Abstract
The aim of this study was to determine the efficacy and pharmacokinetics of bupivacaine in combination with epinephrine or dexmedetomidine after intraperitoneal administration in cats undergoing ovariohysterectomy. Sixteen healthy adult cats (3.3 ± 0.6 kg) were included in a prospective, randomized, masked clinical trial after obtaining owners’ consent. Anesthetic protocol included buprenorphine-propofol-isoflurane. Meloxicam [0.2 mg/kg body weight (BW)] was administered subcutaneously before surgery. Cats were randomly divided into 2 groups to receive 1 of 2 treatments. Intraperitoneal bupivacaine 0.25% (2 mg/kg BW) was administered with epinephrine (BE group; 2 μg/kg BW) or dexmedetomidine (BD group; 1 μg/kg BW) before ovariohysterectomy (n = 8/group). A catheter was placed in the jugular vein for blood sampling. Blood samples were collected for up to 8 h after bupivacaine was administered. Plasma concentrations and pharmacokinetics of bupivacaine were determined using liquid chromatography tandem mass spectrometry (LC-MS/MS) and non-compartmental model, respectively. Pain was evaluated using the UNESP-Botucatu multidimensional composite pain scale (MCPS), the Glasgow composite feline pain scale (GPS), and a dynamic visual analog scale up to 8 h after extubation. Rescue analgesia was provided with buprenorphine if MCPS was ≥ 6. Repeated measures linear models were used for analysis of pain and sedation scores (P < 0.05). Maximum bupivacaine plasma concentrations (Cmax) for BE and BD were 1155 ± 168 ng/mL and 1678 ± 364 ng/mL (P = 0.29) at 67 ± 13 min (Tmax) and 123 ± 59 min (P = 0.17), respectively. Pharmacokinetic parameters and pain scores were not different between treatments (P > 0.05). One cat in the BE group received rescue analgesia (P = 0.30). Intraperitoneal bupivacaine with epinephrine or dexmedetomidine produced concentrations below toxic levels and similar analgesic effects. It is therefore safe to administer these drug combinations in cats undergoing ovariohysterectomy.
Résumé
L’objectif de cette étude était de déterminer la pharmacocinétique de la bupivacaïne avec de l’épinéphrine ou de la dexmedetomidine après son administration intrapéritonéale chez des chats subissant une ovariohystérectomie. Seize chats adultes en bonne santé (3,3 ± 0,6 kg) ont été inclus dans un essai prospectif, randomisé et «à l’aveugle». Le protocole anesthésique comprenait la buprénorphine-propofol-isoflurane. Méloxicam (0,2 mg/kg) a été administré par voie sous-cutanée avant la chirurgie. Un cathéter a été placé dans la veine jugulaire pour l’échantillonnage du sang. La bupivacaïne 0,25 % a été administrée intrapéritonéale (2 mg/kg) avec de l’épinéphrine (BE, 2 μg/kg) ou de la dexmedetomidine (BD, 1 μg/kg) avant l’ovariohystérectomie (n = 8/groupe). Des échantillons de sang ont été prélevés jusqu’à 8 heures après l’administration de bupivacaïne. Les concentrations plasmatiques et les données pharmacocinétiques de la bupivacaïne ont été déterminées par l’aide de la chromatographie liquide-spectrométrie de masse (LC-MS) et la représentation graphique avec un modèle noncompartimentale. La douleur a été évaluée à l’aide de l’échelle composite multidimensionnelle de la douleur (MCPS), de l’échelle composite de Glasgow de la douleur féline (GPS) et d’une échelle visuelle analogique dynamique jusqu’à 8 heures après l’extubation. L’analgésie de secours a été fournie avec la buprénorphine si MCPS ≥ 6. Les modèles linéaires de mesures répétées ont été utilisés pour l’analyse des scores de douleur et de sédation (P < 0,05). Les concentrations plasmatiques maximales de bupivacaïne (Cmax) pour BE et BD étaient de 1155 ± 168 ng/mL et 1678 ± 364 ng/mL (P = 0,29) à 67 ± 13 minutes (Tmax) et 123 ± 59 minutes (P = 0,17), respectivement. Les paramètres pharmacocinétiques et les scores de douleur n’étaient pas différents entre les traitements (P > 0,05). Un chat de BE a reçu analgésie de secours (P = 0,30). La bupivacaïne avec de l’épinéphrine ou de la dexmedetomidine intra-péritonéale a produit des concentrations inférieures aux niveaux toxiques et des effets analgésiques similaires. Il est donc sécuritaire d’administrer ces combinaisons de médicaments chez les chats subissant une ovariohysterectomie.
(Traduit par les auteurs)
Introduction
Intraperitoneal (IP) administration of local anesthetics reduces early postoperative analgesic requirements and pain scores and increases time to first intervention analgesia after abdominal surgery in humans (1,2). For these reasons, the technique has been recommended as part of a multimodal approach after laparoscopic surgery (3,4). In veterinary medicine, the technique has been shown to be a simple, safe, and cost-effective method for reducing pain after ovariohysterectomy in dogs (5–8) and cats (9,10) without adverse effects (6,8,9).
