Abstract
Objectives
Generalized Anxiety Disorder (GAD) is one of the most prevalent mental disorders in the elderly, but its functional neuroanatomy is not well understood. Given the role of emotion dysregulation in GAD, we sought to describe the neural bases of emotion regulation in late-life GAD by analyzing the functional connectivity (FC) in the Salience Network and the Executive Control Network during worry induction and worry reappraisal.
Design, setting and participants
Twenty-eight elderly GAD and thirty-one non-anxious comparison participants were included. Twelve elderly GAD completed a 12-week pharmacotherapy trial. We used an in-scanner worry script that alternates blocks of worry induction and reappraisal. We assessed network FC, employing the following seeds: anterior insula (AI), dorso-lateral prefrontal cortex (dlPFC), the bed nucleus of stria terminalis (BNST), the paraventricular nucleus (PVN).
Results
GAD participants exhibited greater FC during worry induction between the left AI and the right orbito-frontal cortex (OFC), and between the BNST and the subgenual cingulate. During worry reappraisal, the non-anxious participants had greater FC between the left dlPFC and the medial PFC, as well as between the left AI and the medial PFC, while elderly GAD had greater FC between the PVN and the amygdala. Following twelve weeks of pharmacotherapy, GAD participants had greater connectivity between the dlPFC and several prefrontal regions during worry reappraisal.
Conclusion
FC during worry induction and reappraisal points toward abnormalities in both worry generation and worry reappraisal. Following successful pharmacologic treatment, we observed greater connectivity in the prefrontal nodes of the Executive Control Network during reappraisal of worry.
INTRODUCTION
Generalized Anxiety Disorder (GAD) is the most prevalent anxiety disorder in the elderly (1–3). Late-life GAD is associated with decreased quality of life (4, 5), cognitive impairment (6, 7), and increased health care utilization (4). The onset of GAD in the elderly may reflect both the exposure to age-specific stressors and age-specific brain structural changes (e.g., neuronal degeneration and cerebro-vascular disease)(8).
Late-life GAD is relatively understudied, and the underlying structural and functional neuroanatomy has received little attention (9, 10). This is a particularly unfortunate gap in our knowledge given the mediocre treatment response in late-life GAD(8), (11, 12). Compared with midlife GAD, late-life GAD has a poorer response to cognitive behavioral therapy (CBT) (13, 14). To date, there is no published study addressing the neural changes following pharmacotherapy in late-life GAD. The identification of such changes could provide a target for developing targeted treatments for late-life GAD.
Given the role of emotion dysregulation in GAD (15, 16), we were interested in the functional connectivity (FC) in two networks involved in emotion generation and emotion regulation (17): the Salience Network (SN) and the Executive Control Network (ECN). The SN, comprised of anterior insula, dorsal anterior cingulate (ACC), amygdala, ventral tegmental area and the ventromedial nucleus of the thalamus, is involved in monitoring the salience of interoceptive and external events (18, 19). Abnormal SN connectivity has been implicated in anxiety disorders as the neural basis for pathologically enhanced salience detection(20–22), but no studies have focused on its FC during emotion regulation in GAD. The ECN, comprised of dorsolateral prefrontal cortex (dlPFC), ventrolateral PFC, dorsomedial PFC, dorsal ACC, lateral parietal cortex, orbital fronto-insula, dorsal caudate and anterior thalamus (23), is critical for complex cognitive tasks such as working memory, cognitive control and decision-making in the context of goal-directed behavior (18). Recent studies showed that anxious subjects have abnormal dACC response suggesting poor conflict adaptation during an emotional Stoop test (24–26).
Prior research suggested two additional candidate regions that might play a key role in late-life GAD (27–29). The first is the bed nucleus of stria terminalis (BNST), which has been associated with sustained apprehension and considered a key brain region of interest for generalized anxiety (27, 30–32). The second is the paraventricular nucleus (PVN), which is the apex of the HPA axis and has been frequently implicated in stress regulation and autonomic response (29, 33). Recent research on the biology of late-life GAD has linked high cortisol with changes in cognitive domains such as immediate and delayed memory in elderly GAD (6, 34).
Our goal in the present study was to test for differences in FC during worry induction and worry reappraisal between (1) late-life GAD participants and non-anxious comparison participants, (2) late-life GAD participants before and after twelve weeks of antidepressant treatment. We hypothesized that pre-treatment, elderly GAD would have aberrant FC during worry induction and worry reappraisal. More specifically, based on the clinical literature regarding poor emotion regulation in GAD(15) and poor response to CBT in late-life GAD(35), we hypothesized that 1) compared with non-anxious older adults, elderly GAD would have greater connectivity during both worry induction and worry reappraisal in the anxiety-related networks (SN, BNST, PVN); 2) compared with non-anxious older adults, elderly GAD would have reduced FC in the ECN during reappraisal of worry. We further hypothesized that SSRI treatment would improve the FC in both SN and ECN.
METHODS
Participants
Elderly GAD and elderly non-anxious participants were recruited from an ongoing NIMH-funded trial (“Structural and functional neuroanatomy of late-life GAD”). Additionally, the Brain and Behavior Foundation funded a 12-week treatment trial for a sub-sample of the elderly GAD. Elderly GAD participants (age 60 and over) had a principal diagnosis of GAD for at least six months according to the Structured Clinical Interview for DSM-IV (SCID)(36) and a score of 17 or higher on the Hamilton Anxiety Rating Scale (HARS)(37) at the time of first scanning. Participants with other anxiety disorders were included if GAD was the principal diagnosis: 4/28 (14%) GAD participants were diagnosed with another anxiety disorder, including social phobia (n=1), panic disorder (n=2), and post-traumatic stress disorder (n=1). Exclusion criteria: Mini Mental Examination Scale (MMSE)(38) scores of 24 or lower, clinical diagnosis of dementia, Major Depressive Disorder at the time of scanning. Other exclusion criteria were lifetime psychosis or bipolar disorder, increased suicide risk (e.g. current ideation), ongoing psychotherapy, and current antidepressant or anxiolytic use(10).
