ABSTRACT
Bacterial sepsis is a major cause of morbidity and mortality in neonates, especially those involving methicillin-resistant Staphylococcus aureus (MRSA). Guidelines by the Infectious Diseases Society of America recommend the vancomycin 24-h area under the concentration-time curve to MIC ratio (AUC24/MIC) of >400 as the best predictor of successful treatment against MRSA infections when the MIC is ≤1 mg/liter. The relationship between steady-state vancomycin trough concentrations and AUC24 values (mg·h/liter) has not been studied in an Asian neonatal population. We conducted a retrospective chart review in Singapore hospitals and collected patient characteristics and therapeutic drug monitoring data from neonates on vancomycin therapy over a 5-year period. A one-compartment population pharmacokinetic model was built from the collected data, internally validated, and then used to assess the relationship between steady-state trough concentrations and AUC24. A Monte Carlo simulation sensitivity analysis was also conducted. A total of 76 neonates with 429 vancomycin concentrations were included for analysis. Median (interquartile range) was 30 weeks (28 to 36 weeks) for postmenstrual age (PMA) and 1,043 g (811 to 1,919 g) for weight at the initiation of treatment. Vancomycin clearance was predicted by weight, PMA, and serum creatinine. For MRSA isolates with a vancomycin MIC of ≤1, our major finding was that the minimum steady-state trough concentration range predictive of achieving an AUC24/MIC of >400 was 8 to 8.9 mg/liter. Steady-state troughs within 15 to 20 mg/liter are unlikely to be necessary to achieve an AUC24/MIC of >400, whereas troughs within 10 to 14.9 mg/liter may be more appropriate.
KEYWORDS: AUC, MRSA, Monte Carlo simulation, neonatal intensive care unit, neonatal sepsis, neonates, pharmacokinetics, population pharmacokinetic model, target trough, vancomycin
INTRODUCTION
Bacterial sepsis remains a major cause of morbidity and mortality in newborns (1, 2), especially for preterm neonates (3–5). One of the most common pathogens responsible for neonatal sepsis is coagulase-negative Staphylococcus (6). Methicillin-resistant Staphylococcus aureus (MRSA) infections are much less common (7), but they result in high morbidity and mortality (8). In view of these pathogens, vancomycin is commonly administered as first-line therapy (6, 9) due to its good antibacterial activity. Almost 10% of neonates admitted to neonatal intensive care units received at least one dose of vancomycin (10). Despite the long history of use, the optimal use of vancomycin in neonates has not been established (11, 12). Clinical trials on vancomycin use in neonates are lacking (13). Because of the high disease burden associated with MRSA infections (8), optimization of vancomycin pharmacotherapy in neonates for empirical coverage and targeted therapy against MRSA is needed to maximize the likelihood of successful clinical outcomes and minimize the risk of vancomycin toxicity.
In 2011, the Infectious Diseases Society of America (IDSA) published the first set of standardized guidelines regarding vancomycin use for the treatment of health care-associated and community-associated MRSA infections in children and adults (14). The key recommendation is to use therapeutic drug monitoring to ensure sufficient drug exposure. Specifically, a ratio of the steady-state area under the concentration-time curve over the past 24 h to the MIC (AUC24/MIC) of >400 for isolates with a drug MIC of ≤1 mg/liter has been found to be the best predictor of successful treatment against MRSA infections (14–17). This target exposure was derived from adult studies, and there has been no study conducted to validate it in neonates who might exhibit differences in vancomycin pharmacokinetics (PK) and pharmacodynamics. Nevertheless, the target of an AUC24/MIC ratio of >400 serves as a useful reference point for the optimization of vancomycin pharmacotherapy in neonates until more validated pharmacodynamic targets are discovered. For an MIC of ≤1, an AUC24 (mg·h/liter) of >400 is equivalent to an AUC24/MIC of >400.
Intensive blood sampling is the most accurate way to directly monitor AUC24, but this is impractical in clinical settings and ethically unfeasible in neonates. Monitoring steady-state trough concentrations as a surrogate measure of AUC24 would be more viable due to its simplicity (14, 17), and the IDSA recommends steady-state trough concentrations of 15 to 20 mg/liter for children and adults to achieve AUC24 > 400 (14). Thus, determining a steady-state trough target range that is highly predictive of achieving AUC24 > 400 will be a practical starting point to guide clinicians in the monitoring of vancomycin exposure in neonates.
Nephrotoxicity is the main adverse effect of concern with vancomycin use, and the risk is positively associated with trough concentration (18) and therefore drug exposure. Hence, when determining a steady-state trough target range for achieving AUC24 > 400, the magnitude of the AUC24 attained should also be considered. An AUC24 of 700 has been suggested as a conservative upper limit with a minimal risk of vancomycin toxicity in adults (19). Even though the IDSA recommends steady-state trough concentrations of 15 to 20 mg/liter for children and adults to achieve AUC24 > 400 (14), a lower trough target range for neonates that is still able to achieve sufficient drug exposure would be appealing because of the potential reduction in the risk of overexposure and subsequent nephrotoxicity. A population pharmacokinetic study conducted with Caucasian neonates showed that a vancomycin trough concentration of ∼10 mg/liter was enough to reliably achieve AUC24 > 400 (20) in most of the study neonates. This finding was further reinforced by external validation in a follow-up study (21). In this light, higher trough concentrations of 15 to 20 mg/liter may not be necessary to achieve AUC24 > 400 in neonatal patients.