In human medicine, bupivacaine, a long-acting local anesthetic, has been administered intraperitoneally in combination with adjuvant drugs, such as agonists of α2-adrenoreceptors, e.g., dexmedetomidine and epinephrine (11,12). These adjuvant drugs produce local vasoconstriction, which delays systemic absorption and improves the safety and efficacy of the IP local anesthetic (3). The same approach could benefit feline pain management since bupivacaine, dexmedetomidine, and epinephrine are non-controlled drugs that are available worldwide. To the authors’ knowledge, the pharmacokinetics of IP bupivacaine in combination with dexmedetomidine or epinephrine has not yet been determined in cats.
The aims of this study were to determine plasma concentrations and deriving pharmacokinetics from concentration-time data plotting and to evaluate the postoperative analgesic efficacy of bupivacaine in combination with epinephrine or dexmedetomidine after IP administration in cats undergoing ovariohysterectomy. The authors hypothesized that plasma concentrations of bupivacaine would be detected in combination with dexmedetomidine or epinephrine after IP administration, adverse effects would not be observed, and these treatments would provide similar analgesic effects postoperatively. Results of this study were compared with a previous pharmacokinetic study in which bupivacaine alone was administered intraperitoneally in cats undergoing ovariohysterectomy using similar experimental conditions in our laboratory (9).
Materials and methods
The study protocol was approved by the local animal care committee (protocol number 16-Rech-1833) and conducted according to Canadian Council on Animal Care guidelines.
Experimental design and treatment groups
This study was a prospective, randomized, masked clinical trial. Cats were randomly assigned using www.randomization.org (accessed June 28, 2016) to receive 1 of the following 2 treatments by the IP route of administration (n = 8/group): the bupivacaine-epinephrine group (BE) received bupivacaine at 2 mg/kg body weight (BW) (Sensorcaine, bupivacaine HCl 0.5% USP; AstraZeneca, Mississauga, Ontario) and epinephrine at 2 μg/kg BW (Epiclor, Rafter 8; Calgary, Alberta). The bupivacaine-dexmedetomidine (BD) group received bupivacaine at 2 mg/kg BW and dexmedetomidine at 1 μg/kg BW (Dexdomitor; Zoetis, Kirkland, Quebec). In both the BE and BD groups, a solution of bupivacaine 0.5% with epinephrine (1 μg/1 mg of bupivacaine, corresponding to the dose 2 μg/kg BW) or dexmedetomidine (1 μg/kg BW), respectively, was diluted with an equal volume of isotonic sterile saline (0.9% sodium chloride USP; Hospira, Montreal, Quebec), which resulted in a final concentration of 0.25% of bupivacaine.
Study animals
Sixteen adult, mixed-breed, female cats (3.3 ± 0.6 kg) from a local animal shelter were admitted to the veterinary teaching hospital [Centre hospitalier universitaire vétérinaire (CHUV)] of the Faculty of Veterinary Medicine, Université de Montréal for elective ovariohysterectomy, after obtaining the shelter’s written consent. Cats were included in this study if they were considered healthy based on a complete physical examination and normal values for hematocrit and total protein. Exclusion criteria included body weight < 2 kg, cardiac arrhythmias, pregnancy, lactation, body condition score of > 7 or < 3 on a scale from 1 to 9, anemia (hematocrit < 25%), hypoproteinemia (total protein < 59 g/dL), and clinical signs of disease, such as upper tract respiratory infection. Study animals were housed individually in adjacent cages in the cat ward at CHUV.
Experimental procedure
Food but not water was withheld up to 8 h before general anesthesia. Approximately 20 min before induction, a 22-G catheter was inserted aseptically into a cephalic vein. Cats did not receive any premedication and anesthesia was induced using propofol intravenously (IV) (Diprivan 1%; AstraZeneca). Lidocaine 2% (0.05 mL) (Xylocaine; AstraZeneca) was instilled over the arytenoid cartilages and the cats were intubated with an appropriately sized, cuffed endotracheal tube. Anesthesia was maintained with isoflurane (Isoflurane USP; Pharmaceuticals Partners of Canada, Richmond Hill, Ontario) administered in oxygen using a non-rebreathing circuit. Cats were then positioned in dorsal recumbency on a circulating warm water blanket and monitoring [electrocardiogram (ECG), capnography, and pulse oximetry] was recorded every 5 min using a multiparametric monitor (Lifewindow 6000V veterinary multiparameter monitor; Digicare Animal Health, Boynton Beach, Florida, USA). Blood pressure was monitored using a Doppler ultrasound blood flow detector (Doppler Model 811-B; Park Electronics, Aloha, Oregon, USA). A balanced crystalloid solution was administered intravenously at a rate of 10 mL/kg BW per hour throughout anesthesia and surgery. Before surgery began, a 20-G, 1.16-in catheter was aseptically inserted into a jugular vein for blood sampling, fixed with suture, and protected with a bandage. Buprenorphine (Vetergesic; Champion Alstoe Animal Health, York, England) (0.02 mg/kg BW, IV) and meloxicam (Metacam 0.5%; Boehringer Ingelheim, Burlington, Ontario) [0.2 mg/kg BW, subcutaneously (SC)] were administered for analgesia after induction of anesthesia and before surgery.