Assessments and treatment
Thirty-one non-anxious elderly and twenty-eight elderly GAD have been included in this study. Twelve elderly GAD completed the 12-week open pharmacotherapy trial. All participants were psychotropic-free at the time of first scanning. Participants were also evaluated clinically with the Penn State Worry Questionnaire (PSWQ) (39), the Hamilton Depression Rating Scale (HDRS)(40) and the Cumulative Illness Rating Scale, Geriatric (CIRS-G)(41). Following the initial MRI scan, participants were treated with citalopram (titrated to 20 mg/d, as tolerated). All post-treatment participants had a HARS of 14 or lower, which is considered the cutoff point for treatment response (43).
Experimental design
The fMRI block design involved an initial five-minute resting state phase followed by five blocks of the worry task. During the resting state participants were asked to lie still in the scanner, eyes closed and not to think of anything in particular.
To examine the functional neurobiology of worry reactivity and regulation, we used a personalized worry script. The worry script consisted of three individualized worry generating statements alternating with instructions to reappraise worry. During participants’ initial evaluation, we elicited specific worry themes. These themes were used to create sentences that instructed the participant to worry “as hard as s/he can, as s/he usually does it” about that specific theme. In order to standardize the paradigm, participants rehearsed the worry script prior to the experiment and they offered feedback regarding the accuracy of each worry induction and each worry reappraisal sentence. During the experiment, each worry induction/reappraisal statement remained on the screen for one minute. During the worry reappraisal the participant read on the screen a sentence instructing him/her to reappraise the worry theme as discussed prior to the in-scanner experiment.
Data acquisition
We used the pseudo continuous arterial spin labeling (pCASL) sequence (44–47) on the Sieman 3T MR scanner at the University of Pittsburgh Medical Center. Twenty-two slices (slice thickness 4mm, gap = 2 mm) were acquired sequentially from inferior to superior for each volume using a gradient-echo EPI sequence. Interleaved images with and without labeling were acquired with the following parameters: matrix size 64X64, FOV=320×320mm, flip angle=90°, TR/TE = 4000/28 msec. We obtained 80 volumes of ASL images for each participant during rest and 296 volumes of ASL images for each participant during the worry script. A T1-weighted anatomical image was also acquired using a 3D-MPRAGE sequence (TR/TE = 500/11 ms, FOV = 240×240mm, flip angle=9°, slice thickness = 1 mm, matrix = 256×256) for registering functional images to standard MNI space.
Data analysis
The ASL images were processed using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Center for Neuroimaging, London, UK. http://www.fil.ion.ucl.ac.uk/spm/software/spm8) implemented in Matlab version R2012b (Mathworks, Natick, MA). For each subject, the images were realigned, smoothed, and reconstructed for the perfusion and BOLD images by a kinetic model implemented in the algorithm by Wang et al. (ASLtbx, May 2012, http://cfn.upenn.edu/perfusion/software.htm). The estimated whole brain BOLD images were submitted for the seed based connectivity analysis using Conn FC toolbox (http://www.nitrc.org/projects/conn, version 13p) (48).
The voxel-wise connectivity of the whole brain to each seed of interest was estimated by regression, with time series from white matter, CSF and motion included as nuisance covariates. Prior to the regression, the quality of images was evaluated using the Artifact Detection and Correction Tool (ART) version 2.1. (http://www.nitrc.org/projects/art/). The outlier images were detected and scrubbed from subsequent analyses. Individual whole-brain seed-to-voxel connectivity maps were collected and tested for the difference between elderly GAD and non-anxious groups by two-sample t-tests. Pre- and post-treatment difference in FC for the elderly GAD participants was tested by paired t-test. We corrected for multiple comparisons by using Monte Carlo simulations implemented in AlphaSim (version 2.0/2002; http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html)(49). A corrected p ≤ 0.05 was deemed significant.
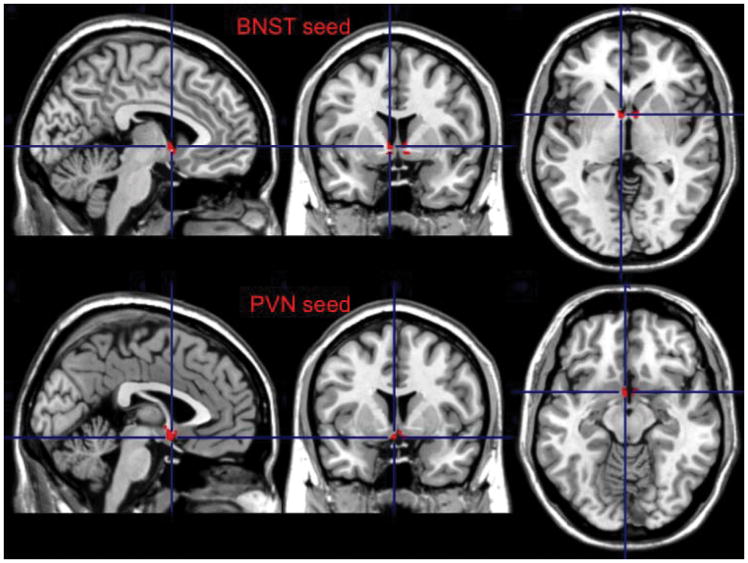
We used as seed the left anterior insula (AI) for the SN, the left dorso-lateral prefrontal cortex (dlPFC, BA 46) for the ECN, the BNST (bilateral), and the PVN. The BNST and the PVN were hand drawn using MRIcron (version 6/2013) on a built in MNI template (ch2better). These non-overlapping regions of interest (ROI) were based on the structures described in the Atlas of the Human Brain (50, 51). The BNST ROI was based on plates 18 (using Talairach reference systems, y=−2.7mm) through 24 (y=+2.7mm) and encompassed the central, medial, lateral, and ventral divisions(50) (see Figure 1). The PVN ROI was based on plates 20 (y=−1.3mm) through 28 (y=8.0mm) and included parvocellular, magnocelluar, dorsal, and posterior subnuclei (50) (see Figure 1). Additional details regarding these ROIs are provided in Table 1.
Figure 1.
The Bed Nucleus of Stria Terminalis (BNST) and Paraventricular Nucleus (PVN) seeds
Table 1.