The primary objective of our study was to determine the minimum vancomycin trough concentration range that is predictive of achieving AUC24 > 400 in our Asian neonatal population in Singapore.
RESULTS
Patient population.
A total of 76 eligible neonates were included in this study and patient characteristics are summarized in Table 1. Seventy (92%) of them were born at gestation less than 37 weeks, and 53 (70%) were of extremely low birth weight (<1,000 g). Vancomycin therapy was initiated after the first 2 weeks of life in majority of the neonates. Of the 429 vancomycin concentrations available for analysis, 58 (14%) had imputed serum creatinine levels (sCr, μmol/liter). There were 217 troughs (10 troughs were below the quantification limit [BQL]), 203 peaks, and 9 random concentrations.
TABLE 1.
Patient characteristics (n = 76)
| Characteristic | No. (%) | Median (IQRa) | Minimum | Maximum |
|---|---|---|---|---|
| Site | ||||
| KK Women's and Children's Hospital | 29 (38) | |||
| Singapore General Hospital | 47 (62) | |||
| Initial dosing regimen | ||||
| 10 mg/kg q8 h | 8 (11) | |||
| 10 mg/kg q12 h | 5 (7) | |||
| 10 mg/kg q24 h | 1 (1) | |||
| 15 mg/kg q6 h | 2 (3) | |||
| 15 mg/kg q8 h | 13 (17) | |||
| 15 mg/kg q12 h | 14 (18) | |||
| 15 mg/kg q18 h | 20 (26) | |||
| 15 mg/kg q24 h | 13 (17) | |||
| Gestational age (wks) | 26.2 (25–28.8) | 23.9 | 40.3 | |
| <30 | 60 (79) | |||
| 30–36 | 10 (13) | |||
| ≥37 | 6 (8) | |||
| Postmenstrual age at initiation of vancomycin therapy (wks) | 30.1 (27.9–36.2) | 25.1 | 57.5 | |
| <30 | 37 (49) | |||
| 30–36 | 22 (29) | |||
| ≥37 | 17 (22) | |||
| Postnatal age at initiation of vancomycin therapy (days) | 17.4 (11.4–40.6) | 4 | 223.7 | |
| ≤14 | 23 (30) | |||
| >14 | 53 (70) | |||
| Birth wt (g) | 828 (699–1,076) | 320 | 3,655 | |
| wt at initiation of vancomycin therapy (g) | 1,043 (811–1,919) | 320 | 6,590 | |
| sCr at initiation of vancomycin therapy (μmol/liter) | 43 (36–59) | 7.5 | 130 | |
| Apgar scoreb | ||||
| 1 min | 5 (4–6) | 1 | 9 | |
| 5 min | 8 (7–9) | 3 | 10 | |
| Male | 44 (58) | |||
| Race | ||||
| Chinese | 39 (51) | |||
| Malay | 15 (20) | |||
| Indian | 13 (17) | |||
| Other | 9 (12) | |||
| SGA/IUGRc | 16 (21) | |||
| Coadministered drugs | ||||
| Gentamicin | 14 (18) | |||
| Amikacin | 13 (17) | |||
| Ibuprofen | 5 (7) | |||
| Indomethacin | 1 (1) | |||
| Furosemide | 17 (22) |
IQR, interquartile range.
n = 75; one neonate had missing data for the 1- and 5-min Apgar scores.
SGA/IUGR, small for gestation age/intrauterine growth restriction.
Population pharmacokinetic analysis and model evaluation.
A one-compartment model with first-order elimination adequately described the vancomycin concentration-time course in the study subjects. An exponential error model for between-subject variability (BSV) and a combined proportional-additive error model for residual unexplained variability (RUV) resulted in the largest decrease in the objective function value (OFV; i.e., the best model fit) for the base model.
All covariates tested in univariate analysis were supported by individual Bayesian PK parameter estimates and residual versus covariate plots. The final population model selected after covariate screening and forward addition/backward elimination comprised the equations CL (liters/h) = 0.0519 · (WT/1,000)3/4 · (PMA/30.1)2.4 · (43/sCr)0.246 · exp[η∼N(0, σ2 = 0.0623)] and V (liters/kg) = 0.498 · (WT/1,000) · exp[η∼N(0, σ2 = 0.00926)] (ΔOFV = −758.8, ΔBSVCL = −92.2%, and ΔBSVV = −50.3%, compared to the base model). Details of this final model are presented in Table 2. Even though a final model incorporating a sigmoid maximum effect maturation function for PMA (PMA = postmenstrual function in weeks), WT (WT = weight in grams; power model), and sCr (power model) had the best model fit (ΔOFV = −767.3), it was not chosen because of unacceptably large standard errors and 95% confidence intervals for clearance in liters per hour (CL = clearance in liters/h; data not shown).
