Abstract
The aim of this study was to evaluate the prevalence of hip and elbow dysplasia in a group of growing Labrador retrievers fed a fish-based diet enriched with nutraceuticals with chondroprotective properties. The puppies ranged from 3 to 12 mo of age and were divided into 2 groups, each fed a different diet. The control diet consisted of a high quality, chicken-based dog food, while the test diet was a fish-based dog food, enriched with nutraceuticals. Hip and elbow joints were radiographed and scored at 6 and 12 mo of age. Overall, 42 dogs completed the study. At 12 mo of age, no differences were found between the groups in the prevalence of hip and elbow dysplasia, although dogs fed the fish-based food enriched with nutraceuticals had a less severe grade of osteoarthritis at 12 mo. It was concluded that the fish-based diet with nutraceuticals did have beneficial effects on the development of severe osteoarthritis.
Résumé
L’objectif de la présente étude était d’évaluer la prévalence de dysplasie de la hanche et du coude dans un groupe de chiens Labrador en croissance nourris avec une diète à base de poisson enrichie de neutraceutiques ayant des propriétés chondroprotectrices. L’âge des chiots variait de 3 à 12 mois et ils ont été divisés en deux groupes, chacun étant nourri avec une diète différente. La diète témoin consistait d’un aliment de haute qualité pour chien à base de poulet, alors que la diète test était un aliment pour chien à base de poisson et enrichi avec des neutraceutiques. Les articulations des hanches et des coudes ont été radiographiées à 6 et 12 mois d’âge. Un total de 42 chiens a complété l’étude. À 12 mois d’âge, aucune différence n’a été trouvée entre les groupes dans la prévalence de dysplasie de la hanche et du coude, bien que les chiens nourris avec la diète à base poisson enrichie de neutraceutiques avaient un score d’ostéoarthrite moins sévère à 12 mois. Il a été conclu que la diète à base de poisson enrichie de neutraceutiques avait des effets bénéfiques sur le développement d’ostéoarthrite sévère.
(Traduit par Docteur Serge Messier)
Hip and elbow dysplasia are frequent non-traumatic orthopedic diseases in growing dogs. These disorders are associated with joint incongruity and joint cartilage injury and eventually lead to osteoarthritis (OA) (1,2). Energy intake and calorific imbalance may play a role in the etiopathogenesis of developmental diseases (2). Dietary additives, including glucosamine, chondroitin sulphate, and polyunsaturated fatty acids, can potentially modify some of the underlying processes of OA by modulating inflammatory response, providing nutrients for cartilage repair, and protecting against oxidative damage (3–6). Green-lipped mussel (Perna canaliculus) contains a unique omega-3 fatty acid that appears to act as a dual inhibitor of arachidonic acid oxygenation (7) and has been shown to have beneficial effects on adult dogs diagnosed with osteoarthritis (7). Devil’s claw (Harpagophytum procumbens) and Boswellia serrata are plants with documented anti-inflammatory and analgesic activity (8,9). Finally, fish represent a source of animal protein that is free from antibiotic residues, particularly oxytetracycline, which seems to exert a cytotoxic and pro-inflammatory effect on human and canine lymphocytes (10,11).
The aim of this study was to evaluate the effect of a commercially available, fish-based dog food supplemented with glucosamine, chondroitin sulphate, fish oil-derived fatty acids, whole freeze-dried green-lipped mussel powder (P. canaliculus), Boswellia serrata, and devil’s claw (H. procumbens) on development of hip and elbow OA, secondary to dysplasia, in growing Labrador retrievers during their first year of life and to compare it with a control diet.
A blind, randomized, controlled clinical evaluation was planned to test the effect of the 2 different diets. The primary outcome was an evaluation of the difference in prevalence of OA in hip and elbow joints, secondary to dysplasia, at 12 mo of age in the 2 test groups. The secondary outcome was a composite measure representing overall severity of OA and included scores for the following single factors: elbow OA; medial coronoid disease; ulnar subtrochlear sclerosis; humeral radioulnar incongruity; hip OA; coxofemoral incongruity; distraction index (DI) (6 mo); and Norberg angle (12 mo). The secondary outcome was measured at 2 endpoints (6 and 12 mo).