Coordinates of the BNST and PVN Regions of Interest
| ROIs | Center of Mass | X-extent (mm) | Y-extent (mm) | Z-extent (mm) | Volume (mm3) |
|---|---|---|---|---|---|
| Left BNST | −6.6, 5.15, −3.19 | −19.5, −2 | 3, 8 | −10.5, 7.5 | 223.5 |
| Right BNST | 7.55, 5.1, −2.79 | 3, 23.5 | 3, 8 | −9, 8 | 234 |
| PVN | 0.72, 4.49, −9.36 | −5.5, 7.5 | 0, 8 | −15.5, 2 | 225.25 |
ROI= region of interest. BNST=Bed Nucleus of Stria Terminalis. PVN=Paraventricular Nucleus.
For the clinical and demographic data, the Mann-Whitney U test was used to test if there were any differences between the distribution of the following variables the non-anxious and elderly GAD groups: age, education, HDRS, PSWQ, HARS, and CIRS-G. The sex and race differences were assessed by the chi-square test.
RESULTS
Clinical and demographic data are presented in Table 2. A summary of the regions (including the peak MNI coordinates and clusters sizes) that showed significant FC effects is presented in Table 3. Unless specified, all the findings presented below survived multiple comparison correction.
Table 2.
Clinical and demographic characteristic of the sample
| Variable | Non-anxious elderly comparison participants N=31 |
Elderly GAD (pre-treatment) N=28 |
Test Statistic (p-value) |
|---|---|---|---|
| Age | 69 (12.50) | 64 (6.75) | U=546, p=0.089 |
| Sex | 17F (55%) | 19F (68%) | X2(df=1)=0.574, p=0.449 |
| Race | 27 W (87%) | 27W (96%) | p=0.356* |
| PSWQ | 32 (10) | 58.50 (21.25) | U=42.5, p<0.001 |
| RBANS | 108 (20) | 103.5 (16.5) | U=553.5, p=0.070 |
| HARS | 3 (2) | 19 (3.25) | U=0, p<0.001 |
| HDRS | 1 (2) n=21** |
13 (4) n=21** |
U=0, p<0.001 |
| CIRS.G | 4.5 (2.5) n=20** |
6 (4.25) n=20** |
U=148, p=0.160 |
All data are median and IQR range. U= Mann-Whitney U test; F= female. W= white; PSWQ=Penn State Worry Questionnaire; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status; HDRS=Hamilton Depression Rating Scale HARS= Hamilton Anxiety Rating Scale. CIRS.G=Cumulative Illness Rating Scale, Geriatrics.
Fisher’s exact test;
missing data
Table 3.
Summary of functional connectivity data
| Condition | Seed | Controls vs. GAD (x/y/z; kE) | Pre- vs. post-treatment GAD (x/y/z; kE) | ||
|---|---|---|---|---|---|
| Controls > GAD | GAD > Controls | Pre > Post | Post > Pre | ||
| Worry Induction | Left anterior insula | Right OFC: (10/56/−12; kE =388; t=3.83; df=54) | Left precentral gyrus [BA6]: (−54/2/42; kE=163; t=6.3, df=11) Left medial frontal gyrus [BA6]: (−2/34/42; kE=95; t=6.0; df=11) Left subgenual cingulate [BA25]: (−7/22/−14; kE=149; t=5.43; df=11) |
Right postcentral gyrus [BA5]: (18/−46/72; kE=76; t=5.1; df=11) | |
| Left BA46 (dlPFC) | Left insula: (−34/−8/12; kE=730; t=3.99; df=54) Right insular cortex [BA13]: (40/−7/13; kE=288; t=4.00; df=54) |
Left superior frontal gyrus [BA10]: (−22/63/−2; kE=174; t=3.71; df=54) Cerebellar vermis: (6/−52/−2; kE 261; t=4.14; df=54) Left fusiform gyrus: (−35/−66/−14; kE=263; t=3.44; df=54) |
Left inferior frontal gyrus [BA45]: (−54/20/12; kE=55; t=7.3; df=11) Right OFC [BA47]: (46/34/−12; kE=275; t=6.7; df=11) |
||
| BNST | Left thalamus: (−18/−22/10; kE=190; t=4.35; df=54) | Anterior cingulate (subgenual) [BA24]: (4/28/−6; kE=172; t=3.95; df=54) | Left insula [BA13]: (−38/−20/10; kE=146; t=7.8; df=11) Right supramarginal gyrus [BA2]: (48/−33/42; kE =456; t=7.1; df=11) |
Left frontal medial gyrus: (−11/37/38; kE=527; t=6.6;df=11) Left frontal superior gyrus: (−18/50/30; kE=527;t= 5.6; df=11) Left lingual gyrus: (−15/−76/−7; kE=60; t=5.68; df=11) Left inferior frontal gyrus [BA46]: (−44/40/16; kE=52, t=5.2; df=11; p=0.00014, threshold p<0.00012) |
|