Ovariohysterectomy was carried out by the same veterinarian (BPM) using a ventral midline incision as reported in a previous study (10). The individual withdrew the test drug (BE or BD) solution in a sterile manner using a 3-mL syringe attached to a 22-G, 1.16-in catheter and divided equally into 3 parts to be administered intraperitoneally. Specifically, each part of the solution was instilled (“splashed”) over the right and left ovarian pedicles and at the caudal aspect of the uterine body immediately after the first venous blood sample had been collected (time point 0, baseline). The ovariohysterectomy was done approximately 2 min later. Duration of surgery (time elapsed from the first incision until placement of the last suture), anesthesia (time elapsed from beginning to cessation of isoflurane administration), and time to extubation (time elapsed from cessation of isoflurane administration until extubation) were recorded for each cat.
Blood sampling and analysis of bupivacaine in plasma
Venous blood samples (1.5 mL) were collected immediately before IP administration of BE or BD (time point 0, baseline) and at 2, 5, 10, 15, 20, 30, 60, 120, 240, 360, and 480 min after administration. These sampling time points were chosen based on findings from previous studies in humans and cats (9,13,14). Hematocrit and total protein were re-evaluated after the last time point to exclude anemia and hypoproteinemia. Samples were collected while the cats were under general anesthesia, with the exception of the following time points: 60, 120, 240, 360, and 480 min. Blood was transferred to EDTA-containing tubes and immediately placed into a container with ice. Samples were kept on ice for 15 to 30 min and then centrifuged at 3500 × g for 10 min. Plasma was separated and stored frozen (−80°C) until analysis.
Pharmacokinetics of bupivacaine
Plasma bupivacaine concentrations were determined using a liquid chromatography tandem mass spectrometry (LC-MS/MS) method as described in a previous study (15). Pharmacokinetic parameters of bupivacaine in cat plasma were calculated using a non-compartmental method (16). The following variables were calculated: area under the plasma concentration-time curve from time zero to the last measured time point (AUC0 − t; ng h/mL); terminal elimination rate constant (λz; 1/h); area under the plasma concentration- time curve from zero (0) hours extrapolated to infinity (∞) (AUC0 − ∞; ng h/mL); terminal elimination half-life (T1/2; h); relative clearance indexed by bioavailability (CL/F; L h/kg); and volume of distribution indexed by bioavailability (Vz/F; L/kg).
Postoperative pain and sedation
Analgesia and sedation were evaluated as part of postoperative care. Pain was evaluated by 1 observer (JB), who was not aware of treatment groups, using a multidimensional composite pain scale (UNESP-Botucatu MCPS) (17) and the Glasgow composite feline pain scale (GPS) (18). Pain and sedation were also evaluated using a dynamic visual analog scale (DIVAS pain and DIVAS sedation, respectively) (19) at 60 min before induction of anesthesia (time 0; baseline) and at 0.5, 1, 2, 3, 4, 6, and 8 h after surgery. Rescue analgesia was provided with buprenorphine (0.02 mg/kg IV BW) if MCPS was ≥ 6. Although data collected after the rescue analgesia was administered were not included in the statistical analysis, cats were monitored continuously to determine the requirement for additional analgesia. A second dose of buprenorphine (0.02 mg/kg BW) was administered intramuscularly (IM) to cats at the end of the study or at any time point if needed.
Statistical analysis
Statistical analyses were carried out with standard software (SAS, Version 9.3; SAS Institute, Cary, North Carolina, USA). Data were tested for normality with a Shapiro-Wilk test. Demographic data for each treatment group were analyzed using equal variances t-tests. Plasma drug concentrations and peak plasma drug concentrations-time values were normalized using a log base of 10. Peak plasma-time values were analyzed using an equal-variances t-test. Repeated measures linear models were carried out with time as the within-subject factor and treatment as the between-subject factor, and adjusted with the Benjamini-Hochberg test. The number of cats receiving rescue analgesia was analyzed in temporal changes within their treatment group (response variable ‘number of rescues’) using a 1-way X2 test, followed by pairwise comparisons between analgesic treatment groups. Values of P < 0.05 were considered significant. Sample size calculations were not done since the same study design and methodology were used as reported in a previous study (9).
Results
Body condition score, body weight, hematocrit, and total protein (before and after surgery), duration of anesthesia and surgery, and time to extubation are shown in Table I. None of these variables was significantly different between the BE and BD groups. The dose (mean ± SD) of propofol administered for induction of anesthesia was 10.2 ± 3.1 mg/kg BW (10.8 ± 3.3 mg/kg BW and 9.7 ± 3.1 mg/kg BW for BE and BD groups, respectively, P > 0.05). All cats were discharged from hospital 24 h after surgery without postoperative complications. No signs of local anesthetic toxicity were recorded.
Table I.
