ABSTRACT
Meropenem-vaborbactam is a fixed combination of the novel β-lactamase inhibitor vaborbactam and the carbapenem antibiotic meropenem, developed for the treatment of serious infections caused by drug-resistant Gram-negative bacteria. The safety, tolerability, and pharmacokinetics (PK) of vaborbactam and meropenem following single and multiple ascending doses of each study drug administered alone or combined were evaluated in 76 healthy adult subjects in a randomized, placebo-controlled, double-blind study. Subjects were enrolled in 1 of 5 dose cohorts (receiving 250 to 2,000 mg vaborbactam and/or 1,000 to 2,000 mg meropenem) alone or in combination. No subjects discontinued the study due to adverse events (AEs), and no serious AEs were observed. The pharmacokinetics of meropenem and vaborbactam were similar when given alone or in combination; all evaluated plasma PK exposure measures (peak plasma concentration, area under the plasma concentration-time curve [AUC] from time zero to the last measurable concentration area under the plasma concentration-time curve, and AUC from time zero to infinity) were similar for the study drugs alone versus those in combination, indicating no pharmacokinetic interaction between meropenem and vaborbactam. Across all treatments, 47 to 64% of an administered meropenem dose and 75 to 95% of vaborbactam was excreted unchanged in the urine over 48 h postdose. Meropenem and vaborbactam, when given alone or in combination, have similar pharmacokinetic properties, with no plasma or urine PK drug-drug interactions, and are well tolerated. These findings supported further clinical investigation of the combination product. (This study is registered at ClinicalTrials.gov under registration no. NCT01897779.)
KEYWORDS: meropenem, vaborbactam, pharmacokinetics, beta-lactamase, drug interaction
INTRODUCTION
Beta-lactam antibiotics are among the most useful classes of antibiotics to treat Gram-negative infections. The growth in resistance to beta-lactam antibiotics, including the carbapenems, among Enterobacteriaceae is a tipping point in the growth of Gram-negative infections, as they are becoming untreatable by modern antimicrobial agents. The World Health Organization considers new drug development for carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, and carbapenem-resistant Enterobacteriaceae to be “Priority 1: Critical” (1). The United States Centers for Disease Control and Prevention (CDC) consider carbapenem-resistant Enterobacteriaceae an urgent antimicrobial resistance threat (2). It is critically important that new antibiotics with efficacy against carbapenem-resistant bacteria are developed.
Meropenem is a broad-spectrum injectable carbapenem antibiotic used to treat a wide variety of infections. The spectrum of activity includes many Gram-positive and Gram-negative bacteria and anaerobic bacteria. Recent nonclinical and clinical studies have demonstrated that meropenem doses of 2 g every 8 h (q8h) administered by prolonged 3-h infusion provides optimized pharmacokinetic-pharmacodynamic (PK-PD) exposures that are associated with improved bacterial killing and clinical response (3).
Vaborbactam (previously known as RPX7009) is a beta-lactamase inhibitor that restores the activity of carbapenems against Enterobacteriaceae producing serine carbapenemases, particularly the K. pneumoniae carbapenemase (KPC) enzyme. It is a member of a new cyclic boronic acid class of beta-lactamase inhibitors (4). Meropenem in combination with vaborbactam has been found to be highly active against Gram-negative pathogens, including KPC-producing, carbapenem-resistant Enterobacteriaceae (5, 6).
The primary objective of this study was to assess the safety, tolerability, and pharmacokinetics of vaborbactam and meropenem administered alone and in combination following single and multiple doses to healthy adult subjects to support further clinical development.
RESULTS
Demographics.
Baseline subject demographics are shown in Table 1. A total of 80 subjects were enrolled in the study, with 76 completing the study. Each of the subjects in cohorts 1 to 5 contributed 4 sampling profiles to the plasma PK analysis data set (days 1, 4, 7, and 14).
TABLE 1.
Demographic and baseline characteristics
| Parameter | Value(s) for cohort: |
|||||
|---|---|---|---|---|---|---|
| 1a (n = 30) | 2b (n = 9) | 3c (n = 13) | 4d (n = 14) | 5e (n = 14) | All subjects (n = 80) | |
| Age, yr | ||||||
| Mean (SD) | 24.7 (4.3) | 27.9 (7.5) | 28.2 (8.0) | 27.6 (9.6) | 25.8 (7.3) | 26.3 (6.9) |
| Median (range) | 25.0 (18–38) | 27.0 (21–44) | 25.0 (22–50) | 24.5 (19–50) | 22.5 (19–42) | 25.9 (18–50) |
| Sex, n (%) | ||||||
| Male | 21 (70.0) | 9 (100.0) | 8 (61.5) | 9 (64.3) | 10 (71.4) | 57 (71.3) |
| Female | 9 (30.0) | 0 | 5 (38.5) | 5 (35.7) | 4 (28.6) | 23 (28.8) |
| Race, n (%) | ||||||
| White | 29 (96.7) | 9 (100.0) | 11 (84.6) | 14 (100.0) | 13 (92.9) | 76 (95.) |
| Pacific Islander | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (1.3) |
| Asian | 1 (3.3) | 0 | 1 (7.7) | 0 | 1 (7.1) | 3 (3.8) |
| BMI (kg/m2), mean (SD) | 24.7 (2.3) | 24.2 (2.7) | 24.5 (2.4) | 23.1 (1.4) | 23.6 (2.7) | 24.1 (2.4) |
Meropenem, 1,000 mg; vaborbactam, 250 mg.
Meropenem, 1,000 mg; vaborbactam, 1,000 mg.
Meropenem, 1,000 mg; vaborbactam, 1,500 mg.
Meropenem, 1,000 mg; vaborbactam, 2,000 mg.
Meropenem, 2,000 mg; vaborbactam, 2,000 mg.
Pharmacokinetics. (i) Plasma concentration-time profiles.
