ABSTRACT
We previously demonstrated that the rate and extent of an antimicrobial agent's bactericidal effects were coupled to the bacterial replication rate, the latter of which was modulated with the sodium chloride concentration. Herein, we describe the results from a 24-h one-compartment in vitro infection model study that was designed to demonstrate that an antimicrobial agent's bactericidal effects could be amplified when it is administered with a pharmaceutical agent that increases the bacterial replication rate. The antimicrobial and growth-promoting agents selected were levofloxacin and norepinephrine, respectively. The challenge isolate was Escherichia coli JMI 21711R (levofloxacin MIC, 8 mg/liter). Within the in vitro infection model, a human levofloxacin concentration-time profile (half-life, 7 h) was simulated and the challenge isolate was subjected to an ineffective monotherapy exposure (free-drug area under the concentration-time curve over 24 h divided by the MIC [AUC/MIC] ratio of 6) with and without norepinephrine as a continuous infusion (275 mg/liter). Samples were collected from the model during the course of the study for bacterial density determinations and drug concentration assay using liquid chromatography-tandem mass spectrometry (LC-MS/MS). As expected, the norepinephrine and no-treatment control arms failed immediately, followed by the levofloxacin monotherapy arm, which failed slowly over time. The levofloxacin-epinephrine regimen resulted in a 2-log10 CFU reduction in bacterial density over the first 6 to 8 h of the study, which was followed by regrowth of a highly levofloxacin-resistant subpopulation (MIC, 64 mg/liter). These data demonstrate that increasing the rate of bacterial replication with a pharmaceutical product in combination with antimicrobial therapy represents an opportunity to increase the rate and magnitude of bactericidal effect.
KEYWORDS: bacterial replication, pharmacokinetics-pharmacodynamics, therapy duration
INTRODUCTION
We previously evaluated the relationship between the bacterial replication rate and the rate and extent of bactericidal effects in the context of an effective antibiotic exposure (1). In brief, the studies utilized in vitro infection models (a one-compartment, 24-h model; a hollow-fiber, 10-day model) where the bacterial replication rate was modulated using sodium chloride. The challenge isolate was Staphylococcus aureus ATCC 29213, and the study drug was levofloxacin. We demonstrated that the bacterial replication rate was coupled to the rate and extent of the antimicrobial bactericidal effects and that the duration of therapy could be modulated by altering the bacterial replication rate. The conclusion for this study was that a proof of concept was provided for new adjunctive therapies to antibiotics that increase the rate and extent of bacterial killing and decrease the duration of therapy.
To build upon our previous findings, we chose to use a pharmaceutical agent, rather than modifying the growth medium, as described above with sodium chloride, to modulate the bacterial replication rate. Catecholamine-type inotropic agents have been reported to increase bacterial replication. Specifically, dopamine, epinephrine, and norepinephrine have been reported to increase bacterial replication in vitro (2, 3, 4, 5) and ex vivo (6). The authors of these studies acknowledged the potential clinical implications of their findings, which was that this interaction between microbe and drug represents an unappreciated risk factor for morbidity and mortality in critically ill patient populations treated with catecholamine agents.
Based upon our aforementioned work (1), we hypothesized exactly the opposite. That is, the interaction between microbe and drug represents an opportunity to increase the rate and magnitude of bactericidal effect through the coadministration of a catecholamine and antimicrobial regimen. This hypothesis is plausible, as most antibiotics require replicating bacteria to exert their bactericidal effects (7) and rapidly growing bacteria stimulate improved host immune response (8). Herein, we describe studies specifically designed to test this hypothesis using norepinephrine and levofloxacin.
RESULTS
In vitro susceptibility studies and growth rate studies.