Body condition score (BCS), body weight, hematocrit, and total protein (before and after surgery), duration of anesthesia and surgery, and time to extubation in cats undergoing ovariohysterectomy after intraperitoneal administration of bupivacaine-epinephrine (BE) or bupivacaine-dexmedetomidine (BD).
| Variables | Population n = 16 |
Group BE n = 8 |
Group BD n = 8 |
P-value |
|---|---|---|---|---|
| BCS (1 to 9) | 5 ± 0 | 5 ± 0 | 5 ± 1 | 0.57 |
| Weight (kg) | 3.3 ± 0.6 | 3.2 ± 0.6 | 3.3 ± 0.6 | 0.91 |
| Hematocrit before (%) Normal range (28 to 47) |
37 ± 4 | 36 ± 5 | 38 ± 4 | 0.70 |
| Hematocrit after (%) Normal range (28 to 47) |
31 ± 4 | 29 ± 4 | 32 ± 4 | 0.45 |
| Total protein before (g/dL) Normal range (59 to 81) |
73 ± 8 | 72 ± 4 | 73 ± 10 | 0.79 |
| Total protein after (g/dL) Normal range (59 to 81) |
62 ± 5 | 61 ± 5 | 63 ± 6 | 0.40 |
| Duration of anesthesia (min) | 58 ± 15 | 60 ± 21 | 56 ± 8 | 0.81 |
| Duration of surgery (min) | 19 ± 2 | 19 ± 3 | 19 ± 1 | 0.62 |
| Time to extubation (min) | 7 ± 6 | 5 ± 3 | 8 ± 8 | 0.58 |
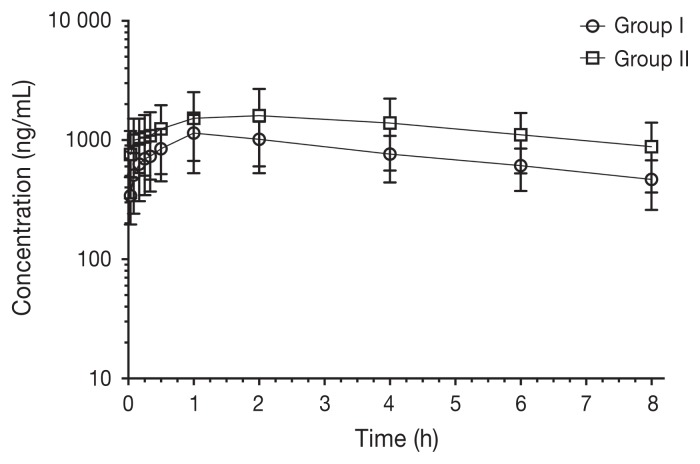
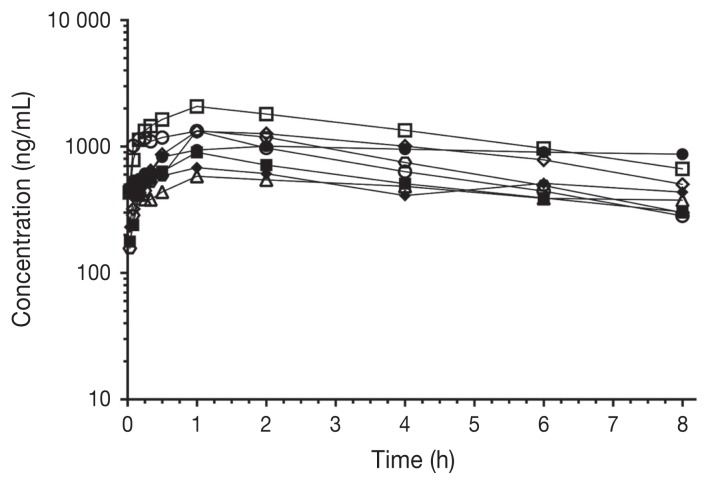
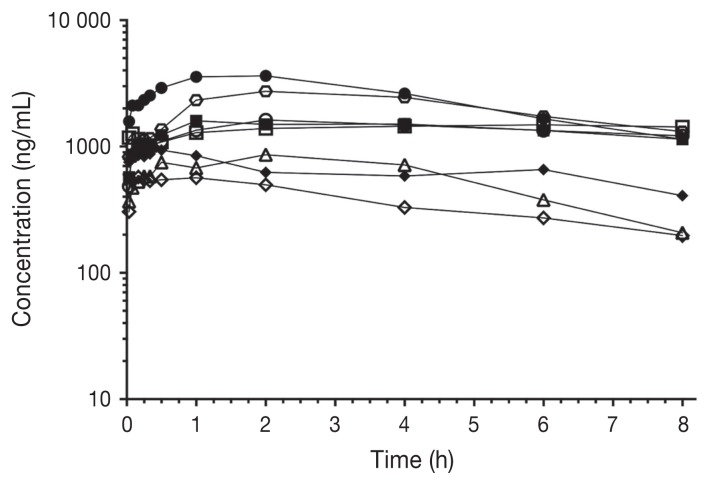
Pharmacokinetic parameters of bupivacaine after IP administration are shown in Table II. Plasma concentrations over time (ng/mL) for each group are shown in Figure 1. Individual plasma concentrations over time (ng/mL) are shown in Figure 2 and Figure 3 for the BE and BD groups, respectively. Maximum bupivacaine plasma concentration (Cmax) for the BE and BD groups were 1155 ± 168 ng/mL and 1678 ± 364 ng/mL (P = 0.29) at 67 ± 13 min (Tmax) and 123 ± 59 min (P = 0.17), respectively.