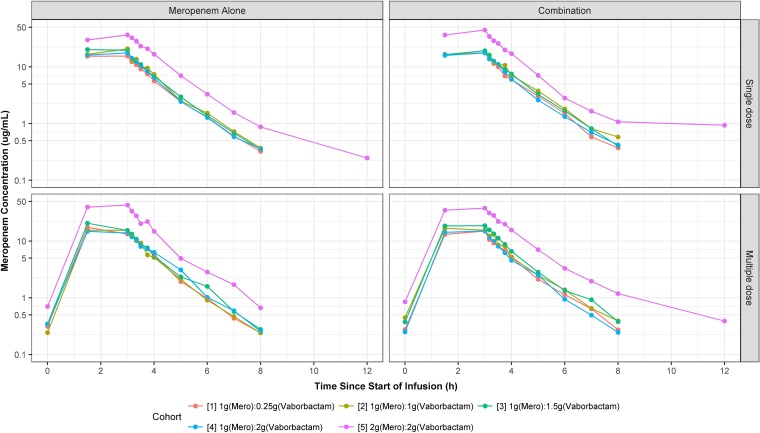
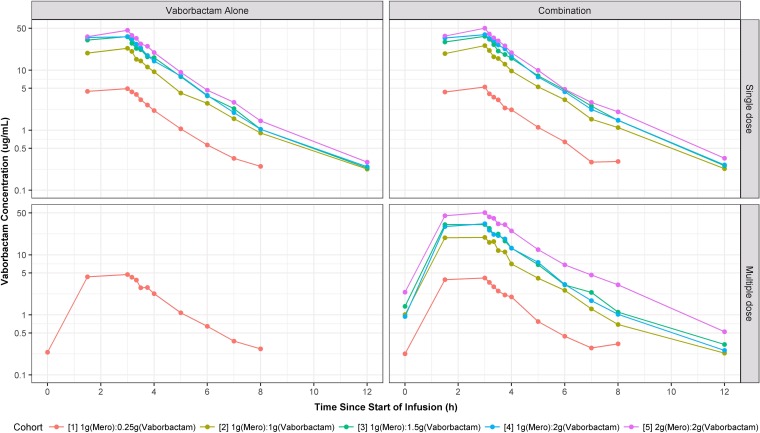
Plots of the median plasma concentration-time profiles for all cohorts are provided in Fig. 1 and 2. Within each cohort, no apparent differences in plasma concentrations of meropenem and vaborbactam were seen regardless of dosing phase (i.e., single versus multiple dosing and study drugs given alone versus combined). During the infusions, median plasma concentrations for vaborbactam and meropenem rapidly increased, with the time of maximum concentration (Tmax) occurring immediately at the end of the 3-h infusion. After completion of the infusion, median plasma concentrations of meropenem and vaborbactam rapidly declined in either a mono- or multiexponential fashion, with geometric mean half-lives ranging from 0.9 to 1.3 h and 1.1 to 1.9 h for meropenem and vaborbactam, respectively. All plasma samples were below the limit of quantification (BLQ) for meropenem and vaborbactam after the 12-h time point.
FIG 1.
Plots of median meropenem plasma concentration-time profiles, stratified by cohort, paneled by treatment and dosing strategy.
FIG 2.
Plots of median vaborbactam plasma concentration-time profiles, stratified by cohort, paneled by treatment and dosing strategy.
(ii) Plasma pharmacokinetic parameters.
Qualitative comparisons of values for meropenem geometric mean peak plasma concentration (Cmax), area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0–t), and AUC from time zero to infinity (AUC0–inf) by cohort suggest that these PK exposure measures increased in only a slightly greater than dose-proportional manner over the evaluated dose range (1,000 to 2,000 mg) (Table 2). For example, in the multiple-dosing phases, the geometric mean AUC0–t in subjects who received 1,000 mg (cohorts 1 to 4) ranged from 47 to 67 μg · h/ml, while the geometric mean AUC0–t in subjects who received 2,000 mg in cohort 5 ranged from 134 to 136 μg · h/ml. The trend was not as consistent with vaborbactam, but qualitative comparisons across evaluated dose levels (250 to 2,000 mg) was suggestive of a similar phenomenon.
TABLE 2.
Pharmacokinetic parameters of meropenem and/or vaborbactam following single or multiple dosesb
| Cohort/dosea and treatment | n |
Cmax (μg/ml) |
t1/2 (h) |
AUC0–inf (μg · h/ml), single dose | AUC0–t (μg · h/ml), multiple dose |
Vss (liters) |
CLT (liter/h) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single dose | Multiple dose | Single dose | Multiple dose | Single dose | Multiple dose | Single dose | Multiple dose | ||||
| Meropenem PK | |||||||||||
| 1/1,000:250 | |||||||||||
| Meropenem alone | 32 | 16.9 (44.7) | 16.5 (58.0) | 1.0 (42.8) | 1.0 (55.5) | 51.6 (43.3) | 49.3 (55.7) | 24.9 (52.7) | 25.7 (77.3) | 19.4 (43.3) | 20.3 (55.6) |
| Combination | 16 | 18.6 (32.4) | 15.7 (34.5) | 1.0 (28.8) | 1.0 (33.4) | 56.1 (30.5) | 47.1 (32.5) | 25.2 (49.5) | 29.4 (62.8) | 17.8 (30.5) | 21.2 (32.8) |
| 2/1,000:1,000 | |||||||||||
| Meropenem alone | 13 | 18.6 (47.4) | 16.4 (38.4) | 1.0 (35.3) | 0.9 (20.3) | 58.1 (47.7) | 49.9 (39.3) | 23.2 (49.1) | 23.2 (34.4) | 17.2 (47.7) | 20.1 (39.2) |
| Combination | 10 | 19.9 (46.6) | 17.0 (32.1) | 1.1 (45.2) | 0.9 (24.0) | 64.1 (51.0) | 53.9 (36.5) | 20.8 (50.7) | 23.1 (44.2) | 15.6 (51.0) | 18.5 (36.2) |