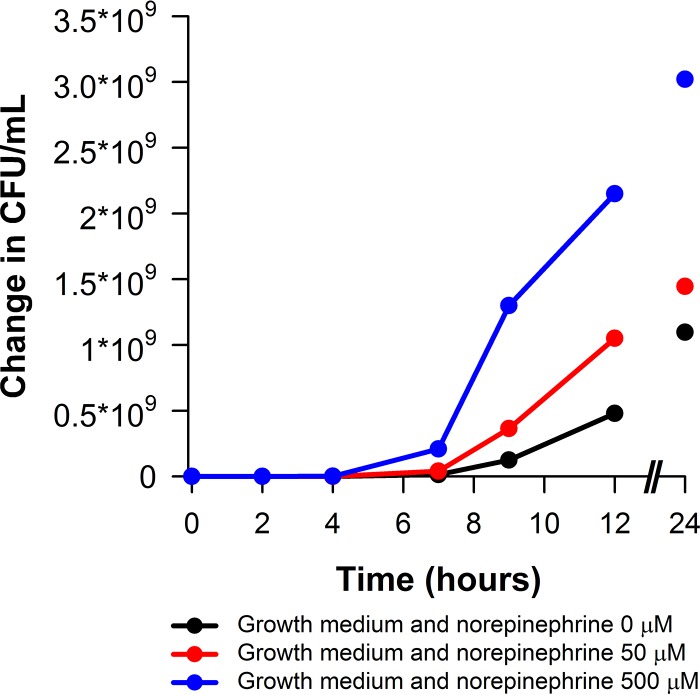
The levofloxacin MIC for Escherichia coli JMI 21711R was 8 mg/liter. Figure 1 shows the impact of the norepinephrine concentration on the change in bacterial density (starting inoculum [time zero], 1 × 106 CFU/ml) in vitro over 24 h for E. coli JMI 21711R. Note that as the concentration of norepinephrine increased, so too did the bacterial replication rate.
FIG 1.
Norepinephrine concentration impact on the growth rate of E. coli JMI 21711R (starting inoculum [time zero], 1 × 106 CFU/ml).
Drug assay and pharmacokinetics.
Assessments of the assay performance demonstrated that the interassay coefficients of variation (CVs) for the quality control samples at concentrations of 1.50, 7.5, and 37.5 μg/ml were 4.32%, 7.41%, and 2.93%, respectively, for norepinephrine. The interassay CVs for the quality control samples at concentrations of 0.300, 1.50, and 7.50 μg/ml were 5.69%, 9.52%, and 6.38%, respectively, for levofloxacin. The correlation coefficient value ranged from 0.9943 to 0.9960 and 0.9940 to 0.9954 for norepinephrine and levofloxacin, respectively.
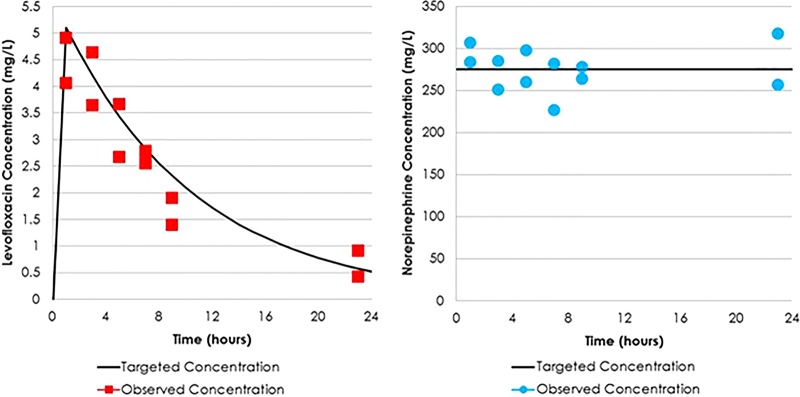
The targeted and observed levofloxacin and norepinephrine concentrations are presented in Fig. 2A and B, respectively. The observed levofloxacin and norepinephrine concentrations were concordant with those expected. These data indicate that the targeted drug concentration for each study agent was well simulated within the one-compartment in vitro infection model.
FIG 2.
Targeted versus observed levofloxacin (A) and norepinephrine (B) concentrations in the one-compartment in vitro infection model.
One-compartment infection model studies.