Based on a published study on hip dysplasia (12), a sample size of 17 dogs/group was calculated in order to meet the minimum requirement for providing 80% confidence in difference detection at a significance level of 0.05. It was determined that 25 dogs/group was an appropriate initial sample size, which accounted for a dropout rate of 10%. All veterinarians followed guidelines established for good clinical practice and owners provided informed consent for participation.
Either the sire or dam of each litter had previously scored with mild elbow or hip dysplasia. Inclusion criteria for the study were: i) birth in the spring/summer season with the pre-weaning period spent on a farm; ii) participation in off-leash exercise on soft ground; and iii) consumption of the same starter food (processed chicken meal, ground rice, fish meal, with added vitamins, 0.5% colostrum, and 0.047% glucosamine). Exclusion criteria included the presence of acute traumatic injuries or systemic diseases, poor owner compliance, adverse food reactions, and food palatability. Dogs that underwent joint surgery or were lost to followup were also excluded.
At birth, litters of puppies were assigned collars of different colors. At 2 mo, colors were changed to labels from a number sequence. Dogs were distributed equally into 2 gender-balanced groups and were then randomly allocated into the 2 test groupings using a computer- generated randomization list (GraphPad Prism 7; GraphPad Software, San Diego, California, USA) by one author (FDI) and the breeder. At 2 mo, all puppies underwent a complete physical and orthopedic examination by private practice vets. The results of both hip and elbow orthopedic examinations were used as a baseline.
Dogs in the control group were fed a high quality food for puppies, while dogs in the treatment group were fed a specific fish-based food for puppies, supplemented with nutraceuticals. These feeding regimes were maintained until the dogs reached 12 mo of age. Both foods were commercially available kibble. The control and the nutraceutical-supplemented diets were carefully adjusted to provide similar calorific intake and to satisfy the nutritional requirements of growing dogs and puppies. The analytical composition of the diets was: crude protein 26%; carbohydrates 34.2%; crude oils and fats 13% (omega 3, 2.21% and omega 6, 2.18%); crude fiber 2.5%; crude ash 8%; and metabolized energy, 3 464 kcal/kg. The control food contained dehydrated chicken meal as the main protein source. The dietary test food contained fish meal and combined kibble and cold-pressed tablets mixed in the same package, with the tablets making up 6% to 7% w/w of the completed food. The tablets consisted of hydrolyzed fish and vegetable protein, supplemented with vegetal glucosamine, chondroitin sulphate, chitosamine, Boswellia serrata, devil’s claw (H. procumbens), green-lipped mussel (P. canaliculus), and omega-3/6 fatty acids in a ratio of 1:1.
Owners were instructed to supply an age-adjusted amount of food 3 times a day and to control the puppy’s activities by avoiding prolonged or intense physical activity, such as walking up stairs or running after a ball or a stick at high speed (2). Owners were telephoned when their puppies reached 4, 8, 10, and 30 mo of age to answer a questionnaire about feeding patterns, physical exercise and housing, difficulties in jumping/walking, or perception of lameness. The interviewer (FM) was unaware of the group assignment at the time of the interviews.
Body weight and body condition (scale from 1 to 5) (6) were monitored throughout the study (at birth and at 4, 6, 8, 10, 12, and 30 mo). Dogs were clinically and radiographically evaluated at 6 and 12 mo. The clinical assessment involved evaluation by an observer (AV), blind to the randomization of dog subjects, who assigned a score ranging from 0 to 3 (none, mild, moderate, severe) for various orthopedic variables (lameness, range of motion, swelling, pain), based on a previous report (1).