| PVN | Middle frontal gyrus [BA6]: (−24/−12/52; kE=577; t=4.25; df=54) | Left OFC [BA11]: (−26/28/−18; kE=35; t=6.4; df=11) Right supramarginal gyrus [BA40]: (51/−32/42; kE=601; t=6.6; df=11) Left insula [BA13]: (−42/−20/10, kE=74; t=5.79; df=11) Right middle frontal gyrus [BA8]: (32/13/42; kE=246; t=5.4; df=11) |
Left medial frontal gyrus [BA9]: (−15/33/30; kE =224; t=5.3; df=11) Left lingual gyrus [BA18]: (−9/−76/−5, kE =187; t=5.3; df=11) |
||
| Worry Reappraisal | Left anterior insula | Left medial frontal gyrus [BA6]: (−8/−16/54, kE=469; t=4.61; df=54) dACC [BA24]: (−6/−12/43; kE=469; t=3.85; df=54) Right superior frontal gyrus: (18/44/34; t=4.44; kE=80) Middle frontal gyrus: (28/−14/48; kE=289; t=4.26; df=54) |
Left temporal pole [BA38]: (−48/16/−12; kE=187; t=4.49; df=54) Left hippocampus: (−30/−38/0; kE=285; t=4.28; df=54) Right superior frontal gyrus [BA6]: (18/20/60; kE=126; t=4.20; df=54) |
Left anterior cingulate [BA32]: (−7/35/28; kE=202; t=12.7; df=11) Left middle temporal gyrus: (−44/4/−37; kE=294; t=9.1; df=11) Right temporal pole [BA38]: (42/16/−38; kE=75; t=7.4; df=11) |
Cerebellar vermis: (−1/−44/−6; kE=138; t=8.5; df=11) |
| Left BA46 (dlPFC) | Right medial frontal gyrus [BA9]: (12/40/26; kE=173; t=4.41; df=54) Right superior frontal gyrus [BA10]: (9/59/−7; kE=146; t=3.46; df=54) |
Left fusiform gyrus: (−18/−34/−16; kE=91; t=4.59; df=54) | Left fusiform gyrus: (−40/−42/−16; kE=301; t=11.6; df=11) Left rectus [BA11]: (−1/21/−24; kE=155; t=9.1; df=11) Left limbic lobe (Uncus): (−32/−2/−34; kE=107; t=8.4; df=11) |
Right supramarginal gyrus [BA40]: (46/−36/60; kE=166; t=13.2; df=11) Right superior frontal gyrus [BA11]: (18/51/−18; kE=52; t= 7.2; df=11) Right inferior frontal gyrus [BA47]: (54/19/0.6; kE=101; t= 6.9; df=11) Middle cingulate gyrus: (7/−32/41; kE=102; t=6.6; df=11) Right cuneus: (12/−98/16; kE=66; t=7.7; df=11) |
|
| BNST | Left medial frontal gyrus: (−18/56/0; kE=52; t=4.08; df=54) | Left frontal middle gyrus [BA6]: (−25/7/63; kE=203; t=7.4; df=11) | Right lingual gyrus [BA19]: (24/−68/−2.70; kE=355; t=8.9; df=11) Right frontal superior gyrus [BA10]: (34/62/16; kE= 179, t=5.4; df=11; p=0.0003, threshold p<0.00017) |
||
| PVN | Right amygdala: (22/−4/−18; kE=217; t=4.88; df=54) | Right transverse temporal gyrus [BA41]: (55/−24/12, kE=97; t= 6.5; df=11) Left putamen: (−24/−4/4, kE=56; t=7.1; df=11) Middle cingulate gyrus [BA31]: (−6/−30/40, kE=125, p=0.0002, threshold p<0.00017; t= 4.5; df=11) |
Right superior frontal gyrus: (21/55/14, kE=115, p=0.0002, threshold p<0.00017; t=5.7; df=11) | ||
BA = Brodmann Area; BNST = bed nucleus of the stria terminalis; dACC = dorsal anterior cingulate cortex; GAD = generalized anxiety disorder; OFC = orbitofrontal cortex; PVN = periventricular nucleus of the hypothalamus. In italics, results that did not survive multiple comparison correction. All other results are significant are a corrected p ≤ 0.05. t=T-test (peak-level); df= degrees of freedom
A. Group differences in functional connectivity between non-anxious and anxious participants
A.1. Differences during worry induction (Figure 2)
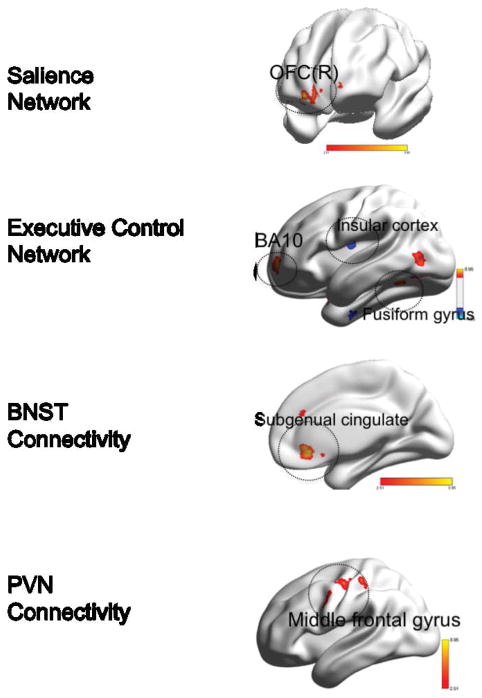
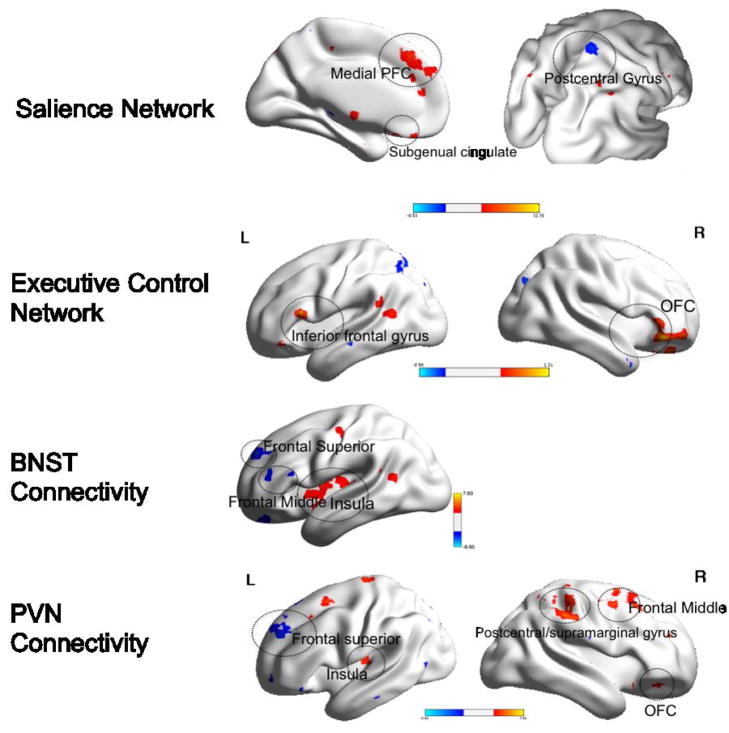
Figure 2. Group differences in functional connectivity during worry induction between elderly GAD participants and elderly non-anxious participants.
Red: GAD>non-anxious comparison, blue: non-anxious comparison>GAD. BNST= bed nucleus of stria terminalis. PVN= paraventricular nucleus. OFC(R)=orbito-frontal gyrus, right. BA= Brodmann Area. Visualized using BrainNet Viewer, version 1.42 (64).Salience Network: OFC(R) (t=3.83; df=54). Executive Control Network: BA10 (t=3.71; df=54); Insular cortex (t=4.00; df=54); Fusiform gyrus (t-3.44; df=54). BNST Connectivity: Subgenual cingulate (t=3.95; df=54). PVN Connectivity: Middle frontal gyrus (t=4.25; df=54).
