in U.S. | 119 vs 366 | 56 vs 57 | Outpatients (OPAT in-home, skilled nursing facility, and rehabilitation facility) with any MSSA infection; chronic renal failure in 10% vs 7.1%; liver dysfunction in 6% vs 6% | Nafcillin | Increase in SCr level of ≥0.5 mg/dl or 50% from baseline | Increase in ALT level of >100 U/liter | ANC of <1,000 | 29 vs 28 (planned) |
| 25 | Retrospective cohort | 1996–2001 | 27 sites in U.S. | 203 vs 295 | NA | Outpatients (OPAT in-home health organization and physician-based or hospital-based programs) with any MSSA infections | Nafcillin and oxacillin at 10 g/day | NA |
Comparisons are presented as the cefazolin arm versus the antistaphylococcal penicillin arm. NA, not available; MSSA, methicillin-susceptible Staphylococcus aureus; BSI, bloodstream infection; ICU, intensive care unit; APACHE II, acute physiology and chronic health evaluation II; ESRD, end-stage renal disease; SCr, serum creatinine; ALT, alanine transaminase; AST, aspartate transaminase; ANC, absolute neutrophil count; GFR, glomerular filtration rate; OPAT, outpatient parenteral antimicrobial therapy; LFTs, liver function tests.
TABLE 2.
Quality assessment of included studies
Study outcomes.
All study outcomes are reported as outcomes with cefazolin versus antistaphylococcal penicillins, with a relative risk (RR) of <1 favoring cefazolin. When multiple definitions or categories were provided in the published investigations, we utilized the least conservative definition or category to include the number of events in the meta-analyses (Table 1). For the primary outcomes of nephrotoxicity and hepatotoxicity, we used conservative definitions in sensitivity analyses that were based on an increase in the serum creatinine level or glomerular filtration rate of ≥50% and an alanine transaminase (ALT) or aspartate transaminase (AST) level >5 × the upper normal limit, respectively. With the exception of nafcillin discontinuation due to adverse drug reactions (ADRs), we were not able to perform any subgroup or sensitivity analyses on the outpatient outcomes since there were fewer than 2 studies per outcome.
Nephrotoxicity and acute interstitial nephritis.
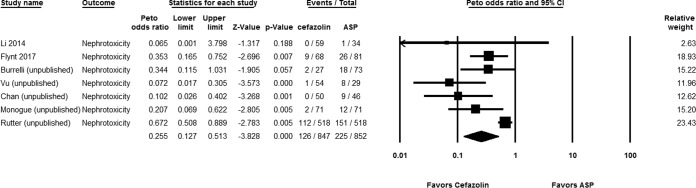
Out of 847 hospitalized patients on cefazolin versus 852 patients on antistaphylococcal penicillins, 126 (14.88%) and 225 (38.66%), respectively, experienced nephrotoxicity (Peto odds ratio [OR], 0.255 [95% confidence interval {CI}, 0.127 to 0.513; P < 0.001]; I2 = 0%; Q = 4.753 [P = 0.002]) (Fig. 2). Subgroup analysis showed that the reduced nephrotoxicity with cefazolin was significant in comparisons with both nafcillin (Peto OR, 0.249 [95% CI, 0.117 to 0.530; P < 0.001]; I2 = 0%; Q = 4.970 [P < 0.001]) and oxacillin (Peto OR, 0.030 [95% CI, 0.002 to 0.762; P = 0.033]; I2 = 0%; Q = 0.135 [P = 0.713]). Sensitivity analyses based on data from the published studies (Peto OR, 0.333 [95% CI, 0.158 to 0.702; P = 0.004]; I2 = 0%; Q = 0.642 [P = 0.423]) and using the more conservative definition defined above (Peto OR, 0.451 [95% CI, 0.2019 to 0.928; P = 0.031]; I2 = 0%; Q = 4.606 [P = 0.141]) showed consistent findings. None of the patients (n = 83) on cefazolin had acute interstitial nephritis, compared to 12/136 (8.82%) of patients on antistaphylococcal penicillins (Peto OR, 0.189 [95% CI, 0.053 to 0.675; P = 0.010]; I2 = 0%; Q = 0.450 [P = 0.799]).
FIG 2.