Hip and elbow joints were radiographed at 6 and 12 mo of age under sedation. Two trained operators (SM and AV, both with PhDs in Domestic Animal Orthopedics), who were blind to the randomization of dogs, carried out the radiographic examinations. Elbow joints were radiographed in 45° flexed mediolateral and craniocaudal projections (1). Hip joints were radiographed in the standard ventrodorsal hip-extended view. At 6 mo of age, an extra radiograph was done in ventrodorsal projection with distraction. A trained radiologist (GG) evaluated all the radiographs independently, unaware of which group the dog on the radiograph belonged to. At 6 and 12 mo of age, the following radiological changes in the elbow were assessed: osteophyte grade; medial coronoid disease grade; ulnar subtrochlear sclerosis; and humeral radioulnar incongruity. These were assessed based on the International Elbow Working Group System (IEWG) (13) and grades are provided in Table I. For hip joints, the following items were scored according to the British Veterinary Association/Kennel Club scheme (14): shape; position of femoral head; cranial acetabular rim; femoral head and neck exostosis; and Norberg angle. These grades are listed and described in Table II. At 6 mo, the distraction index (DI) was calculated. A DI value of < 0.4 was considered normal, > 0.7 was considered abnormal, and values of 0.41 to 0.69 were considered unreliable (15) (Table II). At 12 mo of age, the Norberg angle was calculated (14).
Table I.
Assessments of radiographic analyses of elbow joints.
| Assessment | Grade | Description |
|---|---|---|
| Elbow osteophyte | 0 | No osteophytes |
| 1 | Largest osteophytes < 2 mm | |
| 2 | Largest osteophytes 2 to 5 mm | |
| 3 | Largest osteophytes > 5 mm | |
| Medial coronoid disease | 0 | Normal coronoid |
| 1 | Mild change in coronoid shape | |
| 2 | Marked change in coronoid shape | |
| 3 | Fragmented coronoid | |
| Ulnar subtrochlear sclerosis | 0 | No sclerosis |
| 1 | Mild sclerosis, trabecular pattern still seen | |
| 2 | Moderate sclerosis, trabecular pattern slightly unclear | |
| 3 | Severe sclerosis, trabecular pattern cannot be seen | |
| Humeral radioulnar incongruity | 0 | No incongruity |
| 1 | Mild incongruity < 2 mm | |
| 2 | Moderate incongruity 2 to 3 mm | |
| 3 | Severe incongruity > 3 mm |
Table II.
Assessments of radiographic analyses of hip joints.
| Assessment | Grade | Description |
|---|---|---|
| Shape | 0 | Normal |
| 1 | Femoral head does not fit in a circle due to bone loss | |
| 2 | Obvious bone loss and distinct exostosis giving a slight conical appearance | |
| 3 | Very gross remodelling with marked bone loss and much new bone | |
| Position of femoral head | 0 | Femoral head is well centered in the acetabulum or lies medially to the DAE |
| 1 | Center of femoral head is superimposed on the DAE | |
| 2 | Center of femoral head is just lateral to the DAE | |
| 3 | Center of femoral head is well lateral to, and just touches, the DAE | |
| Cranial acetabular | 0 | Sharp, clean-cut junction of rim the DAE and CRAE |
| 1 | Very small exostosis | |
| 2 | Small exostosis or very small facet | |
| 3 | Gross exostosis and/or facet and/or moderate bilabiation | |
| Femoral head and neck exostosis | 0 | Smooth rounded profile |
| 1 | Slight exostosis in ring form and/or dense vertical line adjacent to trochanteric fossa (Morgan line) | |
| 2 | Distinct exostosis in ring form | |
| 3 | Massive exostosis giving a mushroom-like appearance | |
| Norberg angle | 0 | ≥ 105° |
| 1 | ≥ 100° to < 105° | |
| 2 | ≥ 95° to < 100° | |
| 3 | < 95° |
DAE — dorsal acetabular edge; CRAE — cranial acetabular edge.