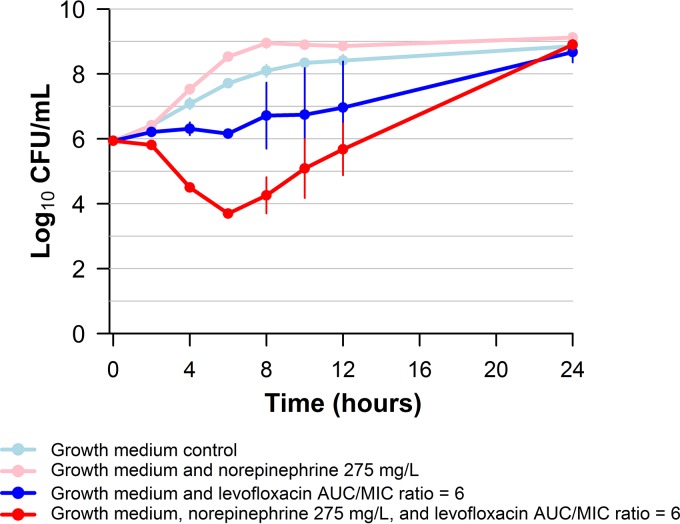
Figure 3 shows the impact of norepinephrine on the change in bacterial density in vitro over the study period relative to that in the no-treatment control arm. First, note that the bacteria in the no-treatment control arm grew well but that those in the norepinephrine-containing control arm grew faster. Second, note that the rate (slope, 0 to 4 h) and extent (24-h number of CFU) of cell kill was greater in the norepinephrine-levofloxacin treatment arm than in the arm with levofloxacin alone. Although there was a 2-log10 CFU reduction from the count at the baseline in the norepinephrine-levofloxacin treatment arm by 6 to 8 h, significant regrowth was observed by 24 h.
FIG 3.
Impact of norepinephrine on the rate and extent of levofloxacin bactericidal activity against E. coli JMI 21711R in a 24-h one-compartment in vitro infection model.
We suspected that the regrowth in the norepinephrine-levofloxacin treatment arm was due to amplification of a drug-resistant bacterial subpopulation.
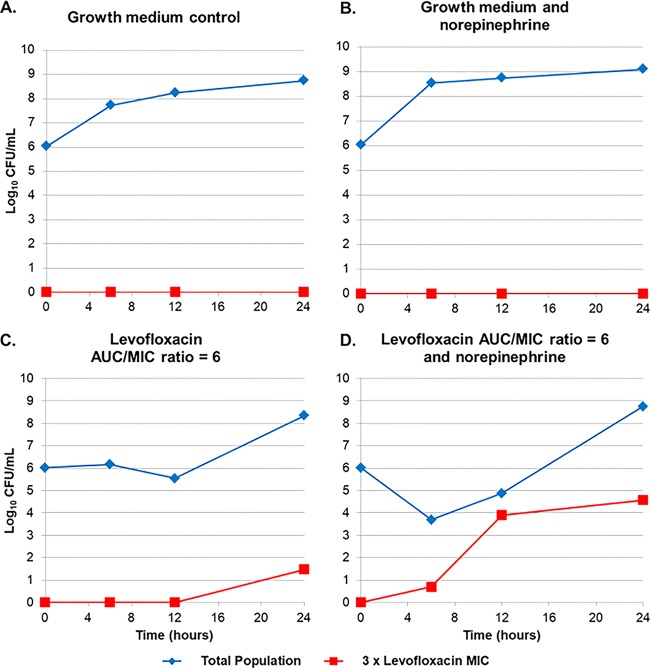
To confirm this explanation, samples from the in vitro infection model system experiments, the results for which are shown in Fig. 3, were cultured on agar plates containing levofloxacin at 0 and 3 times the baseline MIC value (baseline MIC, 8 mg/liter; MIC off drug-containing agar plates, 24 mg/liter). Figure 4 shows the change in bacterial density of a levofloxacin-resistant bacterial subpopulation over time. Note in Fig. 4A and B that there was essentially no difference in the total bacterial population and the drug-resistant subpopulation over time between the growth medium alone and the growth medium plus norepinephrine control arms. However, there was a significant difference in the total bacterial population and the drug-resistant subpopulation over time between the growth medium plus levofloxacin and the growth medium plus levofloxacin and norepinephrine arms (Fig. 4C and D). In the growth medium plus levofloxacin arm, there was essentially no change in the total and drug-resistant subpopulation over the first 12 h of the study, and each increased by 2-log10 CFU over the remainder of the study. In contrast, in the growth medium plus levofloxacin and norepinephrine arm, there was an initial 2-log10 CFU reduction in the total bacterial population over the initial 6 to 8 h of the study but a marked increase in the drug-resistant subpopulation, such that by 12 h it had nearly replaced the entire bacterial population. Thereafter, the total bacterial population grew to match that of the no-treatment control arm (approximately 9 log10 CFU/ml), while the drug-resistant bacterial subpopulation remained stable (4 log10 CFU/ml). This is not surprising, given that the concentrations of levofloxacin were low after 12 h and were insufficient to eliminate the drug-susceptible bacterial subpopulation or to further amplify the drug-resistant subpopulation.