TABLE 2.
Final model parameter estimates versus bootstrap evaluationa
| Population PK parameterb | Final modelc |
Bootstrapd (n = 5,000) |
|||
|---|---|---|---|---|---|
| Estimate | % relative SE | 95% CI | Median | 95% CI | |
| CL (liters/h) | 0.0519 | 2.9 | 0.0490–0.0550 | 0.0519 | 0.0490–0.0552 |
| V (liters/kg) | 0.498 | 2.7 | 0.472–0.524 | 0.498 | 0.474–0.526 |
| PMA exponent | 2.4 | 10 | 1.932–2.868 | 2.39 | 1.93–2.90 |
| sCr exponent | 0.246 | 25.2 | 0.125–0.367 | 0.253 | 0.145–0.407 |
| BSV | |||||
| BSVCL (% CV) | 25.4 | 13.0 | 24.9 | 18.6–31.6 | |
| BSVV (% CV) | 9.6 | 30.0 | 9.62 | 3.20–14.0 | |
| RUV | |||||
| RUVproportional (variance) | 0.0223 | 32.4 | 0.0211 | 0.0109–0.0373 | |
| RUVadditive (variance) | 2.21 | 27.1 | 2.05 | 1.01–3.42 | |
SE, standard error; CI, confidence interval.
CL, clearance; V, volume of distribution; PMA, postmenstrual age (in weeks); sCr, serum creatinine level (μmol/L); BSV, between-subject variability; CV, coefficient of variation; RUV, residual unexplained variability.
Final model: CL = 0.0519 · (WT/1,000)3/4 · (PMA/30.1)2.4 · (43/sCr)0.246; V = 0.498 · (WT/1,000).
Bootstrap success rate = 100%.
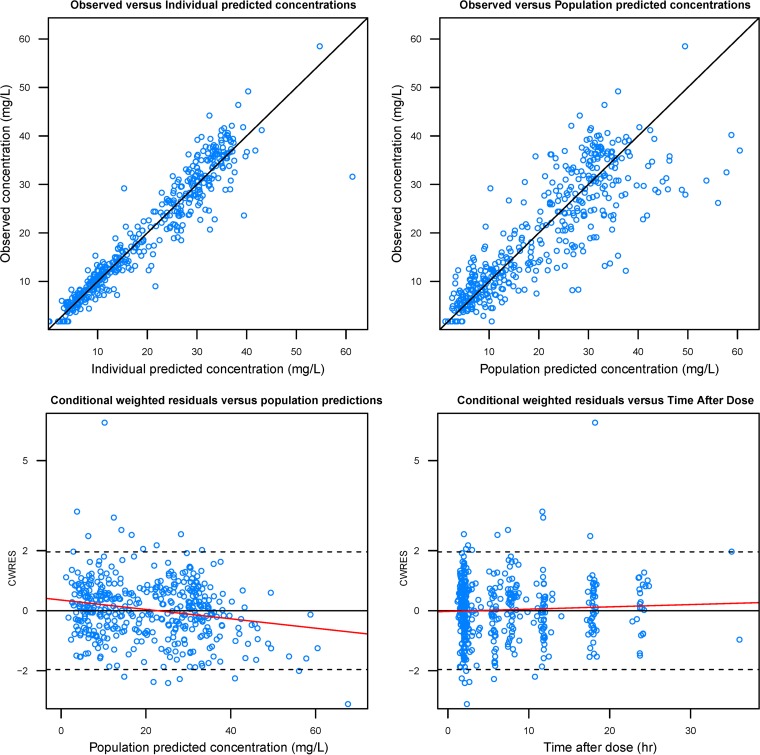
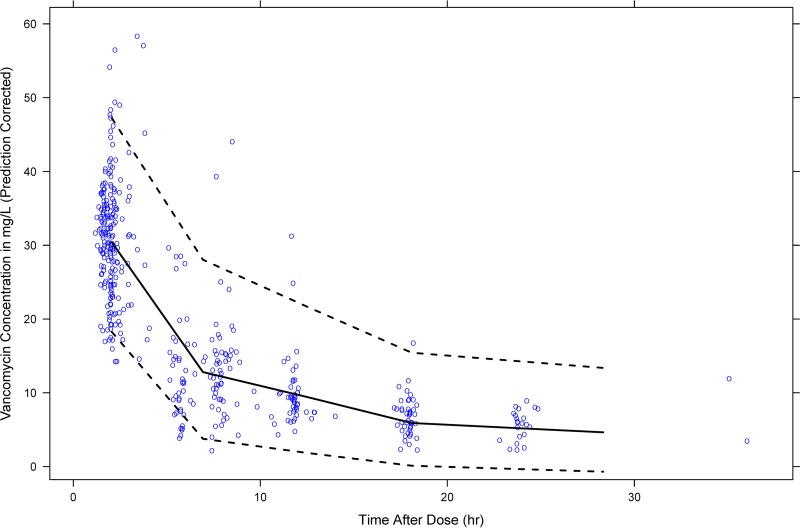
Standard goodness-of-fit plots of the final model did not display any obvious systematic bias (Fig. 1). Median values and 95% confidence intervals generated by bootstrap analysis concurred with their respective estimated values from the original data set, indicating the stability and robustness of the final population model (Table 2). The resulting prediction-corrected visual predictive check (VPC) is shown in Fig. 2. Most of the observed vancomycin concentrations were distributed around the median predicted value, with only 7.69% falling outside the 95% prediction interval.