Differences in the SN connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC between the left AI and the right orbito-frontal cortex (OFC). No areas where the GAD showed lower FC during worry induction survived multiple comparison correction.
Differences in the ECN connectivity
Compared with non-anxious participants, GAD participants exhibited the following differences: greater FC between the left dlPFC and cerebellar vermis, the left fusiform gyrus, and the left superior frontal gyrus (BA 10) and lower FC between the left dlPFC and the left and right insular cortex (BA13).
Differences in the BNST connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC between the BNST and subgenual cingulate (BA 24) and lower FC between the BNST seed and the left thalamus.
Differences in the PVN connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC during worry induction between the PVN and the middle frontal gyrus (BA 6). The results in the opposite direction (non-anxious>GAD) did not survive multiple comparisons correction.
In summary, during worry induction elderly anxious participants had greater connectivity between the left insula seed and the OFC, between the left dlPFC and the fusiform gyrus and the prefrontal cortex (BA10), between BNST seed and the subgenual cingulate, and between the PVN seed and the middle frontal gyrus (BA6).
A.2. Differences during worry reappraisal (Figure 3)
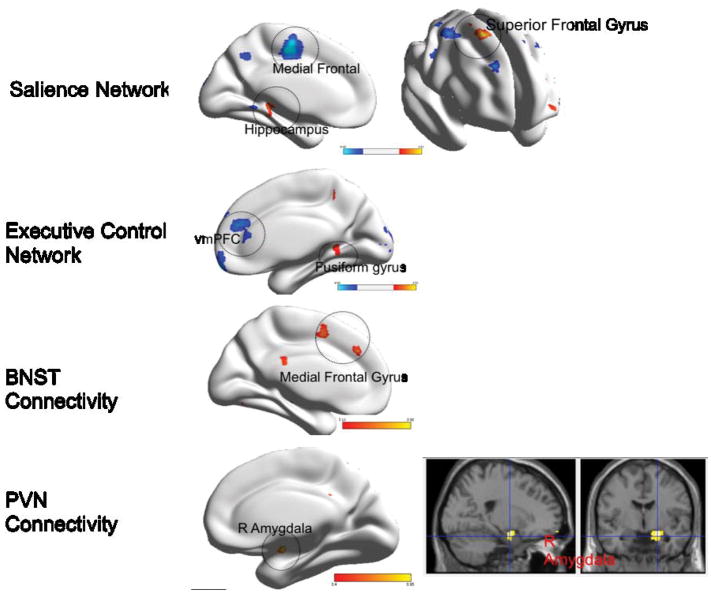
Figure 3. Group differences in functional connectivity during worry reappraisal between elderly GAD participants and non-anxious comparison participants.
Red: GAD>non-anxious comparison, blue: non-anxious comparison>GAD. BNST= bed nucleus of stria terminalis. PVN= paraventricular nucleus. vmPFC= ventromedial prefrontal cortex. BA= Brodmann Area. Visualized using BrainNet Viewer, version 1.42(64). Salience Network: Medial Frontal (t=4.26; df=54); Hippocampus (t=4.28; df=54); Superior frontal gyrus (t=4.44; df=54). Executive Control Network: vmPFC (t=4.41; df=54); Fusiform gyrus (t=4.59; df=54). BNST Connectivity: Medial frontal gyrus (t=4.08; df=54). PVN Connectivity: Amygdala(R) (t=4.88; df=54).
Differences in the SN connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC between the left AI and the left temporal pole (BA 38), left hippocampus, and right superior frontal gyrus (BA 6), and lower FC between the left AI and several frontal regions: left medial frontal (BA 6), dorsal ACC (BA 24), and middle frontal gyrus.
Differences in the ECN connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC between the left dlPFC and the left fusiform gyrus, and lower FC between the left dlPFC and the right PFC (BA 9, BA 10).
Differences in the BNST connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC between the BNST and left medial frontal gyrus.
Differences in the PVN connectivity
Compared with non-anxious participants, GAD participants exhibited greater FC during worry reappraisal between the PVN and the right amygdala. The results for the opposite direction (non-anxious > GAD) did not survive multiple comparisons correction.
In summary, during worry reappraisal, elderly anxious participants had greater connectivity between the left insula seed and the temporal cortex (temporal pole and hippocampus) and BA6, between the dlPFC seed and the fusiform gyrus, between the BNST and the medial frontal gyrus, and between the PVN and the right amygdala. Non-anxious participants had greater connectivity during worry reappraisal between the insula seed and multiple prefrontal regions and between the dlPFC seed and the right prefrontal cortex.
B. Within-subject differences in FC for elderly GAD participants before and after 12 weeks of pharmacotherapy
B.1. Differences during worry induction (Figure 4)
Figure 4. Within group differences in functional connectivity during worry induction between pre- and post-treatment elderly GAD participants.
Red: pre>post-treatment. Blue: post>pre-treatment. BNST= bed nucleus of stria terminalis. PVN= paraventricular nucleus. L=left, R=Right. OFC=orbito-frontal cortex. PFC=prefrontal cortex. Visualized using BrainNet Viewer, version 1.42(64). Salience Network: Medial PFC (t=6.0; df=11); Subgenual cingulate (t=5.43; df=11); Postcentral gyrus (t=5.1; df=11). Executive Control Network: Inferior frontal gyrus (t=7.3; df=11); OFC (t=6.7; df=11). BNST Connectivity: Frontal superior gyrus (t=5.6; df=11); Frontal middle (t=6.6; df=11); Insula (t=7.8; df=11). PVN Connectivity: Frontal superior (t=5.3; df=11); Insula (t=5.79, df=11); Postcentral/supramarginal gyrus (t=6.6; df=11); Frontal middle (t=5.4, df=11); OFC (t=6.4; df=11).
Differences in the SN connectivity
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited lower FC between the left AI and the following regions: left precentral gyrus (BA 6), left medial frontal gyrus (BA 6), and left subgenual cingulate (BA 25).
Differences in the ECN connectivity
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited lower FC between the left dlPFC and the left inferior frontal gyrus (BA 45) and right OFC (BA 47).