| 3/1,000:1,500 | |||||||||||
| Meropenem alone | 19 | 20.9 (34.8) | 23.3 (59.1) | 0.89 (31.5) | 0.87 (33.2) | 64.6 (38.2) | 67. (55.9) | 18.9 (40.1) | 17.2 (51.5) | 15.5 (38.2) | 14.9 (55.9) |
| Combination | 14 | 20.9 (34.8) | 23.3 (59.1) | 0.89 (31.5) | 0.87 (33.2) | 64.6 (38.2) | 67. (55.9) | 21.3 (47.8) | 18.5 (52.7) | 15.6 (48.4) | 15.5 (48.0) |
| 4/1,000:2,000 | |||||||||||
| Meropenem alone | 19 | 17.3 (37.4) | 15.9 (43.9) | 1.0 (47.5) | 1.0 (52.6) | 53.7 (43.0) | 50.7 (32.7) | 25.0 (47.0) | 25.2 (42.4) | 18.6 (43.0) | 19.7 (32.7) |
| Combination | 14 | 17.5 (23.7) | 15.8 (29.2) | 0.9 (45.5) | 1.1 (39.8) | 55.0 (27.0) | 47.9 (20.7) | 23.8 (29.7) | 25.5 (31.8) | 18.2 (27.0) | 20.9 (20.7) |
| 5/2,000:2,000 | |||||||||||
| Meropenem alone | 20 | 40.9 (53.6) | 46.0 (59.6) | 1.1 (54.90 | 1.0 (46.0) | 125.7 (48.9) | 133.7 (56.4) | 22.2 (52.8) | 20.2 (47.9) | 15.9 (48.9) | 15.0 (56.4) |
| Combination | 16 | 45.7 (35.7) | 42.5 (48.5) | 1.3 (82.6) | 1.2 (56.5) | 139.3 (45.8) | 135.7 (46.8) | 21.7 (38.2) | 21.0 (42.5) | 14.4 (45.8) | 14.9 (45.8) |
| Vaborbactam PK | |||||||||||
| 1/1,000:250 | |||||||||||
| Vaborbactam alone | 40 | 5.2 (41.8) | 4.9 (37.0) | 1.1 (57.2) | 1.1 (50.3) | 17.2 (42.7) | 16.2 (46.8) | 22.2 (48.6) | 23.2 (46.0) | 14.5 (42.7) | 15.3 (45.9) |
| Combination | 16 | 5.3 (40.2) | 4.6 (39.5) | 1.1 (52.0) | 1.2 (38.6) | 17.0 (38.3) | 14.6 (39.8) | 24.3 (49.8) | 27.0 (66.1) | 14.7 (38.3) | 16.9 (39.4) |
| 2/1,000:1,000 | |||||||||||
| Vaborbactam alone | 5 | 21.7 (42.3) | 1.5 (69.6) | 75.4 (48.0) | 21.9 (59.0) | 13.3 (48.0) | |||||
| Combination | 10 | 23.3 (46.1) | 19.9 (29.7) | 1.5 (49.8) | 1.3 (61.8) | 79.4 (46.2) | 68.4 (39.0) | 20.2 (41.8) | 22.5 (29.3) | 12.6 (46.2) | 14.8 (37.4) |
| 3/1,000:1,500 | |||||||||||
| Vaborbactam alone | 7 | 36.3 (41.0) | 1.2 (49.3) | 117.8 (36.6) | 21.1 (68.4) | 12.7 (36.6) | |||||
| Combination | 14 | 36.9 (37.0) | 32.6 (32.5) | 1.3 (44.7) | 1.4 (61.2) | 124.2 (40.4) | 114.7 (38.4) | 19.9 (38.2) | 19.4 (36.7) | 12.1 (40.4) | 13.3 (37.3) |
| 4/1,000:2,000 | |||||||||||
| Vaborbactam alone | 7 | 38.0 (28.8) | 1.3 (54.8) | 125.8 (35.1) | 22.8 (28.3) | 15.9 (35.0) | |||||
| Combination | 14 | 40.0 (22.7) | 34.7 (34.6) | 1.4 (46.7) | 1.5 (56.3) | 132.8 (29.2) | 113.2 (29.8) | 23.8 (26.3) | 26.8 (35.0) | 15.1 (29.2) | 17.9 (28.7) |
| 5/2,000:2,000 | |||||||||||
| Vaborbactam alone | 8 | 49.5 (57.4) | 1.4 (37.7) | 151.9 (54.5) | 21.8 (43.5) | 13.2 (54.5) | |||||
| Combination | 16 | 50.1 (42.6) | 54.7 (47.1) | 1.9 (61.9) | 1.6 (50.5) | 165.3 (45.2) | 192.6 (45.0) | 22.0 (41.0) | 19.3 (36.8) | 12.1 (45.2) | 10.7 (43.1) |
Doses are expressed as meropenem (mg):vaborbactam (mg).
Values shown are geometric means (geometric coefficient of variation); AUC0–t, area under the plasma concentration-time curve from time zero to the last measurable concentration; AUCinf, area under the plasma concentration-time curve from time zero to infinity; CLT, total clearance; Cmax, maximum drug concentration in serum; fe, fraction of administered dose excreted over the urine collection interval, expressed as a percent; t1/2, terminal half-life over the sampling period; Vss, steady-state volume of distribution.
(iii) Clearance.