FIG 1.
Standard diagnostic plots of the final population pharmacokinetic model. A solid black line indicates a line of unity, and a solid red line indicates a trend line.
FIG 2.
Prediction-corrected VPC for the final population pharmacokinetic model. The 50th (bold black line) and the 2.5th and 97.5th (dashed black lines) percentiles of simulated concentrations were overlaid on observed concentrations (opened blue circles). Note that 7.69% of the observed concentrations fell outside the 95% prediction interval.
Assessing the relationship between trough concentration and AUC24.
A total of 184 observed trough concentrations were included for analysis. Thirty-three trough concentrations were excluded because they were either taken before the third dose of a new dosing regimen or the time elapsed, since the start of a new dosing regimen was <24 h (unable to calculate AUC24). The median number of half-life (t1/2) values elapsed at the time of trough concentration measurement was 13.4, with 84% of trough concentrations measured at >5 t1/2 (at actual steady state).
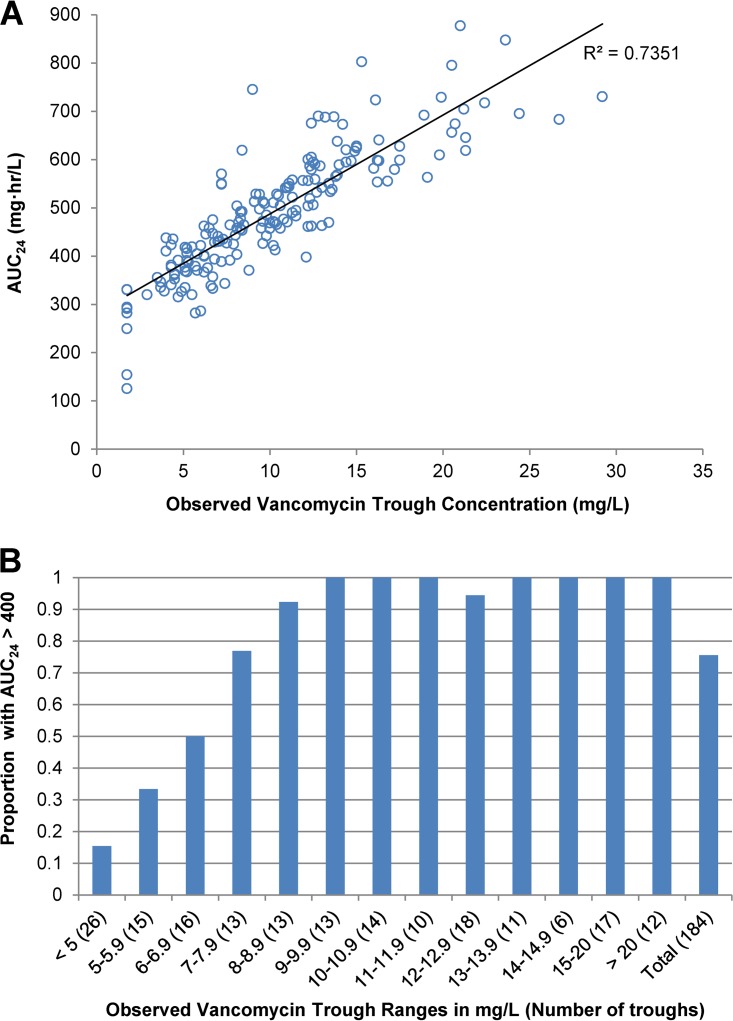
The median AUC24 across all 184 observed trough concentrations was 474 (range, 126 to 877), with 76% of troughs achieving AUC24 > 400. Trough concentrations correlated positively to AUC24 (r2 = 0.74) (Fig. 3A). The proportions of trough concentrations achieving AUC24 > 400 in different trough ranges are shown in Fig. 3B. It was found that 8 to 8.9 mg/liter is the minimum trough concentration range predictive of achieving AUC24 > 400.
FIG 3.
(A) Vancomycin AUC24 versus observed vancomycin trough concentration. (B) Proportion of observed trough concentrations that achieved AUC24 > 400 across different trough concentration ranges. AUC24, steady-state area under the concentration-time curve over the past 24 h.