Table II.
Pharmacokinetic parameters of bupivacaine after intraperitoneal administration of bupivacaine-epinephrine (BE) or bupivacaine-dexmedetomidine (BD) (n = 8/group) in adult cats undergoing ovariohysterectomy.
| Population n = 16 |
Group BE n = 8 |
Group BD n = 8 |
||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Parameter | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range |
| AUC0 − t (ng h/mL) | 7004.3 ± 3302.8 | 3694.8 to 11 150.9 | 6126.5 ± 2290.2 | 3694.8 to 10 571.1 | 10 256.1 ± 5849.8 | 2962.0 to 19 680.8 |
| AUC0 − ∞ (ng h/mL) | 24 292.2 ± 25 918.5 | 7571.9 to 82 378.1 | 15 953.7 ± 12 849.7 | 7841.8 to 46 569.4 | 31 375.9 ± 24 859.8 | 5179.2 to 82 378.1 |
| CL/F (L h/kg) | 0.148 ± 0.083 | 0.024 to 0.264 | 0.168 ± 0.069 | 0.043 to 0.255 | 0.132 ± 0.129 | 0.024 to 0.386 |
| λze (1/h) | 0.1102 ± 0.0827 | 0.0203 to 0.2447 | 0.1295 ± 0.0730 | 0.0243 to 0.2274 | 0.1164 ± 0.0773 | 0.0203 to 0.2447 |
| t1/2 (h) | 11.3 ± 10.2 | 2.8 to 34.1 | 8.9 ± 8.6 | 3.0 to 28.5 | 10.5 ± 10.3 | 2.8 to 34.1 |
| Vz/F (L/kg) | 1.618 ± 0.906 | 0.645 to 3.116 | 1.634 ± 0.899 | 0.645 to 3.116 | 1.195 ± 0.777 | 0.308 to 2.599 |
AUC0 − t — Area under the plasma concentration-time curve from time zero to the last measured time point; AUC0 − ∞ — Area under the plasma concentration-time curve from time zero extrapolated to infinity; CL/F — Relative clearance indexed by bioavailability; λze — Terminal elimination rate constant; t1/2 — Terminal elimination half-life; Vz/F — Volume of distribution indexed by bioavailability.
Figure 1.
Plasma concentrations of bupivacaine after IP administration of bupivacaine-epinephrine (BE group) or bupivacaine-dexmedetomidine (BD group) solutions (n = 8/group) in adult female cats undergoing ovariohysterectomy. Time point 0; immediately before administering bupivacaine solutions.
Figure 2.
Individual plasma concentrations of bupivacaine after IP administration of bupivacaine-epinephrine (BE group) to cats (n = 8) undergoing ovariohysterectomy. Time 0; time of bupivacaine-epinephrine administration. Each symbol represents an individual cat.
Figure 3.
Individual plasma concentrations of bupivacaine after IP administration of bupivacaine-dexmedetomidine (BD group) to cats (n = 8) undergoing ovariohysterectomy. Time 0; time of bupivacaine-dexmedetomidine administration. Each symbol represents an individual cat.
The mean elimination half-life was 11.3 ± 10.2 h (8.9 ± 8.6 h and 10.5 ± 10.3 h; BE and BD groups, respectively). The clearance indexed by bioavailability (CL/F) was 0.148 ± 0.083 L h/kg (0.168 ± 0.069 L h/kg and 0.132 ± 0.129 L h/kg; BE and BD groups, respectively). The volume of distribution indexed by bioavailability (Vz/F) was 1.618 ± 0.906 L/kg (1.634 ± 0.899 L/kg and 1.195 ± 0.777 L/kg; BE and BD groups, respectively). Pharmacokinetic parameters were not different between treatments (P > 0.05).