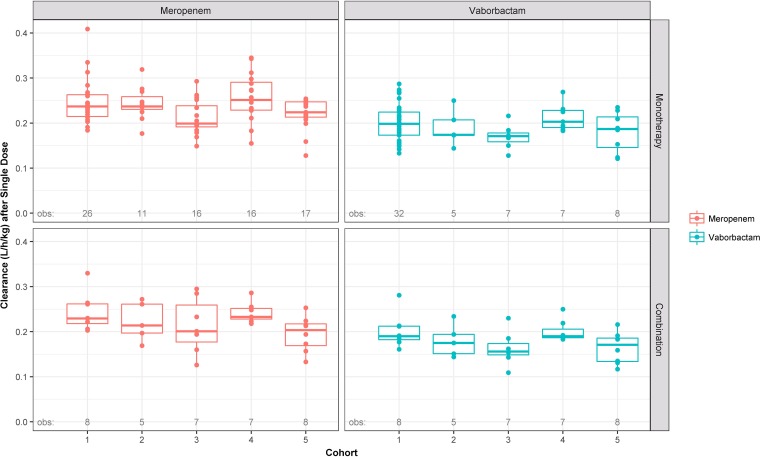
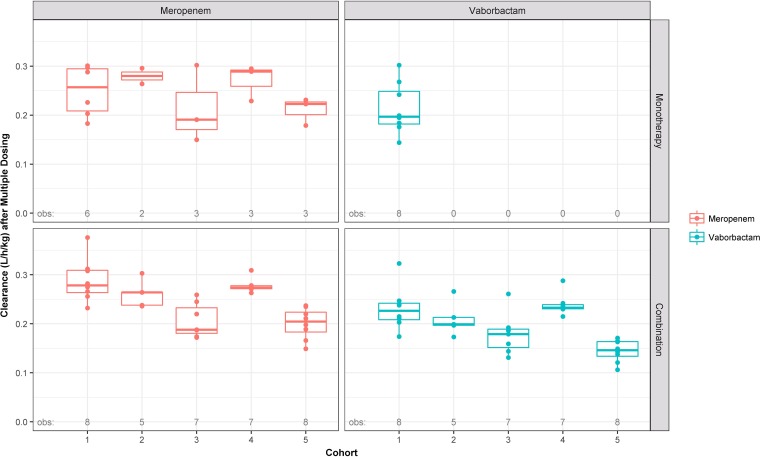
Plasma clearance of both vaborbactam and meropenem was relatively rapid (geometric means across cohorts ranged from 10.7 to 17.9 and 14.4 to 21.2 liters/h, respectively). Graphical depictions of the distributions of weight-normalized plasma clearance (liters/hour/kilogram of body weight) across cohorts, paneled by single or multiple dosing and alone or combination treatment, are provided in Fig. 3 and 4 for meropenem and vaborbactam, respectively. In general, plasma clearance of vaborbactam and meropenem was similar regardless of treatment administration (alone versus combined), indicating that concomitant administration of vaborbactam and meropenem does not affect the plasma PK of either drug (Table 2).
FIG 3.
Box-and-whisker plots of weight-normalized meropenem and vaborbactam plasma clearance following a single dose, stratified by cohort, paneled by treatment and dosing strategy when administered alone or in combination. Circles are the individual points. The horizontal line is the median, the box is the interquartile range (IQR), vertical lines (whiskers) extend to the last values within 1.5 times the IQR, and solid circles are values outside the whiskers.
FIG 4.
Box-and-whisker plots of weight-normalized meropenem and vaborbactam plasma clearance following multiple doses, stratified by cohort, paneled by treatment and dosing strategy when administered alone or in combination. Circles are the individual points. The horizontal line is the median, the box is the interquartile range (IQR), vertical lines (whiskers) extend to the last values within 1.5 times the IQR, and solid circles are values outside the whiskers.
Although there was a trend for the median renal clearance to be lower with combination therapy, the overall range of values was similar across all treatments (single versus multiple dose, alone or combined) (Tables 3 and 4).
TABLE 3.
Meropenem urine PK parameters
| Cohort/dosea and treatment | n | Median (range) amt (mg) excreted as intact meropenem |
Median (range) % of dose excreted as intact meropenem |
Median (range) renal clearance (liter/h) |
|||
|---|---|---|---|---|---|---|---|
| Single dose (collected over 48 h) | Multiple dose (collected over 24 h) | Single dose (collected over 48 h) | Multiple dose (collected over 24 h) | Single dose | Multiple dose | ||
| 1/1,000:250 | |||||||
| Meropenem alone | 32 | 562.8 (358.1–687.4) | 603.5 (503.6–710.8) | 56.3 (35.8–68.7) | 60.4 (50.4–71.1) | 11.4 (5.4–14.9) | 12.6 (8.7–17.9) |
| Combination | 16 | 608.8 (521.3–695.6) | 635.5 (458.4–1299) | 60.9 (52.1–69.6) | 63.6 (45.8–129.9) | 10.5 (8.7–13.1) | 13.5 (10.1–28.0) |
| 2/1,000:1,000 | |||||||
| Meropenem alone | 13 | 534.4 (421.8–613.7) | 545.7 (462.2–629.3) | 53.4 (42.2–61.4) | 54.6 (46.2–62.9) | 8.9 (6.8–11.1) | 10.8 (10.2–11.3) |
| Combination | 10 | 529.4 (437.3–581.2) | 537.8 (423.4–685.4) | 52.9 (43.7–58.1) | 53.8 (42.3–68.5) | 8.1 (6.6–9.7) | 10.4 (8.6–10.8) |
| 3/1,000:1,500 | |||||||
| Meropenem alone | 19 | 558.4 (470.9–619.6) | 606.1 (600.5–611.9) | 55.8 (47.1–62.0) | 60.6 (60.1–61.2) | 8.5 (6.7–10.2) | 10.1 (6.6–11.0) |
| Combination | 14 | 499.7 (346.4–650.1) | 538.6 (376.9–888.5) | 50.0 (34.6–65.0) | 53.9 (37.7–88.8) | 6.9 (4.6–11.4) | 8.7 (5.5–14.5) |
| 4/1,000:2,000 | |||||||
| Meropenem alone | 19 | 563.4 (409.2–734.9) | 564.6 (540.7–602.8) | 56.3 (40.9–73.5) | 56.5 (54.1–60.3) | 10.9 (6.8–15.1) | 11.2 (9.8–12.4) |
| Combination | 14 | 486.6 (384.9–567.4) | 512.4 (88.9–645.9) | 48.7 (38.5–56.7) | 51.2 (8.9–64.6) | 8.6 (7.0–11.6) | 10.5 (1.9–14.1) |
| 5/2,000:2,000 | |||||||
| Meropenem alone | 20 | 1,069 (723.0–1,345) | 1,084 (913.5–1,343) | 53.4 (36.2–67.2) | 54.2 (45.7–67.2) | 8.0 (4.6–11.9) | 8.9 (6.1–10.0) |
| Combination | 16 | 937.8 (844.8–1,191) | 1,120 (829.4–1,202) | 46.9 (42.2–59.6) | 56.0 (41.50–60.1) | 6.9 (5.2–8.9) | 8.1 (5.8–9.4) |
Expressed as meropenem (mg): vaborbactam (mg).