The results of the Monte Carlo simulation sensitivity analysis showed that in general, as PMA increased from 24 weeks to 46 weeks, the minimum trough concentration range predictive of achieving AUC24 > 400 also increased from 7 to 7.9 mg/liter to 9 to 9.9 mg/liter. In a combined data set pooling all 160,000 trough concentrations from the 32 simulations, 8 to 8.9 mg/liter was identified as the minimum trough concentration range predictive of achieving AUC24 > 400. The pertinent results of analyses using observed and simulated trough concentrations are compared in Table 3. Almost 100% of both observed and simulated troughs between 10 to 20 mg/liter achieved AUC24 > 400. For trough concentrations between 10 and 14.9 mg/liter, none of the observed troughs and only three (<0.1%) of the simulated troughs had a AUC24 of >700. The maximum achieved AUC24 was 716 for simulated troughs. In contrast, for trough concentrations between 15 to 20 mg/liter, 3 (17.6%) observed and 6,360 (24.5%) simulated troughs had an AUC24 of >700, and the maximum AUC24 values were 803 and 841 in observed and simulated troughs, respectively.
TABLE 3.
Comparison of results from analyses of observed and simulated trough concentrations
| Trough type | AUC24/trough achievementa | Trough concn |
|||
|---|---|---|---|---|---|
| All troughs (mg/liter) | 8–8.9 mg/liter | 10–14.9 mg/liter | 15–20 mg/liter | ||
| Observed | Minimum trough range predictive of AUC24 > 400 | 8–8.9 | |||
| No. of troughs | 184 | 13 | 59 | 17 | |
| % troughs achieving AUC24 > 400 | 76 | 92 | 98 | 100 | |
| Median AUC24 (range) | 464 (370–619) | 542 (398–690) | 610 (553–803) | ||
| Simulated | Minimum trough range predictive of AUC24 > 400 | 8–8.9 | |||
| No. of troughs | 160,000 | 9,833 | 40,570 | 25,954 | |
| % troughs achieving AUC24 > 400 | 77 | 97 | 100 | 100 | |
| Median AUC24 (range) | 446 (372–546) | 543 (403–716) | 666 (530–841) | ||
AUC24, steady state area under the concentration-time curve over the past 24 h.
DISCUSSION
In the present study, we evaluated the relationship between the steady-state vancomycin trough concentration and the AUC24 in a predominantly Asian neonatal population. The major finding was that a minimum trough concentration range of 8 to 8.9 mg/liter was predictive of achieving AUC24 > 400 in >90% of actual and simulated neonates. Previously, Frymoyer et al. conducted another neonatal vancomycin population pharmacokinetic study with similar methodology in 249 Caucasian neonates with a median gestational age of 34 weeks, a median birth weight of 2,000 g, and a median PMA of 39 weeks at vancomycin initiation (20). These authors reported that a minimum trough concentration of ∼10 mg/liter would be enough to ensure sufficient vancomycin exposure. This is reasonably consistent with what we have found.
An AUC24 of 700 has been proposed to represent a conservative upper limit of safe vancomycin exposure with minimal risk of nephrotoxicity (19). For trough concentrations between 10 and 14.9 mg/liter, AUC24 > 400 was achieved reliably, and a negligible amount of troughs exceeded an AUC24 of 700. In contrast, trough concentrations between 15 and 20 mg/liter achieved AUC24 > 400 reliably as well, but a significantly larger proportion of troughs exceeded an AUC24 of 700. These findings suggest that higher trough concentrations within the range of 15 to 20 mg/liter may potentially lead to an increased risk of vancomycin overexposure and subsequent toxicity. This is consistent with the findings from a systematic review and meta-analysis conducted by Van et al., where it was found that adult and pediatric patients with vancomycin troughs above 15 mg/liter had a higher risk of nephrotoxicity than patients with troughs lower than 15 mg/liter (22). Similarly, a retrospective study conducted by Bhargava et al. concluded that there is a positive correlation between increasing vancomycin trough concentrations and increasing serum creatinine levels. Vancomycin trough concentrations above 15 mg/liter were found to be associated with a higher incidence of acute kidney injury in neonates (23). Trough concentrations lower than 10 mg/liter should also be avoided because of an increased risk of selecting for vancomycin-intermediate or vancomycin-resistant isolates (24–26). Hence, a trough target range of 10 to 14.9 mg/liter may be more appropriate for the treatment of MRSA infections in neonates when the vancomycin MIC is ≤1.
In our developed population pharmacokinetic model, vancomycin CL was predicted by WT, PMA, and sCr, whereas V was predicted by WT alone. The use of these values in predicting CL and V is based on a current understanding of how size, maturation, and renal function, respectively, affect the PK of renally excreted drugs in neonates (27–29) and was also consistent with the covariates incorporated into published neonatal population pharmacokinetic models of renally excreted drugs, as evidenced in a review by Wilbaux et al. (30). The typical neonate in our study (PMA = 30.1 weeks, sCr = 43 μmol/liter, WT = 1,000 g) is predicted to have a CL of 0.0519 liter/h/kg and a V of 0.498 liter/kg, which is comparable to values reported in the literature (20, 31–34). Our final population model was also internally validated by bootstrap analysis and VPC.