FIG 4.
Total and levofloxacin-resistant E. coli populations over 24 h in the one-compartment in vitro infection model with and without norepinephrine.
DISCUSSION
Our aim was to demonstrate that a pharmaceutical agent that increases the bacterial replication rate amplifies the rate and extent of bactericidal activity of an antimicrobial regimen. Given the growing concern surrounding antibiotic resistance among Gram-negative bacilli (9), the challenge isolate that we selected for these studies was a multidrug-resistant (MDR) E. coli clinical isolate. We also thought that it was important that the antibiotic exposures simulated in the in vitro infection model approximated those typically observed in patients following a standard dosing regimen. The catecholamine norepinephrine was selected as the agent to stimulate bacterial replication.
As mentioned earlier, a wealth of evidence demonstrating that inotropic catecholamine agents are potent stimulators of bacterial growth has been generated (2, 3, 4, 5, 6). Previous authors have viewed this finding as a potential threat to patient well-being and have suggested that the administration of inotropic agents may result in increased morbidity. We are unaware of any evidence in the literature to support this concern. However, given that most patients receiving dopamine, epinephrine, or norepinephrine reside in critical care units, where many other interventions are administered simultaneously, the lack of clinical evidence may simply be due to a low signal-to-noise ratio.
Therefore, we searched the literature for such evidence in a less critically ill patient population, patients with Parkinson's disease treated with the catecholamine precursors levodopa and droxidopa. In a randomized, double-blind, placebo-controlled trial evaluating 361 patients with Parkinson's disease, infections were significantly more common among patients receiving levodopa at 600 mg daily than among those receiving placebo (P = 0.01) (10). Similar findings have been reported for droxidopa, a precursor to norepinephrine. In two open-label studies evaluating 422 droxidopa-treated patients with symptomatic neurogenic orthostatic hypotension, urinary tract infections were among the most commonly reported adverse reactions (15%) (11). The significance of these findings is reflected within the prescribing information of levodopa and droxidopa (11, 12). While the Food and Drug Administration acknowledges the increased risk of respiratory and/or urinary tract infections in levodopa- and droxidopa-treated patients, neither the pharmaceutical companies nor drug regulators suggest a cause for the increased infection rates associated with levodopa or droxidopa use. However, it may be due to increasing bacterial replication in an at-risk patient population. The mechanism by which catecholamine agents increase the bacterial replication rate has not been fully elucidated. One possible explanation involves the formation of a catecholamine-transferrin or catecholamine-lactoferrin complex (13), which thereby reduces their iron-carrying capacity and which increases the free iron concentrations supporting bacterial growth.
In our study, there was over a 2-log10 CFU difference in bacterial density between the norepinephrine-levofloxacin and levofloxacin monotherapy arms by 6 to 8 h which favored the combination regimen. However, in the norepinephrine-levofloxacin treatment arm, there was significant regrowth of a levofloxacin-resistant bacterial subpopulation by 24 h. There are two important inferences that can be made about these findings.
First, these data support the hypothesis that a pharmaceutical agent can be used to modulate the bacterial replication rate and increase the bactericidal activity of a treatment regimen. Second, and just as importantly, the amplification of a drug-resistant bacterial subpopulation may require the addition of a second antimicrobial regimen active against this bacterial mass. It remains to be determined how the second regimen should be administered to optimize bactericidal activity. For instance, one could imagine administering the antimicrobial agents concurrently or sequentially with differing effects. Studies of a longer duration using a hollow-fiber in vitro infection model and in vivo confirmation are needed to further support or refute the hypothesis put forth herein. Future studies can also be designed to investigate the most effective strategies for administering combination therapy to prevent regrowth.
Finally, given their effects on heart rate, blood pressure, glucose homeostasis, and the overall impact on the sympathetic nervous system, catecholamine agents are not optimal adjunctive therapy candidates to antibiotics to increase bactericidal activity and to decrease therapy duration. Therefore, it will be important to identify other bacterial growth rate modulators for further study.