Statistical analyses were conducted with statistical software SPSS version 22.0 for Mac (IBM, Chicago, Illinois, USA). Comparison of the prevalence of hip and elbow dysplasia between the 2 groups was analyzed by a chi-squared test. Binary logistic regression was used to determine any associations between the control group and secondary outcomes (using data composites at 6 and 12 mo), including gender (female versus male) as a dependent variable to control for eventual imbalances between groups. Probabilities with 95% confidence intervals (CIs) were used to quantify the strength of these associations. The Hosmer-Lemeshow statistic was used in order to assess goodness-of-fit of the model. Correlations between composite measures for severity of hip and elbow OA were calculated using the Spearman rank correlation coefficient. Mann-Whitney U-test was used to compare clinical assessment and radiological scores for each single factor previously mentioned, between test and control groups at the 2 endpoints (6 and 12 mo of age). P < 0.05 was considered significant.
Fifty Labrador retriever puppies, 26 males and 24 females [mean age: 60.04 ± 0.13 d standard deviation (SD); mean weight: 7 ± 1 kg], from 6 different litters from the same breeder, were enrolled. Twenty-five dogs were allocated to the control group and 25 to the treatment group. A total of 42 dogs completed the study (22 males and 20 females), 20 dogs in the control group, 22 in the treatment group. Eight dogs were excluded because of poor owner compliance (n = 6) or refusal of the food (n = 2). Overall, 93% of the puppies were kept both indoors and outdoors, with 7% kept outdoors only. All dogs were normal at physical and orthopedic examination at 2 mo. None of the dogs had been neutered or spayed or had undergone major surgery. The birth weight was 375 ± 50 g.
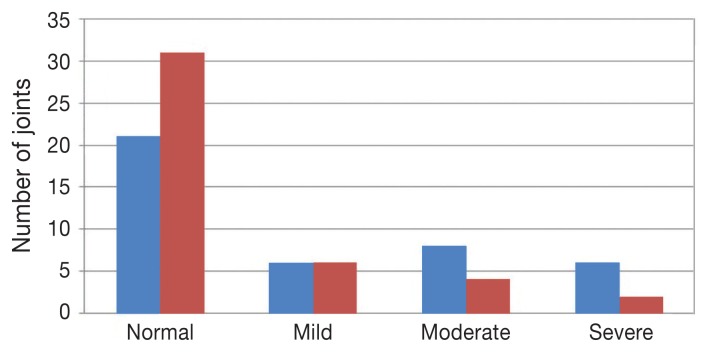
There were no differences in body weight or body condition score between the 2 groups at any time point. Overall, only 3 dogs had clinical signs. No differences were found between the 2 groups at 6 and 12 mo of age for both the pelvic and thoracic limb. No significant differences were found between males and females in the incidence of hip/elbow disease or body weight in either group. There was no significant difference in prevalence of hip and elbow dysplasia in either group, which were the same at 6 and 12 mo. In the univariate analysis, the control diet was associated with a more severe OA at 12 mo of age [odds ratio (OR): 1.17; 95% CI: 1.01 to 1.36; P = 0.038] (Figure 1). The result did not change when adjusted for gender (OR: 1.19; 95% CI: 1.01 to 1.4; P = 0.036). At 6 mo, there was no association between diet group and OA severity in univariate analysis or when adjusting for gender. Severity of elbow dysplasia was not correlated to severity of hip joint dysplasia at any time point. At 12 mo, dogs in the control group were more severely affected by osteoarthritis in the elbow joint and had a higher grade of elbow joint incongruity than dogs in the test group. At 12 mo, dogs fed the test diet had higher Norberg angle values. No significant difference was found for the other single factors. The telephone questionnaire at 30 mo showed no differences in the presence of lameness than at the assessment at 12 mo.
Figure 1.
Influence of diet on the distribution of osteoarthritis grade of dogs at 12 mo. The worst elbow and hip joint of each dog was considered. Blue indicates the control diet and red indicates the test diet.
Dietary supplements can potentially modify some of the underlying processes involved in OA by modulating the inflammatory response, providing nutrients for cartilage repair, and protecting against oxidative damage (7,16). It has been reported that calorie-restricted diets are useful in counteracting the progression of osteoarthritis (17).