In the next stage of analysis, a plot of AUC24 versus the observed trough concentration showed that there was an expected positive correlation between drug exposure and trough concentration. However, because of interindividual variability, the AUC24 achieved for a particular trough concentration ranged up to 2-fold. Hence, it would not be accurate to simply use the best-fit line to identify the trough above which AUC24 is >400. Instead, we applied a probabilistic framework wherein all trough concentrations were divided into different ranges, and the proportion of troughs achieving AUC24 > 400 per range was calculated in order to find the minimum trough concentration range that was predictive of achieving AUC24 > 400. A Monte Carlo simulation sensitivity analysis was also performed, where 160,000 simulated steady-state trough concentrations underwent the same analysis as that used for the observed troughs. The final results from both analyses were similar, which further reinforces our findings that a steady-state trough range of 8 to 8.9 mg/liter or higher would be predictive of achieving AUC24 > 400 in >90% of neonates.
Our study was not without limitations. First, there was a lack of intensive blood sampling and huge variability in the dosing regimens (including dose adjustments) across all neonatal patients. However, the population pharmacokinetic approach is well suited for the determination of parameters based on sparse sampling, and it was able to handle the complicated data structure that was encountered in our study. Next, we utilized imputed sCr levels which might be inaccurate to some extent and cause model misspecification. However, based on the results of the simulations, good correlation was demonstrated between a decrease in vancomycin exposure and a decrease in sCr, which matches our expectations. An additional limitation encountered during our analysis of the relationship between trough concentration and AUC24 was the relatively low number of observed troughs in each trough range. The findings derived may thus not be truly representative of the general neonatal population. Furthermore, 16% of the troughs were not at steady state. In order to overcome these two issues, we simulated a significantly larger number of trough concentrations, all of which were at steady state. The results from the analysis of the simulations were largely in agreement with those of the observed concentrations, which strengthen our findings. Next, if the MIC is ≥2 for more resistant MRSA isolates, our findings would not be applicable because unacceptably high vancomycin exposures (AUC24 > 800) would be needed to achieve AUC24/MIC > 400. For such cases, an alternative agent is recommended instead of vancomycin (17). We also acknowledge that our major finding, i.e., 8 to 8.9 mg/liter is the minimum steady-state trough concentration predictive of achieving AUC24 > 400, may not apply to all dosing regimens. For regimens with a fixed total daily dose, the AUC24 remains constant regardless of dosing interval, assuming a constant CL and V. In this case, the minimum trough concentration corresponding to AUC24 > 400 would be inversely related to the dose interval. Nevertheless, such dosing regimens are not commonly practiced, and we believe that our findings are still clinically relevant in the context of fixed “mg/kg/dose” regimens. Lastly, we did not assess the relationship between trough concentrations and clinical outcomes for MRSA infections because of the low number of study neonates with positive MRSA cultures.
In conclusion, we found that a minimum steady-state vancomycin trough concentration range of 8 to 8.9 mg/liter is predictive of achieving AUC24/MIC > 400 if the MIC is ≤1 in our predominantly Asian neonatal population. Therefore, a target trough concentration between 10 and 14.9 mg/liter may be sufficient to ensure the efficacy of vancomycin in treating MRSA infections and to minimize the risk of nephrotoxicity. More studies are required to confirm our findings and to investigate the association between vancomycin trough concentrations and clinical outcomes in the treatment of MRSA infections in neonates. An external validation of our final population pharmacokinetic model should also be conducted to verify its generalizability and predictive performance. With the establishment of the appropriate vancomycin trough target required to ensure efficacy and safety, further research can be carried out to develop optimal vancomycin dosing regimens in neonates.
MATERIALS AND METHODS
A retrospective chart review of vancomycin therapy and therapeutic drug monitoring was conducted for neonates born between January 2011 and December 2016 at the KK Women's and Children's Hospital (KKH) and Singapore General Hospital (SGH). Approval to conduct this study was granted by the SingHealth Centralised Institutional Review Board (reference 2016/2759).
All vancomycin doses were administered via infusion over a nominal duration of 1 h. Therapeutic drug monitoring was typically performed at the third dose of a new dosing regimen where steady state was assumed to be attained. Trough concentrations were taken within 30 min preceding a dose, whereas peak concentrations were taken 1 h after the end of infusion of a dose. Random concentrations were also considered if the timings of blood takings were documented.
Subjects and data collection.