Differences in the connectivity of the BNST
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the BNST and the left frontal middle and superior gyri as well as the left lingual gyrus and lower FC between the BNST and the left insula and right supramarginal gyrus (BA 2).
Differences in the connectivity of the PVN
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the PVN and the left medial frontal gyrus (BA 9) and the left lingual gyrus (BA 18) and lower FC between the PVN and the left OFC (BA 11), the supramarginal gyrus (BA 40), left insula (BA 13), and middle frontal gyrus (BA 8).
In summary, during worry induction, elderly GAD had lower connectivity in the salience network and the executive control network after 12 weeks of treatment, while they had greater connectivity between the BNST and PVN seeds and several frontal regions.
B.2. Differences during worry reappraisal (Figure 5)
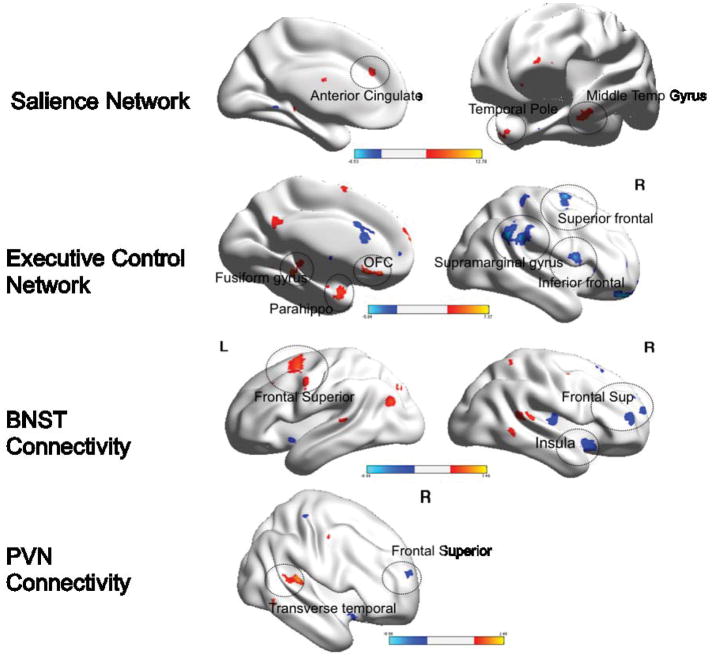
Figure 5. Within group differences in functional connectivity during worry reappraisal between pre- and post-treatment elderly GAD participants.
Red: pre>post-treatment. Blue: post>pre-treatment. BNST= bed nucleus of stria terminalis. PVN= paraventricular nucleus. L=left, R=Right. OFC=orbito-frontal cortex. Parahippo=parahippocampal cortex; PFC=prefrontal cortex. Visualized using BrainNet Viewer, version 1.42(64). Salience Network: Anterior cingulate (t=12.7; df=11); Temporal pole (t=7.4; df=11); Middle Temporal Gyrus (t=9.1; df=11). Executive Control Network: Fusiform gyrus (t=11.6; df=11); Parahippocampus (t=8.4; df=11); OFC (t=9.1; df=11); Superior frontal (t=7.2; df=11); Supramarginal gyrus (t=13.2, df=11); Inferior frontal (t=6.9; df=11). BNST Connectivity: Frontal superior (L) (t=7.4, df=11); Insula (t=8.9; df=11); Frontal superior (R) (t=5.4; df=11). PVN Connectivity: Transverse temporal (t=6.5; df=11); frontal superior (5.7; df=11).
Differences in the SN connectivity
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the left AI and the cerebellar vermis, as well as the supplemental motor area and the superior frontal cortex (the last did not survive multiple comparison correction) and lower FC between the left AI and the left anterior cingulate (BA 32), left middle temporal gyrus, and the left temporal pole (BA 38).
Differences in the ECN connectivity
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the left dlPFC and the right supramarginal gyrus (BA 40), right superior frontal gyrus (BA 11), right inferior frontal gyrus (BA 47), middle cingulate gyrus, and right cuneus and lower FC between the left dlPFC and the left fusiform gyrus, left rectus (BA 11), and left parahippocampal gyrus (uncus).
Differences in the connectivity of the BNST
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the BNST and the right lingual gyrus (BA 19) and right frontal superior gyrus (BA 10) (did not survive multiple comparison correction) and lower FC between the BNST and the left frontal superior gyrus (BA 6).
Differences in the connectivity of the PVN
Compared with themselves pre-treatment, the post-treatment GAD participants exhibited greater FC between the PVN and prefrontal cortex (right superior frontal gyrus) (this finding did not survive multiple comparison correction) and lower FC between the PVN and the right transverse temporal gyrus (BA 41), left putamen, and middle cingulum (BA 31) (did not survive multiple comparison correction).
In summary, during worry reappraisal, elderly GAD had greater connectivity between the dlPFC and several prefrontal areas after 12 weeks of treatment.
DISCUSSION
Compared with non-anxious participants, elderly GAD show multiple differences in the FC in networks involved in both emotion generation and emotion regulation. Following successful pharmacologic treatment of late-life GAD, we observed several significant changes in the same networks (see Table 4, Fig 6).
Table 4.
Proposed deficits in emotion regulation in late-life GAD
| Domain | Deficit | Functional connectivity findings |
|---|---|---|
| Emotion Generation (Worry Induction) | Excessive attribution of negative affective value to worry statements | Greater Insula-OFC connectivity during worry induction in pre-treatment GAD (compared with non-anxious participants and with post-treatment GAD) |
| Excessive attribution of threat | Greater BNST-Insula connectivity during worry induction in GAD | |
| Emotion Regulation (Worry reappraisal) | Failure to reappraise worry | Lower insula-prefrontal connectivity during worry reappraisal in GAD |
| Lower prefrontal connectivity (BA46-BA10/BA9) in GAD during worry reappraisal | ||
| Greater prefrontal connectivity in post-treatment GAD during worry reappraisal | ||
| Rigid executive network control | Similar nodes connected during both induction and reappraisal of worry in GAD | |
| Increased stress response during reappraisal | Greater BNST-subgenual cingulate connectivity in pre-treatment GAD during worry induction | |
| Greater PVN-Amygdala connectivity in pre-treatment GAD during worry reappraisal |
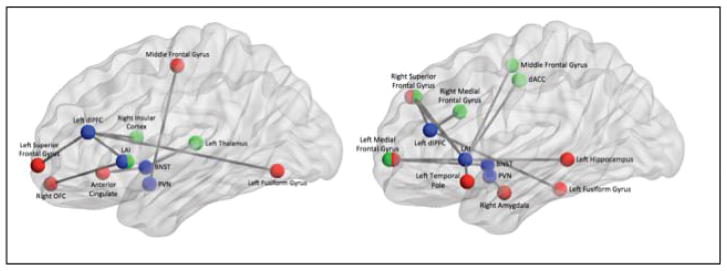
Fig 6. Summarized findings showing differences in functional connectivity between non-anxious participants and elderly GAD during worry induction (left) and during worry reappraisal (right).