Forest plot showing the Peto odds ratios of nephrotoxicity for patients receiving cefazolin versus antistaphylococcal penicillin (ASP) (12–14, 16, 18, 20, 21).
In studies of outpatients, a statistically significant reduced risk of nephrotoxicity was found with cefazolin compared to antistaphylococcal penicillins (Peto OR, 0.372 [95% CI, 0.192 to 0.722; P = 0.003]; I2 = 0%; Q = 0.136 [P = 0.712]).
Hepatotoxicity.
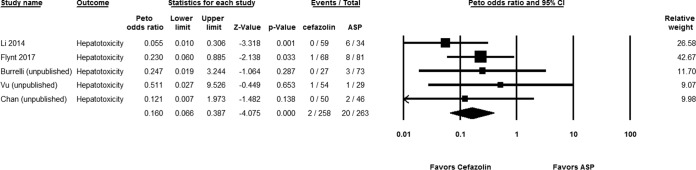
For the studies of hospitalized patients, 2 out of 258 patients (0.78%) on cefazolin experienced hepatotoxicity, compared to 20 out of 263 patients (7.6%) who received antistaphylococcal penicillins (Peto OR, 0.160 [95% CI, 0.066 to 0.387; P < 0.001]; I2 = 0%; Q = 2.508) (Fig. 3). Consistent observations were found in sensitivity analyses based on published studies (Peto OR, 0.125 [95% CI, 0.031 to 0.495; P = 0.003]; I2 = 0%; Q = 1.000 [P = 0.201]) and the conservative definition (Peto OR, 0.121 [95% CI, 0.015 to 0.968; P = 0.047]; I2 = 0%; Q = 1.000 [P = 0.199]). The statistically significant differences persisted with subgroup analysis of cefazolin compared to either nafcillin (Peto OR, 0.252 [95% CI, 0.087 to 0.731; P = 0.011]; I2 = 0%; Q = 1.878 [P = 0.598]) or oxacillin (Peto OR, 0.049 [95% CI, 0.010 to 0.240; P < 0.001]; I2 = 0%; Q = 0.161 [P = 0.688]).
FIG 3.
Forest plot showing the Peto odds ratios of hepatotoxicity for patients receiving cefazolin versus antistaphylococcal penicillin (ASP) (12–14, 18, 21).
In the studies of outpatients, a statistically significant reduced risk of hepatotoxicity was found with cefazolin compared to antistaphylococcal penicillins (Peto OR, 0.313 [95% CI, 0.156 to 0.627; P = 0.001]; I2 = 0%; Q = 0.212 [P = 0.645]).
Discontinuation due to ADRs.
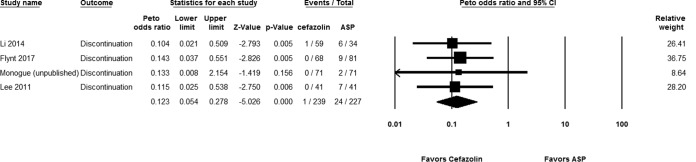
Out of 269 hospitalized patients, 4 (1.49%) in the cefazolin group had therapy interrupted due to ADRs, compared to 31 out of 277 patients (11.19%) in the antistaphylococcal penicillin group (Peto OR, 0.192 [95% CI, 0.089 to 0.414; P < 0.001]; I2 = 0%; Q = 3.840 [P = 0.321]) (Fig. 4). We found consistent findings in our sensitivity analysis of published studies (Peto OR, 0.122 [95% CI, 0.052 to 0.287; P < 0.001]; I2 = 0%; Q = 0.099 [P = 0.952]) and in our subgroup analysis of nafcillin (Peto OR, 0.221 [95% CI, 0.088 to 0.555; P = 0.001]; I2 = 0%; Q = 2.810 [P = 0.267]). Oxacillin was used in only one study (18), which had a statistically significant finding by itself.
FIG 4.
Forest plot showing the Peto odds ratios of drug discontinuation due to adverse reactions for patients receiving cefazolin versus antistaphylococcal penicillin (ASP) (14, 16–18).