No significant differences in the prevalence of hip and elbow OA were identified between the groups in this study. As a secondary outcome, however, severity of OA differed between groups. Dogs fed a fish-based dog food enriched with nutraceuticals had a less severe grade of OA at 12 mo of age. This outcome is in agreement with the results of a recent study, in which oral administration of hyaluronic acid, hydrolyzed collagen, glucosamine, chondroitin sulphate, and gamma oryzanol between 3 to 20 mo significantly decreased the prevalence of clinical signs of elbow OA in a cohort of Labrador retriever dogs (1). Although supplementing with specific chondro-protective properties may have no effects on phenotypic expression of hip and elbow dysplasia, it may have a cumulative protective effect against the progression of radiographic osteoarthritic changes (1). This hypothesis is supported by a recent review of studies on dietary supplements for managing OA in dogs (16).
The use of fish instead of chicken could be another important issue, due to the presence of oxytetracycline residues in poultry bones (18,19). Oxytetracycline residues exert cytotoxic effects, which suggests that the use of poultry bones and deboned meat in pet food is a potential risk (10,18,19). It is possible that the combination of a major supply of omega-3 fatty acids contained in fish oil and fish meal and the cytotoxic effect of oxytetracycline residues in poultry bones could have contributed to the results of the secondary outcome in the present study. However, more studies are needed to test the effects of a single protein source and/or its pollutants on developmental skeletal disease in growing dogs. In this study, no adverse effects could be attributed to the treatment diet, which was also noticeably associated with good product palatability.
The majority of dogs from both groups with radiological diagnoses of OA did not show clinical signs up to 30 mo of age. A combination of strict weight control during growth, associated with moderate exercise, could have played a concomitant role in both groups. Although the results of the secondary outcome may provide some additional information about the effects of a commercially available fish-based dog food enriched with nutraceuticals on the severity of OA, there are some limitations that should be mentioned. Animals were not radiographically followed at 30 mo of age and elbow lesions were not evaluated with computed tomography to detect early lesions, so false negatives were possible (20).
In conclusion, administration of fish proteins and supplementation with nutraceuticals with chondroprotective, anti-inflammatory, and antioxidant properties in the same food did not reduce the prevalence of hip and elbow dysplasia in this sample of dogs. Dogs fed the fish-based food had a less severe grade of OA at 12 mo, however, and therefore this diet did have beneficial effects on the development of severe osteoarthritis.
Table III.
Prevalence of elbow and hip dysplasia between groups. No statistically significant differences were found.
| Control group | Treatment group | |||
|---|---|---|---|---|
|
|
|
|||
| Age | Elbow dysplasia | Hip dysplasia | Elbow dysplasia | Hip dysplasia |
| 6 mo | 23% | 38% | 0.7% | 28% |
| 12 mo | 69% | 38% | 50% | 35% |
Acknowledgments
Sanypet Forza10 USA Corporation provided the complete and balanced nutraceutical dog food used as test diet. Moreover it covered all live expenses for the purchase of consumable materials used in this study. Dr. Sergio Canello, Dr. Gianandrea Guidetti, and Dr. Sara Centenaro are members of Sanypet group. The other authors have no financial or nonfinancial competing interest. The authors thank Dr. Gianluca Amato and Professor Alberto Sabbioni for statistical support, Mr. Fabio Mambelli for technical assistance, and Dr. Frazer Coomber (Bangor University, Wales) for assistance in editing this article.