Data for all eligible neonates were collected from electronic medical records and included demographics, sCr, the complete vancomycin dosing and concentration history, indications for vancomycin use, culture results, and information regarding concomitant nephrotoxic agents. Neonates with ambiguous vancomycin dosing and serum concentrations, congenital kidney disease, major congenital heart disease (diagnosis other than ventricular septal defect, atrial septal defect, or patent ductus arteriosus), acute kidney injury, or unstable renal function and those on extracorporeal membrane oxygenation during vancomycin therapy were excluded. The weight of each neonate at all vancomycin dosing and trough measurement time points was recorded. If weight was not measured at a particular time point, the last measured weight would be carried forward. Serum creatinine levels around the time (±48 h) of all vancomycin concentration measurements were recorded and updated throughout the vancomycin course. If the sCr readings within ±48 h were unavailable, the closest available sCr reading was imputed. Vancomycin concentrations below the quantification limit (BQL) (<3.5 mg/liter) from SGH neonates were imputed as 1.75 mg/liter and included in the analysis. There were no BQL concentrations from KKH.
Population pharmacokinetic analysis.
Population pharmacokinetic analysis was performed using nonlinear mixed effects modeling software NONMEM (NONMEM 7.3; Icon Development Solutions, Ellicott City, MD). A one-compartment model was used as the structural model because most of the vancomycin concentrations were measured after the distribution phase. The first-order conditional estimation method with interaction was implemented throughout the model building and evaluation process. BSV was evaluated based on CL and volume of distribution (V; expressed as liters/kg) using an exponential error model, while RUV was evaluated using proportional, additive, and combined proportional-additive error models.
Based on an understanding of developmental pharmacology and published neonatal population pharmacokinetic models for common neonatal medications, including vancomycin, multiple covariates related to size (weight), maturation (age), and renal function were evaluated for their impact on vancomycin pharmacokinetics (PK) (30). All continuous variables were normalized during the modeling process. The effect of weight (WT; expressed in grams) on the CL and V was explored using allometric (27) and power models. Covariates related to age included gestational age (GA [weeks]), postnatal age (PNA [days]), and postmenstrual age (PMA [weeks]), and their effect on CL was explored using power models and a sigmoid maximum effect maturation function (for PMA only) (28, 35). The only covariate related to renal function, sCr, was assessed for its effect on CL using a power model. Potential final models with incorporated covariates were evaluated via stepwise forward addition/backward elimination to ascertain the statistical significance of each covariate. In the forward addition step, a covariate would be retained after addition to the model if the decrease in OFV was >3.84 [χ2 distribution, P < 0.05, degree of freedom (df) = 1]. A more stringent criterion was used for the backward elimination step, where a covariate would be retained if the increase in OFV after removal of the covariate was >10.83 (χ2 distribution, P < 0.001, df = 1). The final model was selected based on physiological plausibility of population CL and V estimates, acceptable standard errors, and good visual representation of standard diagnostic plots.
Model evaluation.
Nonparametric bootstrap analysis and a VPC were conducted to evaluate the accuracy and stability of the final model using the NONMEM support software Perl-speaks-NONMEM (v4.6.0). For bootstrap analysis, 5,000 bootstrap data sets were generated from the original data set by repeated sampling with replacement. Next, the final model was used to generate parameter estimates for each resampled data set. The medians and 95% confidence intervals of bootstrap parameter estimates were compared to their respective values obtained from the original data set. As for VPC, 5,000 virtual data sets were simulated using the final model. The 2.5th, 50th, and 97.5th percentiles of the simulated concentrations were overlaid on the observed concentrations versus time profile using R version 3.3.1.
Assessing the relationship between trough concentration and AUC24.
Bayesian estimates of CL for all actual neonates were generated by the final model. These CL values were used to calculate the AUC24 (AUC24 = dose over past 24 h/CL) corresponding to all observed vancomycin trough concentrations measured at the third dose and onward of any new dosing regimen, where steady state was assumed to have been achieved in practice. In order to evaluate the attainment of steady state at each trough measurement, the number of half-lives (t1/2 = 0.693 × V/CL) that have passed were computed as the time elapsed since the first dose divided by the t1/2. Next, trough concentrations were divided into different ranges. The proportion of troughs in each range achieving the target vancomycin exposure was calculated by dividing the number of troughs with AUC24 > 400 over the total number of troughs in that range. For a trough range to be predictive of achieving AUC24 > 400, >90% of troughs in that range achieving AUC24 > 400 were selected as the criterion. The minimum trough concentration range that was predictive of achieving AUC24 > 400 was determined.
A Monte Carlo simulation sensitivity analysis was performed to evaluate the impact of various PMA, sCr, and dose interval values on the relationship between steady-state vancomycin trough concentrations and the AUC24. The final population model was used to simulate 32 data sets, each containing 5,000 hypothetical neonates with one steady-state trough concentration per neonate. For each simulation, the PMA, sCr, and dose interval were fixed. The values tested were (i) 24, 32, 40, and 46 weeks for PMA; (ii) 20, 35, 70, 110 μmol/liter for sCr; and (iii) 6, 8, 12, 18, and 24 h for dose interval. All doses were fixed at 15 mg/kg/dose, and dosing regimens for neonates of any particular PMA adhered to SGH and KKH dosing guidelines. The median weight for a given PMA from the Fenton growth chart was used (36). To determine the minimum trough concentration range that was predictive of achieving AUC24 > 400, the method as described above for the study neonates was used for all simulation data sets.