For outpatients, statistically significant lower rates of discontinuation due to ADRs were originally identified in one study (24) but statistical significance did not persist in the meta-analysis of data from that study and the second study (25) (Peto OR, 0.432 [95% CI, 0.127 to 1.474; P = 0.180]; I2 = 0%; Q = 1.000 [P = 0.040]). However, a subgroup analysis showed that the difference was statistically significant compared to nafcillin (Peto OR, 0.267 [95% CI, 0.172 to 0.415; P < 0.001]; I2 = 0%; Q = 0.401 [P = 0.527]).
Neutropenia and thrombocytopenia.
In hospitalized patients, no statistically significant differences between the two groups were identified for rates of neutropenia (Peto OR, 0.853 [95% CI, 0.195 to 3.724; P = 0.832]; I2 = 0.976%; Q = 3.030 [P = 0.203]) or thrombocytopenia (Peto OR, 0.949 [95% CI, 0.052 to 17.202; P = 972]; I2 = 47.965%; Q = 1.922 [P = 0.166]). However, there was a non-statistically significant trend in outpatients toward less neutropenia with cefazolin (Peto OR, 0.512 [95% CI, 0.240 to 1.091; P = 0.083]; I2 = 0%; Q = 0.5 [P = 0.480]).
Hypersensitivity reactions.
In studies of hospitalized patients, no statistically significant difference in the rates of hypersensitivity between the two groups was identified (Peto OR, 1.042 [95% CI, 0.471 to 2.305; P = 0.919]; I2 = 0%; Q = 4.044 [P = 0.543]), but in studies of outpatients, a statistically significant difference favoring cefazolin was found (Peto OR, 0.372 [95% CI, 0.201 to 0.687; P = 0.002]; I2 = 0%; Q = 0.133 [P = 0.715]).
C. difficile infection and diarrhea.
No statistically significant differences between treatment groups were found for the rates of diarrhea in hospitalized patients (Peto OR, 0.651 [95% CI, 0.073 to 5.834; P = 0.701]; I2 = 34.568%; Q = 3.057 [P = 0.217]) or the rates of Clostridium difficile infection in outpatients (Peto OR, 3.729 [95% CI, 0.025 to 566.776; P = 0.608]; I2 = 0%; Q = 1.000 [P = 0.037]).
DISCUSSION
The findings from this systematic review suggest that treatment with cefazolin in adults is associated with a clinically important favorable safety profile compared to antistaphylococcal penicillins because of lower risks of nephrotoxicity, acute interstitial nephritis, hepatotoxicity, hypersensitivity reactions, and discontinuation due to ADRs. In other studies, cefazolin has demonstrated at least equivalent efficacy compared to antistaphylococcal penicillins (26) and improved cost-effectiveness (14). Therefore, cefazolin should be considered a first-line treatment option for MSSA bacteremia.
To our knowledge, this is the first systematic review with meta-analyses to assess the safety of antistaphylococcal penicillins. There was one previous meta-analysis (27). It was reported in 2014, no unpublished work was included, and the authors of that study stated that there were too few reported adverse events to provide meaningful comparisons. We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and included several recent studies. Our primary analyses included both published and unpublished work using less-conservative definitions, but the findings did not change when we limited the analyses to either published studies or more-conservative definitions. This suggests that our findings are robust, but a large randomized controlled trial to assess both efficacy and safety is needed to reduce the likelihood of both measured and unmeasured confounders.
Most patients in our included studies had MSSA bloodstream infections. Since patients with bacteremia are often treated for a prolonged period of time, there is a greater possibility of patients experiencing side effects. Knowing the safer antibiotic option is of great clinical relevance. For example, in a retrospective cohort study by Blot et al., MSSA bacteremia was associated with a long length of stay and a high treatment cost in comparison to cases caused by other organisms (28). It is unclear how these findings could be related to the use of antistaphylococcal penicillins for cases of MSSA bacteremia. Moreover, a recent large multicenter retrospective cohort study by McDanel et al. (29) showed a significantly lower mortality risk with cefazolin than with nafcillin or oxacillin. If this difference in mortality is true, it is possibly due to antibiotic-related ADRs and the associated antibiotic discontinuation rates in light of findings from more-recent studies that suggest equivalent efficacies of cefazolin and antistaphylococcal penicillins (26).