TABLE 4.
Vaborbactam urine PK parameters
| Cohort/dosea and treatment | n | Median (range) amt (mg) excreted as intact vaborbactam |
Median (range) % of dose excreted as intact vaborbactam |
Median (range) renal clearance (liter/h) |
|||
|---|---|---|---|---|---|---|---|
| Single dose (collected over 48 h) | Multiple dose (collected over 24 h) | Single dose (collected over 48 h) | Multiple dose (collected over 24 h) | Single dose | Multiple dose | ||
| 1/1,000:250 | |||||||
| Vaborbactam alone | 40 | 206.2 (124.3–356.5) | 219.1 (49.3–256.6) | 82.5 (49.7–142.6) | 87.6 (19.7–102.6) | 12.2 (6.3–23.7) | 13.1 (3.3–19.7) |
| Combination | 16 | 212.1 (200.1–2,824) | 231.1 (196.9–588.2) | 84.84 (80.0–1,130) | 95.2 (78.8–235.3) | 11.8 (10.1–189.8) | 17.6 (12.5–44.9) |
| 2/1,000:1,000 | |||||||
| Vaborbactam alone | 5 | 810.0 (789.7–922.2) | 81.0 (79.0–92.2) | 11.0 (8.8–14.9) | |||
| Combination | 10 | 830.9 (773.4–682.5) | 858.2 (757.9–930.3) | 83.1 (77.3–86.3) | 85.8 (75.8–93.0) | 10.3 (8.6–12.7) | 11.5 (10.6–16.1) |
| 3/1,000:1,500 | |||||||
| Vaborbactam alone | 7 | 1,233 (1,010–1,400) | 82.2 (67.3–93.3) | 9.8 (8.5–14.0) | |||
| Combination | 14 | 1,128 (858.9–1,324) | 1,284 (1,015–1,728) | 75.2 (57.3–88.3) | 85.6 (67.6–115.2) | 8.6 (6.8–11.0) | 12.3 (8.5–15.6) |
| 4/1,000:2,000 | |||||||
| Vaborbactam alone | 7 | 1,744 (1,591–1,825) | 87.2 (79.6–91.3) | 14.1 (12.2–15.4) | |||
| Combination | 14 | 1,718 (1,341–1,781) | 1,697 (318.9–1,821) | 85.9 (67.1–89.1) | 84.8 (16.0–91.1) | 12.6 (10.5–15.3) | 13.9 (2.8–18.5) |
| 5/2,000:2,000 | |||||||
| Vaborbactam alone | 8 | 1,785 (1,347–2,070) | 89.3 (67.4–103.5) | 10.8 (8.6–14.4) | |||
| Combination | 16 | 1,548 (1,388–1,622) | 1,715 (1,502–1,926) | 77.4 (69.4–81.1) | 85.8 (75.1–96.3) | 10.0 (6.9–11.4) | 8.6 (6.5–12.1) |
Expressed as meropenem (mg): vaborbactam (mg).
(iv) Urinary excretion.
Across cohorts, 47 to 64% of an administered meropenem dose was recovered as unchanged drug in the urine over 48 h postdose, whereas 81 to 95% of the vaborbactam dose was recovered in urine over the same time period (Tables 3 and 4). The overall range of values was similar across all treatments (single versus multiple dosing and alone versus combined).
(v) Drug-drug interaction.
For all plasma PK exposure measures (Cmax, AUC0–t, and AUC0–inf), the 90% confidence intervals (CIs) for the least-squares (LS) geometric mean ratios were completely contained within the bioequivalence window of 0.8 to 1.25 for both vaborbactam and meropenem (Table 5). The results of these statistical comparisons indicate that the exposure to either vaborbactam or meropenem was not different when the study drugs were given alone or in combination.
TABLE 5.
Drug-drug interaction analysis
| Parameter | ANOVA model applied to meropenem PK parametersa |
ANOVA model applied to vaborbactam PK parametersb |
||
|---|---|---|---|---|
| Meropenem alone | Combination | Vaborbactam alone | Combination | |
| Cmax (μg/ml) | ||||
| LS geometric mean | 21.6 | 23.2 | 22.8 | 24.1 |
| LS geometric mean (90% CI) | 19.1, 24.3 | 20.6, 26.1 | 17.5, 29.6 | 18.5, 31.4 |
| LS geometric mean ratio | 1.07 | 1.06 | ||
| LS geometric mean ratio (90% CI) | 1.01, 1.14 | 1.01, 1.11 | ||
| AUC0–t (μg · h/ml) | ||||
| LS geometric mean | 65.5 | 71.2 | 73.6 | 78.8 |
| LS geometric mean (90% CI) | 58.1, 73.4 | 63.2, 80.2 | 56.5, 95.9 | 60.5, 103 |
| LS geometric mean ratio | 1.09 | 1.07 | ||
| LS geometric mean ratio (90% CI) | 1.04, 1.13 | 1.04, 1.11 | ||
| AUC0–inf (μg · h/ml) | ||||
| LS geometric mean | 66.0 | 71.9 | 74.6 | 79.9 |
| LS geometric mean (90% CI) | 58.6, 74.4 | 63.8, 81.1 | 57.4, 96.9 | 61.5, 104 |
| LS geometric mean ratio | 1.09 | 1.07 | ||
| LS geometric mean ratio (90% CI) | 1.05, 1.14 | 1.04, 1.11 | ||
Only those subjects who crossed over from single-dose meropenem to single-dose combination treatment are included in this analysis (n = 35).