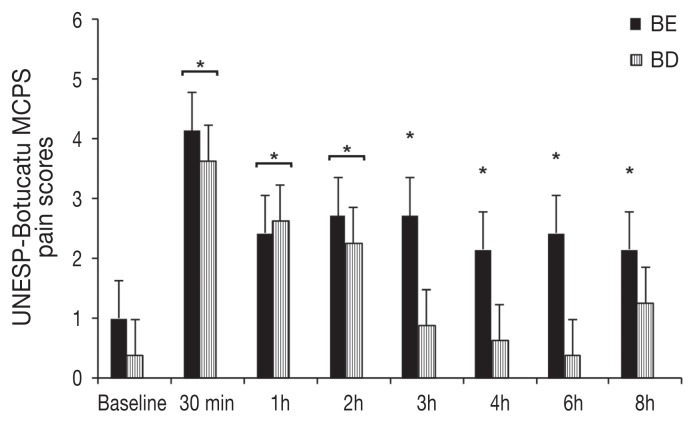
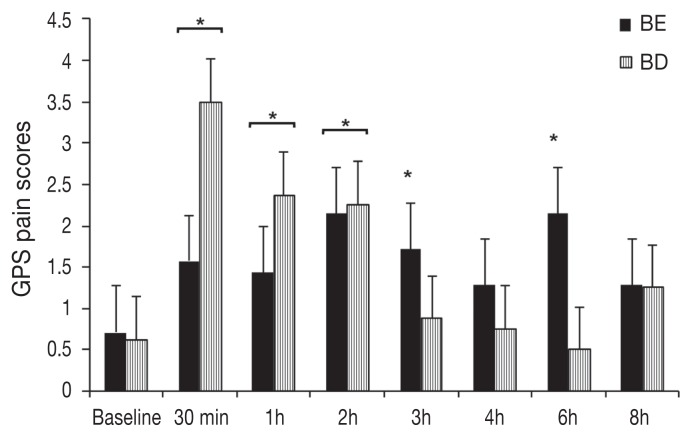
One cat in the BE group required rescue analgesia at 1 h post-extubation. An effect of treatment on the number of cats requiring rescue analgesia was rejected (P = 0.3). Pain and sedation scores were not different between treatments (P > 0.05). The MCPS scores were increased in cats of the BE group at 0.5, 1, 2, 3, 4, 6, and 8 h (P < 0.001 for all time points) and of the BD group at 0.5, 1, and 2 h (P < 0.0003 for all time points) compared with baseline values (Figure 4). The GPS scores were increased in the BE group at 0.5, 1, 2, 3, and 6 h (P < 0.01 for all time points) and in the BD group at 0.5, 1, and 2 h (P < 0.001 for all time points) when compared with baseline values (Figure 5). Dynamic visual analog scale (DIVAS) pain scores were not significantly different when compared with baseline values. Sedation scores (DIVAS) were significantly increased in the BE group at 0.5 h (P < 0.0001) and in the BD group at 0.5 and 1 h (P < 0.0001 for both time points) compared with baseline values.
Figure 4.
Mean ± standard error of mean (SEM) pain scores using the multidimensional composite pain scale (UNESP-Botucatu MCPS). Scores were not significantly different between treatments (P = 0.1487). All cats received buprenorphine (0.02 mg/kg BW IV) and meloxicam (0.2 mg/kg BW SC); BE = intraperitoneal bupivacaine (bupivacaine 0.25%, 2 mg/kg BW) and epinephrine (2 μg/kg BW). BD = intraperitoneal bupivacaine (bupivacaine 0.25%, 2 mg/kg BW) and dexmedetomidine (1 μg/kg BW).
*Significantly increased when compared with baseline values.
Figure 5.
Mean ± standard error of mean (SEM) pain scores using the Glasgow feline composite pain scale (GPS). Scores were not significantly different between treatments (P = 0.9725). All cats received buprenorphine (0.02 mg/kg BW IV) and meloxicam (0.2 mg/kg SC BW); BE = intraperitoneal bupivacaine (bupivacaine 0.25%, 2 mg/kg BW) and epinephrine (2 μg/kg BW). BD = intraperitoneal bupivacaine (bupivacaine 0.25%, 2 mg/kg BW) and dexmedetomidine (1 μg/kg BW).
*Significantly increased when compared with baseline values.
Discussion
Sustained plasma concentrations of bupivacaine were measured after IP administration of BE and BD without adverse effects, as similarly reported in humans (20). Mean maximum plasma concentrations of bupivacaine were not significantly different between the BE and BD groups and were far from those reported to cause convulsive electroencephalogram pattern (20) or fatal arrhythmias (21) in cats. It is therefore safe to administer these drug combinations in cats undergoing ovariohysterectomy. Both treatments resulted in comparable pharmacokinetics. Interestingly, Cmax (BE − 1155 ± 168 ng/mL and BD − 1678 ± 364 ng/mL) was not lower than for bupivacaine alone (1030 ± 497.5 ng/mL) as reported in a previous study using the same technique and dosage regimens (9).
Although it is not appropriate to make comparisons between humans and animals, studies in humans have shown that the BE group decreased Cmax by approximately 50% when compared with bupivacaine alone (14). In addition, clinically relevant differences in Tmax were observed among the BE and BD groups (BE — 67 ± 13 min; BD — 123 ± 59 min) and bupivacaine alone (30 ± 24 min) (9). This was particularly true for the BD group versus bupivacaine alone, where a 4-fold difference for Tmax and approximately 50% difference in mean Cmax were detected. These 2 variables (Tmax and Cmax) are influenced by the relationship between absorption, distribution, and elimination rate constants. Since terminal elimination rate constant and clearance indexed by bioavailability were similar between treatments, it is possible that vasoconstriction produced by epinephrine and dexmedetomidine played a role in delayed absorption and longer terminal elimination half-life in the BE and BD groups compared with bupivacaine alone.