The only antistaphylococcal penicillins used in the studies included in our systematic review were nafcillin and oxacillin. We would expect relatively similar findings with other agents in this class, such as cloxacillin and flucloxacillin, but further studies are needed to confirm this hypothesis. Our primary aim was not to compare the safety of the various antistaphylococcal penicillins. Although our results demonstrate that both nafcillin and oxacillin have an inferior safety profile compared to cefazolin, subgroup analyses according to the type of antistaphylococcal penicillin did not identify significant differences, with the exception of higher nafcillin discontinuation rates due to ADRs in outpatients. A previous study found that nafcillin was associated with a high discontinuation rate due to ADRs compared to oxacillin (30). The results of our analysis are unable to confirm or refute this finding since we did not perform a head-to-head analysis, and the oxacillin subgroup could be simply underpowered.
We did not combine data from studies of hospitalized patients with those involving outpatients because of several differences in heterogeneity, including the frequency and length of monitoring of ADRs as well as the treatment duration. Similarly to hospitalized patients, outpatients who received antistaphylococcal penicillins were at higher risks of nephrotoxicity, hepatotoxicity, and drug discontinuation due to ADRs. However, only outpatients who received antistaphylococcal penicillins were at a higher risk of a hypersensitivity reaction. Some of these ADRs have a delayed onset, which could explain why these ADRs are more likely to be detected in outpatients with more-prolonged follow-up. Despite no statistically significant differences in neutropenia in hospitalized patients, outpatients had a nonsignificant trend toward a higher risk of neutropenia with the use of antistaphylococcal penicillins. In the study using nafcillin, statistical significance was achieved. It is unclear if this is due to differences in the patient population, the type of antistaphylococcal penicillins, or the frequency of monitoring. However, it is worth mentioning that most of the studies (14, 16, 22) that assessed hospitalized patients used these β-lactams for short durations (≤2 weeks).
It is still unclear if individuals with deep-seated infections with a high MSSA burden, such as those with endocarditis, should be treated with cefazolin or antistaphylococcal penicillins. There is consensus that individuals with central nervous system infections should not be treated with cefazolin because of its poor penetration of the blood-brain barrier. For other MSSA infections, the decision by clinicians to use cefazolin or antistaphylococcal penicillins should take into account toxicity as well as efficacy and cost considerations. Another limitation of our analysis was the inability to compare study outcomes based on dose. For those studies that reported cefazolin dosing, standardization of dosing according to renal function was not always well described. Given the safety and concerns about the inoculum effect of cefazolin, it seems prudent to use a higher dose of 2 g q8h in most patients with normal renal function. Given the fact that some study participants may have continued antibiotic therapy after discharge and may have chosen to seek care at a local hospital or clinic instead of the original hospital at which the studies were conducted, uncertainty as to the extent of follow-up after hospital discharge is possible. Since the included studies were observational and antistaphylococcal penicillins are believed by some clinicians to cause more ADRs, clinicians are perhaps more likely to monitor ADRs and attribute some ADRs to these agent rather than cefazolin. Therefore, anchoring bias has to be considered.
In conclusion, this systematic review found that the use of cefazolin in both inpatients and outpatients is associated with low risks of nephrotoxicity, hepatotoxicity, and discontinuation due ADRs compared to antistaphylococcal penicillins regardless of publication status, definition, and type of penicillins. In outpatients, hypersensitivity reactions also occurred less frequently with cefazolin. No significant differences in other adverse events were found, but there was a nonsignificant trend toward lower rates of neutropenia in outpatients. Future large randomized clinical trials should be conducted on the safety of beta-lactam antibiotics. However, our results provide additional evidence that cefazolin should be considered a first-line option for patients with MSSA infections when its efficacy is presumed to be similar to that of antistaphylococcal penicillins.
MATERIALS AND METHODS
The meta-analyses were conducted according to the PRISMA guidelines (31) (see Table S1 in the supplemental material). This study is registered with PROSPERO International Prospective Register of Systematic Reviews (registration number CRD42017069474).
Search strategy and study selection.