Only those subjects who crossed over from single-dose vaborbactam to single-dose combination treatment are included in this analysis (n = 35).
(vi) Dose proportionality.
An assessment of dose proportionality for vaborbactam was conducted. Vaborbactam plasma PK appeared to follow dose proportionality for AUC0–inf, AUC0–t, and Cmax (results not shown). The criteria were not strictly met for all of the comparisons, but the 90% confidence for the power coefficient for AUC0–inf did contain 1.00, and all of the other power coefficients were close to 1.00.
Safety and tolerability.
There were no deaths or other serious adverse events (AEs). One subject in cohort 4 terminated the study due to an AE (thrombophlebitis after 19 doses of 1 g meropenem in combination with 2 g vaborbactam). Two subjects chose to withdraw early from the study due to personal reasons, and one subject was withdrawn early by the principal investigator. The subject withdrawn by the principal investigator was in cohort 1 on meropenem at 1 g alone and was withdrawn due to lack of venous access.
Treatment-emergent adverse events (TEAEs) were reported for 62 of 80 (77.5%) subjects. This included 12 (75%) subjects following administration of placebo, 18 (94.7%) subjects following administration of meropenem alone, 6 (75%) subjects following administration of vaborbactam alone, and 26 (70.3%) subjects following meropenem in combination with vaborbactam. The most common TEAEs in the pooled meropenem/vaborbactam combination group following multiple doses were infusion site phlebitis and infusion site pain (Table 6). There was no evidence for an increasing incidence of related AEs with increasing doses of meropenem or vaborbactam or the combination of meropenem and vaborbactam following multiple-dose administration.
TABLE 6.
Commonly occurring treatment-emergent AEs that occurred in >10% of subjects during multiple-dose treatment
| AE | No. (%) of subjects positive/no. of events |
|||
|---|---|---|---|---|
| Pooled placebo (n = 16) | Pooled meropenem alone (n = 19) | Vaborbactam alone (n = 8) | Pooled meropenem/vaborbactam combination (n = 37) | |
| >1 TEAE | 12 (75.0)/19 | 18 (94.7)/39 | 6 (75.0)/11 | 26 (70.3)/67 |
| Infusion site phlebitis | 4 (25.0)/4 | 7 (36.8)/7 | 2 (25.0)/3 | 23 (62.2)/44 |
| Infusion site pain | 2 (12.5)/3 | 3 (15.8)/3 | 0 | 13 (35.1)/16 |
| Infusion site erythema | 3 (18.8)/3 | 5 (26.3)/7 | 0 | 3 (8.1)/3 |
| Infusion site swelling | 3 (18.8)/3 | 1 (5.3)/2 | 0 | 3 (8.1)/4 |
| Vessel puncture site hematoma | 2 (12.5)/2 | 0 | 0 | 5 (13.5)/5 |
| Fatigue | 0 | 1 (5.3)/1 | 1 (12.5)/1 | 1 (2.7)/1 |
| Catheter site hematoma | 0 | 4 (21.1)/4 | 0 | 4 (10.8)/4 |
| Catheter site erythema | 2 (12.5)/2 | 0 | 0 | 2 (5.4)/2 |
| Catheter site pain | 0 | 0 | 0 | 4 (10.8)/4 |
| Catheter site phlebitis | 0 | 0 | 2 (25.0)/2 | 2 (5.4)/2 |
| Headache | 2 (12.5)/2 | 6 (31.6)/9 | 2 (25.0)/3 | 8 (21.6)/10 |
| Diarrhea | 0 | 2 (10.5)/2 | 0 | 1 (2.7)/1 |
| Erythema | 0 | 0 | 3 (37.5)/3 | 0 |
| Infusion site cellulitis | 0 | 2 (10.5)/2 | 0 | 0 |
| Decreased appetite | 0 | 2 (10.5)/2 | 0 | 0 |
There were no differences in safety as assessed by clinical laboratory tests (hematology, biochemistry, coagulation, and urinalysis) or vital sign assessments between subjects who had received meropenem, vaborbactam, or the combination of meropenem and vaborbactam. No electrocardiogram (ECG) changes were assessed as clinically significant by the principal investigator, and no ECG abnormalities were reported as AEs.
DISCUSSION
The single- and multiple-dose safety, tolerability, and pharmacokinetics of vaborbactam and meropenem, when given alone or in combination, were investigated in healthy adult subjects. Both drugs, at doses of up to 2,000 mg q8h, were well tolerated, and no safety concerns were identified when given alone or in combination. There was no evidence for an increasing incidence of any AE with increasing doses of meropenem or vaborbactam alone or in combination. Both drugs exhibited a similar plasma terminal elimination half-life, and no apparent differences in plasma PK exposure or urine PK parameter measures were seen when each drug was given alone or in combination.
The range of meropenem geometric mean plasma clearance estimates in this study (14.4 to 21.2 liters/h; 13.4 to 20.2 liters/h/70 kg) were generally consistent with those from a previously published report showing a mean meropenem plasma clearance of 15.5 liters/h/70 kg (7). Similarly, meropenem plasma PK exposure estimates in subjects receiving a 3-h infusion were consistent with a previous study conducted in normal, healthy volunteers (8) reporting mean Cmax and AUC0–inf estimates of 39.8 μg/ml and 127 μg · h/ml, respectively, after administration of 2,000 mg of meropenem over 3 to 6 h in normal healthy adults. The same can be said of other calculated meropenem PK parameters, in that they are generally consistent with a previously published report (7).