In conclusion, we hypothesized that increasing the rate of bacterial replication with a pharmaceutical agent in combination with antimicrobial therapy represents an opportunity to increase the rate and magnitude of bactericidal effect. While these data support this hypothesis, it is evident that the treatment of high-density bacterial infections, especially those involving MDR or extremely drug-resistant pathogens, will likely require combination antimicrobial regimens.
MATERIALS AND METHODS
Bacteria and study drug.
E. coli JMI 21711R was the challenge isolate selected for use in these studies. This isolate was categorized as an MDR clinical isolate, as it was known to be resistant to members of three different drug classes (ceftriaxone [MIC ≥ 16 mg/liter], levofloxacin [MIC = 8 mg/liter], and tobramycin [MIC ≥ 16 mg/liter]) (14, 15). The antimicrobial agents tested were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). Norepinephrine was obtained from Sigma-Aldrich (St. Louis, MO).
Media and in vitro susceptibility studies.
Susceptibility studies were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (16) and interpretive criterion recommendations of the United States Committee on Antimicrobial Susceptibility Testing (USCAST, Silverton, OR) (17) using a microdilution method in cation-adjusted Mueller-Hinton broth (BD Laboratories, Franklin Lakes, NJ). These studies were conducted in triplicate over a 2-day period, and the results are presented as the modal values.
Pharmacokinetic-pharmacodynamic in vitro infection model and sample processing.
The one-compartment in vitro model used in this study, which has been described previously (18), consists of a central infection compartment, which contains broth growth medium maintained at a pH of 7.2, the challenge isolate, and magnetized stir bars to ensure the homogeneity of the drug, medium, and organism. The central infection compartment was placed upon a magnetic stir plate within a temperature- and humidity-controlled incubator set to 35°C. Computer-controlled peristaltic pumps were used to infuse drug-free growth medium into the central infection compartment, while at the same time they were used to remove medium through an exit port into a waste container. The challenge isolate was inoculated directly into the central infection compartment, and the test compound was infused with the aid of computer-controlled syringe pumps. Peristaltic pump diffusion rates were set such that the desired concentration-time profile of levofloxacin mimicked human pharmacokinetic profiles, while norepinephrine was simulated over a continuous infusion. Specimens for determination of the number of CFU and drug concentration assay were aseptically collected from the central infection compartment at predetermined time points (see below).
The initial challenge isolate inoculum was 1.0 × 106 CFU/ml in these experiments. The challenge isolate was prepared from a culture grown overnight on Trypticase soy agar plus 5% lysed sheep blood agar plates (BD Laboratories). Isolates were taken from overnight cultures and grown to a mid-logarithmic phase in a Mueller-Hinton broth-containing flask, which was set in a shaking water bath, at 35°C and 125 rotations per minute. The bacterial concentration within the Mueller-Hinton broth-containing flask was determined by optical density measurement and making comparisons to a previously confirmed growth curve for the challenge isolate.
In the antibiotic-containing treatment arms, bacteria were then exposed to changing concentrations of levofloxacin simulating a half-life of 7.0 h and plasma protein binding of 31% in humans (19). Free-drug maximum concentration (Cmax) and area under the concentration-time curve (AUC) values of approximately 5 mg/liter and 48 mg · h/liter, respectively, with and without norepinephrine continuous infusion maintaining a concentration of 275 mg/liter were targeted. This allowed for an AUC/MIC ratio of 6 to be achieved for the above-described E. coli challenge isolate. No-treatment control arms were also included. Specimens (1 ml) were collected for determination of the number of CFU at 0, 1, 2, 3, 4, 6, 8, 12, and 24 h. Samples were centrifuged, washed, and resuspended with sterile normal saline twice, preventing drug carryover, and subsequently cultured onto Trypticase soy agar enriched with 5% sheep blood and Mueller-Hinton agar supplemented with three times the levofloxacin MIC and incubated at 35°C for 24 h. Specimens (1 ml) for drug assay were collected at 1, 3, 5, 7, and 24 h and then immediately frozen at −80°C until assayed for drug concentration.
Drug assay.