The full search strategy is provided in the supplemental material. Two authors of this study (K.E. and S.A.) independently searched the PubMed, Embase, and International Pharmaceutical Abstracts biomedical databases as well as the ClinicalTrials.gov and ClinicalTrialsRegister.eu websites without date or language restrictions through 23 June 2017. In addition, the following infectious disease and pharmacy conference proceedings were searched between 2010 and 23 June 2017: the Infectious Diseases Society of America, the European Congress of Clinical Microbiology and Infectious Diseases, the Interscience Conference on Antimicrobial Agents and Chemotherapy/ASM Microbe, the American Society of Health-System Pharmacists, and the American College of Clinical Pharmacy. The references of the included studies were checked to identify additional studies. Two authors of this study (K.E and S.A.) independently extracted the data regarding study characteristics (Table 1) and any safety endpoints. Any disagreement between the authors was resolved through discussion. Any study that compared the safety of antistaphylococcal penicillins to that of cefazolin in adults was included. One analysis focused on studies of hospitalized patients, whereas the other included outpatients only. Typically, inpatients would be sicker and more likely to have confounding factors, including other medications. In addition, outpatients are monitored less frequently than inpatients. For example, it might be easier to detect nephrotoxicity in inpatients, but this could be missed in outpatients, even if changes in renal function met the definition of nephrotoxicity. Moreover, outpatients are monitored for longer periods, and some adverse events might have been detected later when individuals were outpatients but did not occur while they were inside the hospital. On the other hand, studies of outpatients would not report adverse events that had occurred before discharge from the hospital.
Data analysis.
The primary outcomes were rates of defined nephrotoxicity, defined hepatotoxicity, and discontinuation due to ADRs. Studies that did not provide the definitions for nephrotoxicity and hepatotoxicity were excluded. For primary outcomes, we performed a subgroup analysis according to the type of antistaphylococcal penicillin as well as sensitivity analyses based on both publication status and the more-conservative definitions among the included studies. Secondary outcomes included rates of any other ADRs reported in at least two studies. Heterogeneity (I2) was assessed by using Cochran's chi-squared test. The Peto ORs with 95% CIs were estimated by using random-effects models. The authors of the studies were contacted for missing data. The quality of studies was independently evaluated by using the Newcastle-Ottawa scale (NOS) for observational studies (32). Publication bias was visually assessed by using the funnel plot, and asymmetry was tested by using the Egger test (see Fig. S4 to S6 in the supplemental material). All analyses were conducted by using Comprehensive Meta-Analysis Version 3 software (Biostat, Englewood, NJ, USA).
Supplementary Material
ACKNOWLEDGMENTS
No external funding was received, and we have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01816-17.
REFERENCES
- 1.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infections: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA. 2015. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 4.Bryant RE, Alford RH. 1977. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 237:569–570. doi: 10.1001/jama.1977.03270330059022. [DOI] [PubMed] [Google Scholar]
- 5.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis 128(Suppl):S386–S389. doi: 10.1093/infdis/128.Supplement_2.S386. [DOI] [PubMed] [Google Scholar]
- 6.Nannini EC, Singh KV, Murray BE. 2003. Relapse of type A beta-lactamase-producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 37:1194–1198. doi: 10.1086/379021. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Guerrero ML, de Gorgolas M. 2005. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin Infect Dis 41:127. doi: 10.1086/430833. [DOI] [PubMed] [Google Scholar]
- 8.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible causes of cefazolin treatment failure. Antimicrob Agents Chemother 53:3437–3441. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livorsi DJ, Crispell E, Satola SW, Burd EM, Jerris R, Wang YF, Farley MM. 2012. Prevalence of blaZ gene types and the inoculum effect with cefazolin among bloodstream isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 56:4474–4477. doi: 10.1128/AAC.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zygmunt DJ, Stratton CW, Kernodle DS. 1992. Characterization of four beta-lactamases produced by Staphylococcus aureus. Antimicrob Agents Chemother 36:440–445. doi: 10.1128/AAC.36.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le A, Patel S. 2014. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother 48:870–886. doi: 10.1177/1060028014527820. [DOI] [PubMed] [Google Scholar]