The observed range of median renal clearance values for both vaborbactam (8.6 to 17.6 liters/h) and meropenem (6.9 to 13.5 liters/h) exceeded the reported normal range for glomerular filtration rate (GFR) in healthy subjects (5.4 to 7.2 liters/h), indicating both renal filtration and active tubular secretion for these agents. This finding for meropenem is consistent with that reported in the meropenem drug label (9). Although there was a trend for the median renal clearance values of the study drugs to be lower with combination therapy, the overall range of values was similar across all treatments (single and multiple dosing, alone or combined) for both vaborbactam and meropenem (10). Vaborbactam was developed in combination with meropenem and shows potent in vitro and in vivo activity against carbapenem-resistant KPC-containing strains of Enterobacteriaceae. Overall, this study demonstrated the safety and tolerability of vaborbactam and meropenem when given alone or in combination. Importantly, the study showed similar pharmacokinetic properties for meropenem and vaborbactam at all doses, including that for 2-g doses of each component used in phase 3 clinical trials for the treatment of complicated urinary tract infections, including acute pyelonephritis (ClinicalTrials registration no. NCT02166476) and serious infections due to carbapenem-resistant Enterobacteriaceae, including hospital-acquired and ventilator-associated pneumonia (ClinicalTrials registration no. NCT02168946).
MATERIALS AND METHODS
Study design.
Plasma and urine pharmacokinetics of meropenem alone, vaborbactam alone, and both in combination following single and multiple doses in healthy adult subjects were investigated in a phase 1 healthy-volunteer study (ClinicalTrials registration no. NCT01897779). The study was conducted at a clinical research facility in Adelaide, Australia (CMAX, Royal Adelaide Hospital, North Terrace, Adelaide, Australia). The doses of vaborbactam used in this study were selected using information gained from previous nonclinical and clinical experience (11, 12). Meropenem is approved in many countries for use at doses of up to 2 g q8h (9). Administrations of meropenem or vaborbactam alone as single or repeated doses were used for comparisons with the combination arms.
A total of 80 subjects were enrolled and formed a total of 5 dose cohorts receiving 3-h infusions of the study drugs, with 76 subjects completing the trial. Subjects participated in only one cohort each. Each dose level cohort had 4 treatment arms. The length of participation in the study for each subject (excluding screening) was approximately 14 days. Subjects received single doses on days 1, 2, and 7 and multiple doses on days 8 through 14.
Prior to undergoing any screening or study activities, all subjects signed an informed consent document approved by a local ethics committee. After informed consent was obtained, a medical history, physical examination, and electrocardiography were conducted. Blood was drawn to test for abnormalities and alcohol abuse, and vital signs and concomitant medications were recorded. Male or female (non-child-bearing potential) subjects were included in the study if they were aged 18 to 55 years, had a body mass index (BMI) within the range of 18.5 to 29.9 kg/m2, were a nonsmoker for the previous 6 months, and had no significant illness. Subjects were excluded if they had a history of being HIV, HBsAg, or hepatitis C virus positive, were excessive drinkers (>3 drinks per day or >21 drinks per week), were on any medications other than oral contraceptives, were pregnant or lactating, had a creatinine clearance of <50 ml/min, or had a corrected QT, Fridericia's formula (QTcF) of >450 at screening. Eligible subjects were then randomized via a computerized randomization scheme created by an unblinded statistician. The randomization scheme was available to only the pharmacy staff who prepared the study drug and was not available to the sponsor, study subjects, or members of the staff responsible for the monitoring and evaluation of safety assessments.
This study was conducted in compliance with the protocol and all regulatory requirements in accordance with good clinical practice (GCP), including the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. The protocol, the informed consent document, and all relevant supporting data were reviewed and approved by the Bellberry Human Research Ethics Committee.
Safety.
Safety was assessed throughout the study via medical history, physical examination, monitoring of vital signs, electrocardiograms (ECGs), and clinical safety laboratory work before and through 96 h after dosing. Additionally, safety was monitored by subject self-reports of adverse events (AEs) and specific inquiries to subjects regarding symptoms. Subjects with more than 1 episode of the same adverse event were recorded only once in the per-subject analysis.
After the completion of each cohort, blinded safety data (i.e., physical examinations, vital signs, ECGs, laboratory test results, and AEs), including data from previous cohorts, were reviewed by a Safety Review Committee consisting of the Principal Investigator, the sponsor's medical monitor, and an independent physician who was not an employee of either the investigational site or the sponsor. All cohorts proceeded as planned with dose escalations.
Pharmacokinetic samples.
Blood samples for the measurement of meropenem and vaborbactam were collected at predefined times after each single dose of drug(s) and after the last dose during multiple-dosing periods. Blood samples were obtained at the following times after single-dose administration: predose and 1.5 (midpoint of the infusion), 3, 3.167, 3.333, 3.5, 3.75, 4, 5, 6, 7, 8, 12, and 24 h after the start of the 3-h infusion. After the start of the multiple-dosing period in these cohorts (day 8), predose and end-of-infusion samples were drawn in the morning on days 9, 11, and 13. A full profile was then obtained around the final dose on day 14 at the following times: predose and 1.5, 3, 3.167, 3.333, 3.5, 3.75, 4, 5, 6, 7, 8, 12, and 24 h after the start of the 3-h infusion. Urine for PK analysis was obtained at the following intervals on days 1, 4, 7, and 14: 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h. Additionally, urine was collected over the 24- to 48-h postdose window on days 1, 4, and 14. All blood samples (approximately 2 ml) were obtained via an indwelling intravenous cannula, collected into EDTA-containing tubes, immediately placed on ice, and centrifuged at 3,000 × g for 15 min. All plasma samples were diluted 1:1 with 3-(-N-morpholino) propanesulfonic acid (MOPS) buffer (to stabilize meropenem), thoroughly mixed, and frozen. Urine sample volumes were recorded for each collection period, and an aliquot was removed and diluted 1:1 with MOPS buffer, mixed, and frozen.