The safety and efficacy of IP analgesia depend on many factors, including the use of different local anesthetics or their combinations; doses; concentrations; volumes of injection; mode of administration, (i.e., aerosol, nebulization versus instillation); and addition of other adjuvants such as opioids, etc. Many of these factors have not been investigated in feline medicine and surgery and it is not known how they would change pharmacokinetics and pharmacodynamics of analgesics. Furthermore, modifications of the technique, such as post-incisional versus end of surgery, frequency (single versus multiple doses versus infusions), and site of administration (port-site or directed infiltration versus instillation), could also impact safety and efficacy. Future studies are warranted to investigate these aspects of IP analgesia in feline practice and their effects on pharmacokinetics and pharmacodynamics.
This study had some limitations. Firstly, there was no control group receiving bupivacaine alone. Such a group would have validated our results and highlighted the advantages of using bupivacaine-epinephrine and bupivacaine-dexmedetomidine solutions compared with bupivacaine alone, especially when using a small population of cats with large individual variability. Such a control group was not included in the present study due to financial constraints and because it would have been ethically unacceptable since the pharmacokinetics of bupivacaine after IP administration have already been described in this species (9). Comparisons with historical controls that received IP bupivacaine alone using a similar study design, species of animals (cats), and methodology have been used. Although not ideal, it provides some perspective on these different treatments for IP analgesia. Secondly, the active metabolites of bupivacaine were not analyzed and the clinical relevance of these metabolites in cats is not known. Thirdly, plasma concentrations were measured only up to 8 h after the surgical procedure, at which point they had not returned to pre-administration values, which could have influenced the pharmacokinetics. When the concentration was converted to the natural logarithm of concentration versus time, however, the slope of the curve had a homogenous elimination phase without evidence of drug redistribution that could have influenced the pharmacokinetic profile. Finally, bupivacaine was combined with a single dose of epinephrine and dexmedetomidine. It is not known whether pharmacokinetics would have changed if different dosages had been used.
Both the BE and BD groups produced satisfactory postoperative analgesia when administered in combination with meloxicam and buprenorphine. The number of cats requiring rescue analgesia and pain/sedation scores was similar between the 2 treatments. However, MCPS and GPS pain scores were significantly increased in the BE group (up to 6 to 8 h) and for much longer than in the BD group (up to 2 h) compared with baseline values. Dexmedetomidine has some analgesic properties (22) and it is possible that a bupivacaine-dexmedetomidine solution may provide a slightly better short-acting analgesic effect than a bupivacaine-epinephrine solution. This could not be observed in a study using a small number of cats (type-II error). In addition, the authors cannot conclude that bupivacaine-epinephrine and bupivacaine-dexmedetomidine solutions are better than bupivacaine alone in terms of analgesic efficacy. In theory, prolonged analgesic effects are one of the advantages of using epinephrine and dexmedetomidine as drug adjuvants to local anesthetics for IP analgesia. This could be the subject of a future clinical trial using a larger population of cats.
In conclusion, IP bupivacaine with epinephrine or dexmedetomidine produced concentrations below toxic levels and are safe to administer for IP analgesia in cats. Similar postoperative analgesia was observed for bupivacaine-epinephrine and bupivacaine-dexmedetomidine in combination with meloxicam and buprenorphine.
Acknowledgments
The authors thank Fleur Gaudette from the Pharmacokinetics core facility of the Centre de Recherche, Centre hospitalier de l’Université de Montréal (CRCHUM) for carrying out LC-MS/MS method development, validation, and sample analysis; Guy Beauchamp for statistical analysis; and Faustine Sarceau for technical help during the study. Funding was provided by the American College of Veterinary Anesthesia and Analgesia (ACVAA) Research Foundation; the “Fonds du Centenaire” of the Faculty of Veterinary Medicine, Université de Montréal; and a generous donation by Valeria Rosenbloom and Mike Rosenbloom. Dr. Beatriz Monteiro is a recipient of the Vanier Canada Graduate Scholarship.