- 12.Burrelli CC, Snyder GM, Gold HS, McCoy C, Mahoney MV, Hirsch EB. 2016. Treatment outcomes with nafcillin versus cefazolin for methicillin-susceptible Staphylococcus aureus bloodstream infections, abstr 1071. Abstr IDWeek 2016, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 13.Chan L, Guarascio AJ, Como J, Chan-Tompkins NH. 2016. Retrospective analysis of adverse events (AEs) between nafcillin vs. cefazolin for complicated methicillin susceptible Staphylococcus aureus (MSSA) infections, abstr 407. Abstr ASM Microbe 2016, Boston, MA. [Google Scholar]
- 14.Flynt LK, Kenney RM, Zervos MJ, Davis SL. 2017. The safety and economic impact of cefazolin versus nafcillin for the treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections. Infect Dis Ther 6:225–231. doi: 10.1007/s40121-017-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann W, Christensen C, Cohen SH. 2016. Outcomes and tolerability of nafcillin versus cefazolin for systemic methicillin-susceptible Staphylococcus aureus (MSSA) infections, abstr 1074. Abstr IDWeek 2016, San Diego, CA. [Google Scholar]
- 16.Monogue ML, Ortwine JK, Wei W, Bhavan K. 2015. Nafcillin versus cefazolin for the treatment of methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia, abstr S-912. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]
- 17.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 55:5122–5126. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS II. 2014. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 58:5117–5124. doi: 10.1128/AAC.02800-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel UC, McKissic EL, Kasper D, Lentino JR, Pachucki CT, Lee T, Lopansri BK. 2014. Outcomes of ceftriaxone use compared to standard of therapy in methicillin susceptible Staphylococcal aureus (MSSA) bloodstream infections. Int J Clin Pharm 36:1282–1289. doi: 10.1007/s11096-014-9999-5. [DOI] [PubMed] [Google Scholar]
- 20.Rutter WC, Burgess DS. 2016. Nephrotoxicity and clinical outcomes in patients treated with nafcillin or cefazolin, abstr 302. Abstr IDWeek 2016, San Diego, CA. [Google Scholar]
- 21.Rao SN, Rhodes NJ, Lee BJ, Scheetz MH, Hanson AP, Segreti J, Crank CW, Wang SK. 2015. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 59:5232–5238. doi: 10.1128/AAC.04677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vu BN, Guo Y, Williamson JE, Chung P. 2016. Evaluation of outcomes in patients treated with antistaphylococcal penicillins versus cephalosporins in methicillin-susceptible Staphylococcus aureus bacteremia, abstr 1076. Abstr IDWeek 2016, San Diego, CA. [Google Scholar]
- 23.Lee B, Tam I, Weigel B, Breeze JL, Paulus JK, Nelson J, Allison GM. 2015. Comparative outcomes of beta-lactam antibiotics in outpatient parenteral antibiotic therapy: treatment success, readmissions and antibiotic switches. J Antimicrob Chemother 70:2389–2396. doi: 10.1093/jac/dkv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youngster I, Shenoy ES, Hooper DC, Nelson SB. 2014. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 59:369–375. doi: 10.1093/cid/ciu301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn M, Dalavisio JR, Tice AD, Jiang X. 2005. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South Med J 98:590–595. doi: 10.1097/01.SMJ.0000145300.28736.BB. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Echevarria KL, Traugott KA. 2017. β-Lactam therapy for methicillin-susceptible Staphylococcus aureus bacteremia: a comparative review of cefazolin versus antistaphylococcal penicillins. Pharmacotherapy 37:346–360. doi: 10.1002/phar.1892. [DOI] [PubMed] [Google Scholar]
- 27.Vardakas KZ, Apiranthiti KN, Falagas ME. 2014. Antistaphylococcal penicillins versus cephalosporins for definitive treatment of meticillin-susceptible Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Int J Antimicrob Agents 44:486–492. doi: 10.1016/j.ijantimicag.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. 2002. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 162:2229–2235. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 29.McDanel JS, Roghmann MC, Perencevich EN, Ohl ME, Goto M, Livorsi DJ, Jones M, Albertson JP, Nair R, O'Shea AM, Schweizer ML. 31 March 2017. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: a nationwide cohort study. Clin Infect Dis doi: 10.1093/cid/cix287. [DOI] [PubMed] [Google Scholar]
- 30.Viehman JA, Oleksiuk L-M, Sheridan KR, Byers KE, He P, Falcione BA, Shields RK. 2016. Adverse events lead to drug discontinuation more commonly among patients who receive nafcillin than among those who receive oxacillin. Antimicrob Agents Chemother 60:3090–3095. doi: 10.1128/AAC.03122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 32.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. 7 June 2017. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in metaanalyses. Ottawa Hospital Research Institute, Ottawa, Ontario, Canada: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.