In blue – the four seeds (LAI=left anterior insula, dlPFC=dorso-lateral prefrontal cortex, BNST=bed nucleus of stria terminalis, PVN=paraventricular nucleus. In red-regions of interest that had greater connectivity with the seed for GAD than for non-anxious participants. In green: regions of interest that had greater connectivity with the seed for non-anxious participants than for GAD.
The differences reported in the SN during worry induction indicate a stronger connectivity between the insula and the OFC for the GAD participants. As OFC is involved in anticipating the negative affective value of future events (52), our results may indicate that GAD participants attribute negative affective value to worry statement to a larger degree than the non-anxious participants. This observation about FC has a clinically correlate in the dysphoric nature of worry thoughts and it further supports the model of emotion dysregulation in GAD (16). This model suggests that GAD participants have deficits in emotion generation including a tendency for strong emotional responses mediated by motivational salience to perceive threats (16, 53).
During worry reappraisal, non-anxious participants increased, as expected, the connectivity between the anterior insula and various prefrontal regions (54). In contrast, the GAD participants show only limited insula connectivity with the prefrontal cortex during reappraisal. As the SN assigns salience to emotional or homeostatic stimuli and accesses the ECN for future weighting of behavioral choices (18), the differences noticed between non-anxious and GAD participants indicate aberrant SN connectivity during both worry generation and reappraisal – excessive attribution of negative affect to worry statements followed by failure to engage the prefrontal cortex during reappraisal (Table 4).
The ECN results reveal a different feature of GAD functional networks pathology. Thus, the greater connectivity between dlPFC and insula noticed during induction of worry in non-anxious participants probably reflects the fine-tuning between salience detection and cognitive demand. The same non-anxious participants switch to a robust ‘in-network’ connectivity during reappraisal, tapping into prefrontal regions frequently engaged in emotion reappraisal (55, 56). In contrast to the flexibility displayed by non-anxious participants when switching from induction to reappraisal of worry, GAD participants display a rather rigid connectivity of the dlPFC. Thus, during both induction and reappraisal, GAD participants maintain greater connectivity between the dlPFC and the fusiform gyrus and fail to increase the connectivity between the dlPFC and other prefrontal regions. This lack of flexibility in the ECN, especially with regard to the lack of ‘in-network’ prefrontal connectivity may be linked to the poor CBT response noticed in late-life GAD (13). These results are further supported by the pre-post treatment analysis. Post-treatment GAD participants have greater connectivity between dlPFC and several frontal regions as well as the supramarginal gyrus (57). These results suggest that pharmacotherapy may ameliorate a connectivity deficit during reappraisal in the ECN and consequently promote efficacy of reappraisal and other cognitive restructuring strategies. Although these results need further confirmation on larger samples, we may speculate that sequential treatment strategies (pharmacotherapy followed by CBT) would prove more efficacious in late-life GAD in order to consolidate response and prevent future relapses (58).
The BNST has been implicated in mediating environmental threat monitoring (59, 60). Our results regarding the greater connectivity between BNST and the subgenual cingulate (GAD>non-anxious), as well as between BNST and insular cortex (pre-treatment>post-treatment GAD) outline a possible hyperactive network involving limbic and paralimbic structures implicated in stress response (60) and excessive attribution of threat (53). This excessive salience/sustained apprehension/excessive stress response network received additional support when examining the PVN connectivity.
The most striking result from our analysis is the PVN FC differences noted during worry reappraisal. GAD participants have greater connectivity than non-anxious participants between PVN and the right amygdala during worry reappraisal, a result that suggests an increased autonomic response during reappraisal in GAD. Given PVN’s role in stress response (33), we may speculate that GAD participants perceive cognitive strategies of worry regulation as stress-inducing and anxiety-provoking. Our results suggest that normative cognitive reappraising strategies may be at odds with the engrained strategies used during worry (15) to the point of triggering somatic anxiety in participants who otherwise are notorious for low cardiovascular flexibility (15, 61, 62).
Our study has several strengths: it analyzes a relatively large sample of elderly GAD participants and it uses an age and cognition matched control group. An additional strength of the GAD sample is that participants were psychotropic free at baseline, as well as “purely” anxious (with no other comorbid psychiatric illnesses at the time of scan, including no Major Depressive Disorder). We used a tailored worry induction and reappraisal task, which allowed us to track the neural response correlated with both generation of worry and reappraisal. We have also explored the FC of seeds that are particularly relevant for the neural basis of GAD (BNSD, PVN, Anterior Insula). Some limitations are worth noting also. The pre/post treatment analysis had a reduced sample, limiting the power to observe differences between the two groups. We did not measure autonomic response following induction and reappraisal of worry and thus we cannot correlate the results that suggest increased stress response with autonomic changes. The worry script used to test emotion regulation lacks test-retest reliability data at this time. Although the GAD participants did not satisfy the criteria for major depression, they have significantly higher HDRS scores than the non-anxious participants. However, the mild range of depressive symptoms associated with high PSWQ and HARS scores are prototypical for the clinical presentation of GAD.
Other future directions would involve expanding the study to midlife and young subjects with generalized anxiety, including autonomic measures of stress response, and comparing the post-treatment effects for pharmacologic and psychotherapeutic interventions in generalized anxiety.
Acknowledgments
Supported by NIMH MH 086686, MH 071944, the Brain and Behavior Research Foundation (NARSAD), Young Investigator Award (Dr. Andreescu).
Footnotes
Financial disclosures: Carmen Andreescu, Lei K. Sheu, Dana Tudorascu, James J. Gross, Sarah Walker, and Layla Banihashemi do not have any potential conflict of interest to acknowledge. Howard Aizenstein has received research support from Novartis Pharmaceuticals.