References
- 1.Martì-Angulo S, Garcìa-Lopez N, Dìaz-Ramos A. Efficacy of an oral hyaluronate and collagen supplement as a preventive treatment of elbow dysplasia. J Vet Sci. 2014;15:569–574. doi: 10.4142/jvs.2014.15.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallander MH, Hedhammar A, Trogen ME. Diet, exercise, and weight as risk factors in hip dysplasia and elbow arthrosis in Labrador retrievers. J Nutr. 2006;136:2050–2052. doi: 10.1093/jn/136.7.2050S. [DOI] [PubMed] [Google Scholar]
- 3.Vandeweerd JM, Coisnon C, Clegg P, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med. 2012;26:448–456. doi: 10.1111/j.1939-1676.2012.00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Hielm-Björkman AH, Roine J, Elo K, Lappalainen A, Junnila J, Laitinen-Vapaavuori O. An uncommissioned randomized, placebo- controlled double-blind study to test the effect of deep sea fish oil as a pain reliever for dogs suffering from canine OA. BMC Vet Res. 2012;8:157. doi: 10.1186/1746-6148-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. Int J Rheumatol. 2011 doi: 10.1155/2011/969012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236:59–66. doi: 10.2460/javma.236.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Rialland P, Bichot S, Lussier B, et al. Effect of a diet enriched with green-lipped mussel on pain behaviour and functioning in dogs with clinical osteoarthritis. Can J Vet Res. 2013;77:66–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders M, Grundmann O. The use of glucosamine, devil’s claw (Harpagophytum procumbens), and acupuncture as complementary and alternative treatments for osteoarthritis. Altern Med Rev. 2011;16:228–238. [PubMed] [Google Scholar]
- 9.Guidetti G, Di Cerbo A, Giovazzino A, et al. In vitro effects of some botanicals with anti-inflammatory and antitoxic activity. J Immunol Res. 2016 doi: 10.1155/2016/5457010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Cerbo A, Palatucci AT, Rubino V, et al. Toxicological implications and inflammatory response in human lymphocytes challenged with oxytetracycline. J Biochem Mol Toxicol. 2016;30:170–177. doi: 10.1002/jbt.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo A, Landi R, Rubino V, et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ. 2017 doi: 10.7717/peerj.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GK, Lawler DF, Biery DN, et al. Chronology of hip dysplasia development in a cohort of 48 Labrador retrievers followed for life. Vet Surg. 2012;41:20–33. doi: 10.1111/j.1532-950X.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- 13.Draffan D, Carrera I, Carmichael S, Heller J, Hammond G. Radiographic analysis of trochlear notch sclerosis in the diagnosis of osteoarthritis secondary to medial coronoid disease. Vet Comp Orthop Traumatol. 2009;22:7–15. [PubMed] [Google Scholar]
- 14.Dennis R. Interpretation and use of BVA/KC hip scores in dogs. In Practice. 2012;34:178–194. [Google Scholar]
- 15.Lust G, Williams AJ, Burton-Wurster N, et al. Joint laxity and its association with hip dysplasia in Labrador retrievers. Am J Vet Res. 1993;54:1990–1999. [PubMed] [Google Scholar]
- 16.Comblain F, Serisier S, Barthelemy N, Balligand M, Henrotin Y. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J Vet Pharmacol Ther. 2016;39:1–15. doi: 10.1111/jvp.12251. [DOI] [PubMed] [Google Scholar]
- 17.Kealy RD, Lawler DF, Ballam JM, et al. Evaluation of the effect of limited food consumption on radiographic evidence of osteoarthritis in dogs. J Am Vet Med Assoc. 2000;217:1678–1680. doi: 10.2460/javma.2000.217.1678. [DOI] [PubMed] [Google Scholar]
- 18.Odore R, De Marco M, Gasco L, et al. Cytotoxic effects of oxytetracycline residues in bones of broiler chickens following therapeutic oral administration of a water formulation. Poult Sci. 2015;94:1975–1985. doi: 10.3382/ps/pev141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmieri B, Di Cerbo A, Laurino C. Antibiotic treatments in zoo-technology and effects induced on the food chain of domestic species and, comparatively, the human species. Nutr Hosp. 2014;29:1427–1433. doi: 10.3305/nh.2014.29.6.7350. [DOI] [PubMed] [Google Scholar]
- 20.Lau SF, Wolschrijn CF, Hazewinkel HA, Siebelt M, Voorhout G. The early development of medial coronoid disease in growing Labrador retrievers: Radiographic, computed tomographic, necropsy and micro-computed tomographic findings. Vet J. 2013;197:724–730. doi: 10.1016/j.tvjl.2013.04.002. [DOI] [PubMed] [Google Scholar]