Calibration standards and quality controls were prepared in study matrix and processed concurrently with a collected unknown sample. All samples were assayed simultaneously for both norepinephrine and levofloxacin by liquid chromatography-tandem mass spectrometry on a Sciex 5500 mass spectrometer. Prior to analysis, norepinephrine was derivatized with acetic anhydride while simultaneously precipitating matrix proteins by denaturation with acetonitrile. Analytical curves were linear from 0.500 to 50.0 and 0.100 to 10.0 μg/ml for l-(−)-norepinephrine (+)-bitartrate salt monohydrate and levofloxacin, respectively. The lower limit of quantitation was 0.500 μg/ml and 0.100 μg/ml for l-(−)-norepinephrine (+)-bitartrate salt monohydrate and levofloxacin, respectively.
REFERENCES
- 1.Ambrose PG, VanScoy B, Conde H, McCauley J, Rubino CM, Bhavnani SM. 2017. Bacterial replication rate modulation in combination with antimicrobial therapy: turning the microbe against itself. Antimicrob Agents Chemother 61:e01605-16. doi: 10.1128/AAC.01605-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyte M, Frank CD, Green BT. 1996. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol Lett 139:155–159. [DOI] [PubMed] [Google Scholar]
- 3.Freestone PP, Haigh RD, Williams PH, Lyte M. 1999. Stimulation of bacterial growth by heat-stable norepinephrine-induced autoinducers. FEMS Microbiol Lett 172:53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- 4.Neal CP, Freestone PP, Maggs AF, Haigh RD, Williams PH, Lyte M. 2001. Catecholamine inotropes as growth factors for Staphylococcus epidermis and other coagulase-negative staphylococci. FEMS Microbiol Lett 194:163–169. doi: 10.1111/j.1574-6968.2001.tb09463.x. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell PM, Aviles H, Lyte M, Sonnerfeld G. 2006. Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: importance of inoculum density and role of transferrin. Appl Environ Microbiol 72:5097–5099. doi: 10.1128/AEM.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freestone PP, Hirst RA, Sandrini SM, Sharaff F, Fry H, Hyman S, O'Callaghan C. 2012. Pseudomonas aeruginosa-catecholamine intrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest 142:1200–1210. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- 7.Kohanski MA, Dwyer DJ, Collins JJ. 2010. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith H. 2000. The activities of bacterial pathogens in vivo: based on contributions to a Royal Society discussion meeting, p 3–38. In Smith H, Dorman CJ, Dougan G, Holden DW, Williams P (ed). Imperial College Press, London, United Kingdom. [Google Scholar]
- 9.Boucher HW, Ambrose PG, Chambers HF, Ebright RH, Jezek A, Murray BE, Newland JG, Ostrowsky B, Rex JH, Infectious Diseases Society of America. 2017. White paper: developing antimicrobial drugs for resistant pathogens, narrow-spectrum indications, and unmet needs. J Infect Dis 216:228–236. doi: 10.1093/infdis/jix211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahn S, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow W, Tanner C, Marek K. 2004. Levodopa and the progression of Parkinson's disease. N Engl J Med 351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 11.Lundbeck NA Ltd. 2014. Northera (droxidopa) capsules prescribing information. Lundbeck NA Ltd, Deerfield, IL. [Google Scholar]
- 12.Bristol-Myers Squibb. 2009. Sinemet (carbidopa-levodopa) tablets prescribing information. Bristol-Myers Squibb, Princeton, NJ. [Google Scholar]
- 13.Lyte M, Freestone PP, Primrose PE. 2010. Inter-kingdom signaling in health and infectious disease. In Lyte M, Freestone PE (ed), Microbial endocrinology. Springer-Verlag, New York, NY. [Google Scholar]
- 14.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 15.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011–2012). Antimicrob Agents Chemother 57:6305–6310. doi: 10.1128/AAC.01802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.United States Committee on Antimicrobial Susceptibility Testing. 2017. Quinolone in vitro susceptibility test interpretive criteria evaluations (V1.2). United States Committee on Antimicrobial Susceptibility Testing. https://app.box.com/s/hxo1zbtunse7zse19ibxwza7u190rmla. [DOI] [PMC free article] [PubMed]
- 18.VanScoy BD, Mendes RE, McCauley J, Bhavnani SM, Bulik CC, Okusanya OO, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2013. Pharmacological basis of β-lactamase inhibitor therapeutics: tazobactam in combination with ceftolozane. Antimicrob Agents Chemother 57:5924–5930. doi: 10.1128/AAC.00656-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen Pharmaceuticals. 2014. Levaquin (levofloxacin)® package insert. Janssen Pharmaceuticals, Raritan, NJ. [Google Scholar]