Cited Literature
- 1.Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Flint AJ. Generalised anxiety disorder in elderly patients : epidemiology, diagnosis and treatment options. Drugs Aging. 2005;22:101–114. doi: 10.2165/00002512-200522020-00002. [DOI] [PubMed] [Google Scholar]
- 4.de Beurs E, Beekman AT, van Balkom AJ, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29:583–593. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- 5.Wetherell JLGM, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychol Aging. 2001;16:187–195. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Mantella RC, Butters MA, Dew MA, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 7.Caudle DD, Senior AC, Wetherell JL, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 8.Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009;24:1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohlman J, Price RB, Eldreth DA, et al. The relation of worry to prefrontal cortex volume in older adults with and without generalized anxiety disorder. Psychiatry Res. 2009;173:121–127. doi: 10.1016/j.pscychresns.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreescu C, Gross JJ, Lenze E, et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depress Anxiety. 2011;28:202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohlman J. Psychosocial treatment of late-life generalized anxiety disorder: current status and future directions. Clin Psychol Rev. 2004;24:149–169. doi: 10.1016/j.cpr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Thorp SR, Ayers CR, Nuevo R, et al. Meta-analysis comparing different behavioral treatments for late-life anxiety. Am J Geriatr Psychiatry. 2009;17:105–115. doi: 10.1097/JGP.0b013e31818b3f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covin R, Ouimet AJ, Seeds PM, et al. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord. 2008;22:108–116. doi: 10.1016/j.janxdis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Mennin DS, McLaughlin KA, Flanagan TJ. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J Anxiety Disord. 2009;23:866–871. doi: 10.1016/j.janxdis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mennin DS, Holaway RM, Fresco DM, et al. Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behav Ther. 2007;38:284–302. doi: 10.1016/j.beth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Gross JJ, Sheppes G, Urry HL. Cognition and Emotion Lecture at the 2010 SPSP Emotion Preconference. Cognition & emotion. 2011;25:765–781. doi: 10.1080/02699931.2011.555753. [DOI] [PubMed] [Google Scholar]
- 18.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 20.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Pannekoek JN, Veer IM, van Tol MJ, et al. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Pannekoek JN, Veer IM, van Tol MJ, et al. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter CS, Macdonald AM, Botvinick M, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Chechko N, Wehrle R, Erhardt A, et al. Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLoS One. 2009;4:e5537. doi: 10.1371/journal.pone.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis M, Shi, Changjun The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 28.Yassa MA, Hazlett RL, Stark CE, et al. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flandreau EI, Ressler KJ, Owens MJ, et al. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis M, Young, Lee Lim. Fear and Anxiety: Possible Roles of the Amygdala and Bed Nucleus of Stria Terminalis. Cognition and Emotion. 1998;12:277–305. [Google Scholar]
- 31.Davis M, Walker DL, Miles L, et al. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pego JM, Sousa JC, Almeida OF, et al. Stress and the neuroendocrinology of anxiety disorders. Current topics in behavioral neurosciences. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- 34.Lenze EJ, Mantella RC, Shi P, et al. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19:482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorp SR, Ayers CR, Nuevo R, et al. Meta-analysis Comparing Different Behavioral Treatments for Late-Life Anxiety. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e31818b3f7e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.First MSR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P), 2.0 ed. 1995. [Google Scholar]
- 37.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MFFS, McHugh PR. Mini-Mental State. a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 42.Duff K, Humphreys Clark JD, O’Bryant SE, et al. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23:603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenze EJ, Rollman BL, Shear MK, et al. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301:295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Licht DJ, Jahng GH, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 46.Koziak AM, Winter J, Lee TY, et al. Validation study of a pulsed arterial spin labeling technique by comparison to perfusion computed tomography. Magn Reson Imaging. 2008;26:543–553. doi: 10.1016/j.mri.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Ewing JR, Cao Y, Knight RA, et al. Arterial spin labeling: validity testing and comparison studies. J Magn Reson Imaging. 2005;22:737–740. doi: 10.1002/jmri.20451. [DOI] [PubMed] [Google Scholar]
- 48.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 49.Ward B. AFNI AlphaSim documentation. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 2000. Simulatenous inference for fMRI data. [Google Scholar]
- 50.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3. New York: Academic Press; 2008. [Google Scholar]
- 51.Banihashemi L, Sheu LK, Gianaros PJ. Childhood physical abuse predicts adulthood stressor-evoked activity in the limbic forebrain and hypothalamic regions. Philadelphia: 2012. [Google Scholar]
- 52.Ursu S, Carter CS. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: implications for neuroimaging studies of decision-making. Brain research Cognitive brain research. 2005;23:51–60. doi: 10.1016/j.cogbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin Psychol Rev. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ochsner K, Gross J. Cognitive Aspects of Emotion Regulation. Current Directions in Psychological Science. 2008;17:1–109. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Gross JJ, Thompson RA. Emotion Regulation: Conceptual Foundations. Guilford Press; 2007. [Google Scholar]
- 57.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wetherell JL, Petkus AJ, White KS, et al. Antidepressant Medication Augmented With Cognitive-Behavioral Therapy for Generalized Anxiety Disorder in Older Adults. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez RP, Chen G, Bodurka J, et al. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borkovec TD, Shadick RN, Hopkins M. The nature of normal and pathological worry. In: Rappe RM, Barlow DH, editors. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. 1991. pp. 29–51. [Google Scholar]
- 62.Llera SJ, Newman MG. Effects of worry on physiological and subjective reactivity to emotional stimuli in generalized anxiety disorder and nonanxious control participants. Emotion. 2010;10:640–650. doi: 10.1037/a0019351. [DOI] [PubMed] [Google Scholar]
- 63.Beekman AT, de Beurs E, van Balkom AJ, et al. Anxiety and depression in later life: Co-occurrence and communality of risk factors. Am J Psychiatry. 2000;157:89–95. doi: 10.1176/ajp.157.1.89. [DOI] [PubMed] [Google Scholar]
- 64.Xia M. BrainNet Viewer Manual. 2013 doi: 10.1371/journal.pone.0068910. http://www.nitrc.org/docman/view.php/504/1280/BrainNet. [DOI] [PMC free article] [PubMed]