References
- 1.Buck L, Varras MN, Miskry T, Ruston J, Magos A. Intraperitoneal bupivacaine for the reduction of postoperative pain following operative laparoscopy: A pilot study and review of the literature. J Obstet Gynaecol. 2004;24:448–451. doi: 10.1080/01443610410001685637. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra N, Chanana C, Roy KK, Kumar S, Rewari V, Sharma JB. To compare the efficacy of two doses of intraperitoneal bupivacaine for pain relief after operative laparoscopy in gynecology. Arch Gynecol Obstet. 2007;276:323–326. doi: 10.1007/s00404-007-0337-1. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SM, Karanicolas PJ, Emmerton-Coughlin HM, Kanbur B, Kanbur S, Colquhoun PH. Better late than never? Impact of local analgesia timing on postoperative pain in laparoscopic surgery: A systematic review and metaanalysis. Surg Endosc. 2010;24:3167–3176. doi: 10.1007/s00464-010-1111-1. [DOI] [PubMed] [Google Scholar]
- 4.Mitra S, Khandelwal P, Roberts K, Kumar S, Vadivelu N. Pain relief in laparoscopic cholecystectomy — A review of the current options. Pain Pract. 2012;12:485–496. doi: 10.1111/j.1533-2500.2011.00513.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalchofner Guerrero KS, Campagna I, Bruhl-Day R, Hegamin-Younger C, Guerrero TG. Intraperitoneal bupivacaine with or without incisional bupivacaine for postoperative analgesia in dogs undergoing ovariohysterectomy. Vet Anaesth Analg. 2016;43:571–578. doi: 10.1111/vaa.12348. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DV, Barnes KS, Hauptman JG. Pharmacokinetics of combined intraperitoneal and incisional lidocaine in the dog following ovariohysterectomy. J Vet Pharmacol Ther. 2004;27:105–109. doi: 10.1111/j.1365-2885.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 7.Campagnol D, Teixeira-Neto FJ, Monteiro ER, Restitutti F, Minto BW. Effect of intraperitoneal or incisional bupivacaine on pain and the analgesic requirement after ovariohysterectomy in dogs. Vet Anaesth Analg. 2012;39:426–430. doi: 10.1111/j.1467-2995.2012.00728.x. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter RE, Wilson DV, Evans AT. Evaluation of intraperitoneal and incisional lidocaine or bupivacaine for analgesia following ovariohysterectomy in the dog. Vet Anaesth Analg. 2004;31:46–52. doi: 10.1111/j.1467-2995.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 9.Benito J, Monteiro B, Beaudry F, Lavoie AM, Lascelles BD, Steagall PV. Pharmacokinetics of bupivacaine after intraperitoneal administration in cats undergoing ovariohysterectomy. Am J Vet Res. 2016;77:641–645. doi: 10.2460/ajvr.77.6.641. [DOI] [PubMed] [Google Scholar]
- 10.Benito J, Monteiro B, Lavoie AM, Beauchamp G, Lascelles BD, Steagall PV. Analgesic efficacy of intraperitoneal administration of bupivacaine in cats. J Feline Med Surg. 2016;18:906–912. doi: 10.1177/1098612X15610162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oza VP, Parmar V, Badheka J, Nanavati DS, Taur P, Rajyaguru AM. Comparative study of postoperative analgesic effect of intraperitoneal instillation of dexmedetomidine with bupivacaine and bupivacaine alone after laparoscopic surgery. J Minim Access Surg. 2016;12:260–264. doi: 10.4103/0972-9941.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng A, Swami A, Smith G, Davidson AC, Emembolu J. The analgesic effects of intraperitoneal and incisional bupivacaine with epinephrine after total abdominal hysterectomy. Anesth Analg. 2002;95:158–162. doi: 10.1097/00000539-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Lipscomb GH, Summitt RL, Jr, McCord ML, Ling FW. Serum bupivacaine levels during laparoscopic sterilization using local anesthesia. J Am Assoc Gynecol Laparosc. 1994;2:27–30. doi: 10.1016/s1074-3804(05)80827-8. [DOI] [PubMed] [Google Scholar]
- 14.Narchi P, Benhamou D, Bouaziz H, Fernandez H, Mazoit JX. Serum concentrations of local anaesthetics following intraperitoneal administration during laparoscopy. Eur J Clin Pharmacol. 1992;42:223–225. doi: 10.1007/BF00278490. [DOI] [PubMed] [Google Scholar]
- 15.Gaudette F, Benito J, Steagall P, Beaudry F. Assessment of tandem mass spectrometry and high resolution mass spectrometry for the analysis of bupivacaine in plasma. Biomed Chromatogr. 2015;29:1724–1730. doi: 10.1002/bmc.3485. [DOI] [PubMed] [Google Scholar]
- 16.Rowland M, Tozer TN. Distribution kinetics. In: Rowland M, Tozer TN, editors. Clinical Pharmacokinetics: Concepts and Applications. 3rd ed. Philadelphia, Pennsylvania: Lippincott, Williams & Wilkins; 1995. pp. 317–319. [Google Scholar]
- 17.Brondani JT, Mama KR, Luna SP, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res. 2013;9:143. doi: 10.1186/1746-6148-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo G, Holden E, Reid J, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract. 2014;55:622–629. doi: 10.1111/jsap.12280. [DOI] [PubMed] [Google Scholar]
- 19.Steagall PV, Taylor PM, Rodrigues LC, Ferreira TH, Minto BW, Aguiar AJ. Analgesia for cats after ovariohysterectomy with either buprenorphine or carprofen alone or in combination. Vet Rec. 2009;164:359–363. doi: 10.1136/vr.164.12.359. [DOI] [PubMed] [Google Scholar]
- 20.de Jong RH, Ronfeld RA, DeRosa RA. Cardiovascular effects of convulsant and supraconvulsant doses of amide local anesthetics. Anesth Analg. 1982;61:3–9. [PubMed] [Google Scholar]
- 21.Kasaba T, Shiraishi S, Taniguchi M, Takasaki M. Bupivacaine-induced convulsion is suppressed by MK-801. Reg Anesth Pain Med. 1998;23:71–76. doi: 10.1016/s1098-7339(98)90113-4. [DOI] [PubMed] [Google Scholar]
- 22.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]