Bioanalytical analyses.
Concentrations of meropenem and vaborbactam in plasma and urine were measured by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) at MicroConstants, Inc. (San Diego, CA, USA).
The calibration range of the plasma assay for meropenem and vaborbactam was linear (r2 of ≥0.999) from 0.2 to 100 μg/ml. A total of 5,492 unique samples were analyzed in 59 analytical runs, which all met acceptance criteria for standard curve and quality control (QC) samples. The accuracy of the method was determined by comparing the mean measured concentrations with theoretical concentrations of each analyte in the QC samples. The deviations of the means from theoretical values did not exceed ±1.13% and ±0.93% for meropenem and vaborbactam, respectively. The precision was determined from the percent coefficient of variation (%CV) of the QC sample replicates at each concentration level. The %CV for meropenem and vaborbactam ranged from 6.11% to 6.56% and 3.46% to 17.1%, respectively.
Pharmacokinetic analyses.
Plasma and urine PK parameters for the evaluated analytes were calculated using noncompartmental methods (Phoenix WinNonlin, version 6.3). All postdose plasma concentration values below the lower limit of quantitation (BLQ) were treated as missing, and no imputation was performed.
The pharmacokinetic parameters that were determined included the peak plasma concentration (Cmax), time to maximum plasma concentration (Tmax), area under the plasma concentration-time curve (AUC), total clearance (CLT), renal clearance (CLR), volume of distribution at steady-state (Vss), plasma half-life (t1/2), and the percentage of the administered dose excreted intact in urine (% fe).
Statistical analyses.
Statistical comparisons of the potential for an interaction between vaborbactam and meropenem (comparison of alone versus combination treatment) were done for Cmax, AUC0–t, and AUC0–inf (single-dose comparisons only) using analysis of variance (ANOVA) and mixed-model analyses based on the ln-transformed data. The ANOVA model included sequence, treatment, and period as fixed effects and subject nested within sequence as a random effect. Only those subjects who received both study drugs were included in the analysis. Given the crossover nature of this comparison, data were also pooled and analyzed independently of cohort. Least-squares (LS) means for each treatment and differences between LS means were computed for the ln-transformed PK parameters together with their associated 90% CI using the residual variance derived from the mixed-model ANOVA. The analyses were performed using the SAS PROC MIXED procedure. The exponentiated LS means were presented as geometric means, and the exponentiated differences between LS means were presented as geometric mean ratios (GMR) (combination/alone). Consistent with the approach of 2 one-sided tests to assess bioavailability, the endpoints for each of the designated treatments was the 90% CI for the GMR that are derived from the analyses of the ln-transformed PK parameters Cmax, AUC0–t, and AUC0–inf. The 90% CI for the GMR were derived from the exponentiated confidence limits for the 90% CI for the differences between LS means on the natural log scale. The statistical comparisons of the PK parameters were assessed based on whether the 90% CI for the geometric mean ratios for the defined comparisons were within the 80% to 125% interval, as defined by FDA guidance (13).
Dose proportionality of vaborbactam was assessed using the power model (14): P = μ × doseβ, where P is the value of the PK exposure parameter, μ and β represent constants, and dose is the dose at which the parameter was derived. The power model was fit to the pooled, individual subject data using nonlinear least-squares regression (data were log transformed if appropriate). If the 90% CI of β included 1.0, then linearity was concluded.
ACKNOWLEDGMENTS
This work, and the efforts of J.S.L., E.E. M., D.W., M.N.D., and D.C.G., were funded in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA), under contract no. HHSO100201400002C with Rempex Pharmaceuticals.
We acknowledge writing assistance provided by Starr Grundy of SD Scientific, Inc., that was funded by The Medicines Company.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for the manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
REFERENCES
- 1.World Health Organization. 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/. [Google Scholar]
- 2.Centers for Disease Control and Prevention, Office of Infectious Disease. 2013. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/ Accessed 5 May 2017.
- 3.Grupper M, Kuti JL, Nicolau DP. 2016. Continuous and prolonged intravenous beta-lactam dosing: implications for the clinical laboratory. Clin Microbiol Rev 29:759–772. doi: 10.1128/CMR.00022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN. 2015. Discovery of a cyclic boronic acid beta-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem 58:3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 5.Lapuebla A, Abdallah M, Olafisoye O, Cortes C, Urban C, Quale J, Landman D. 2015. Activity of meropenem combined with RPX7009, a novel beta-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob Agents Chemother 59:4856–4860. doi: 10.1128/AAC.00843-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. 2016. Effect of the beta-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano GL, Hutchison M. 1995. The pharmacokinetics of meropenem. Scand J Infect Dis Suppl 96:11–16. [PubMed] [Google Scholar]
- 8.Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP. 2003. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 23:988–991. doi: 10.1592/phco.23.8.988.32878. [DOI] [PubMed] [Google Scholar]
- 9.AstraZeneca Pharmaceuticals LP 2016. MERREM I.V. (meropenem for injection) Prescribing information. AstraZeneca Pharmaceuticals LP, Gaithersburg, MD. [Google Scholar]
- 10.The Medicines Company. 2017. Vabomere (meropenem and vaborbactam for injection) prescribing information. The Medicines Company, San Diego, CA. [Google Scholar]
- 11.Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. 2016. Phase 1 study of the safety, tolerability, and pharmacokinetics of the beta-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob Agents Chemother 60:6326–6332. doi: 10.1128/AAC.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomovskaya O, Griffith DC, Loutit JS, Dudley MN. 2015. Rationale for dose selection for Carbavance (CVC; meropenem/RPX7009) in phase 3 trials. Open Form Infect Dis 2:794. [Google Scholar]
- 13.U.S. FDA 2001. Guidance for industry–statistical approaches to establishing bioequivalence. U.S. FDA, Washington, DC. [Google Scholar]
- 14.Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey L, McKellar J. 1995. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Ther Innov Regul Sci 29:1039–1048. [Google Scholar]