ACKNOWLEDGMENTS
This research was jointly supported by the Department of Pharmacy, National University of Singapore; the Department of Pharmacy, KK Women's and Children's Hospital, Singapore; and the Department of Pharmacy, Singapore General Hospital, Singapore.
REFERENCES
- 1.Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. 2005. Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis 40:218–224. doi: 10.1086/426444. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK Jr, Smith PB. 2009. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J 28:1052–1056. doi: 10.1097/INF.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlapbach LJ, Mattmann M, Thiel S, Boillat C, Otth M, Nelle M, Wagner B, Jensenius JC, Aebi C. 2010. Differential role of the lectin pathway of complement activation in susceptibility to neonatal sepsis. Clin Infect Dis 51:153–162. doi: 10.1086/653531. [DOI] [PubMed] [Google Scholar]
- 4.Lehtonen L, Gimeno A, Parra-Llorca A, Vento M. 2017. Early neonatal death: a challenge worldwide. Semin Fetal Neonatal Med 22:153–160. doi: 10.1016/j.siny.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID III, Watterberg KL, Saha S, Das A, Higgins RD. 2010. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boghossian NS, Page GP, Bell EF, Stoll BJ, Murray JC, Cotten CM, Shankaran S, Walsh MC, Laptook AR, Newman NS, Hale EC, McDonald SA, Das A, Higgins RD. 2013. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr 162:1120–1124. doi: 10.1016/j.jpeds.2012.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, Laptook AR, Das A, Walsh MC, Hale EC, Newman NS, Schrag SJ, Higgins RD. 2012. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics 129:e914–e922. doi: 10.1542/peds.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MU, Gallagher PG. 2012. Methicillin-resistant Staphylococcus aureus in the neonatal intensive care unit. Semin Perinatol 36:424–430. doi: 10.1053/j.semperi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 10.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. 2006. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 11.Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. 2013. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med 18:28–34. doi: 10.1016/j.siny.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Moellering RC., Jr 2006. Vancomycin: a 50-year reassessment. Clin Infect Dis 42(Suppl 1):S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 13.Pansieri C, Bonati M, Choonara I, Jacqz-Aigrain E. 2014. Neonatal drug trials: impact of EU and US paediatric regulations. Arch Dis Child Fetal Neonatal Ed 99:F438. doi: 10.1136/archdischild-2013-305900. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 15.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez R, Lopez Cortes LE, Molina J, Cisneros JM, Pachon J. 2016. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother 60:2601–2609. doi: 10.1128/AAC.03147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 18.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 19.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frymoyer A, Hersh AL, El-Komy MH, Gaskari S, Su F, Drover DR, Van Meurs K. 2014. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother 58:6454–6461. doi: 10.1128/AAC.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockmann C, Hersh AL, Roberts JK, Bhongsatiern J, Korgenski EK, Spigarelli MG, Sherwin CM, Frymoyer A. 2015. Predictive performance of a vancomycin population pharmacokinetic model in neonates. Infect Dis Ther 4:187–198. doi: 10.1007/s40121-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargava V, Malloy M, Fonseca R. 2017. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr 17:50. doi: 10.1186/s12887-017-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 38:448–451. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 25.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 38:521–528. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 26.Sakoulas G, Gold HS, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM. 2006. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother 57:699–704. doi: 10.1093/jac/dkl030. [DOI] [PubMed] [Google Scholar]
- 27.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 28.Holford N, Heo YA, Anderson B. 2013. A pharmacokinetic standard for babies and adults. J Pharm Sci 102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 29.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NH. 2009. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 30.Wilbaux M, Fuchs A, Samardzic J, Rodieux F, Csajka C, Allegaert K, van den Anker JN, Pfister M. 2016. Pharmacometric approaches to personalize use of primarily renally eliminated antibiotics in preterm and term neonates. J Clin Pharmacol 56:909–935. doi: 10.1002/jcph.705. [DOI] [PubMed] [Google Scholar]
- 31.Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F. 2007. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit 29:284–291. doi: 10.1097/FTD.0b013e31806db3f5. [DOI] [PubMed] [Google Scholar]
- 32.Bhongsatiern J, Stockmann C, Roberts JK, Yu T, Korgenski KE, Spigarelli MG, Desai PB, Sherwin CM. 2015. Evaluation of vancomycin use in late-onset neonatal sepsis using the area under the concentration-time curve to the minimum inhibitory concentration ≥400 target. Ther Drug Monit 37:756–765. doi: 10.1097/FTD.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. 2004. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother 48:1159–1167. doi: 10.1128/AAC.48.4.1159-1167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo YL, van Hasselt JG, Heng SC, Lim CT, Lee TC, Charles BG. 2010. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother 54:2626–2632. doi: 10.1128/AAC.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63:75–84. doi: 10.1111/j.1365-2125.2006.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenton TR, Sauve RS. 2007. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr 61:1380–1385. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]