Abstract
EUS-FNA is often performed in the evaluation of Cystic Pancreatic Lesions (CPL) for a better preoperative characterization. The objective is to identify premalignant lesions as Mucinous Cystic Neoplasms, and/or a malignant transformation of them (adenocarcinoma). The role of cytological evaluation in this setting is discouraging and intracystic markers analysis, mainly CEA, lacks of a good specificity for the detection of mucinous neoplasms. New devices and approaches have emerged to overcome these problems as the cytology brush (Echobrush), the small mini-biopsy foceps, the cystoscopy and the needle Confocal LASER Endomicroscopy (nCLE), showing in some studies good rates of accuracy for distinguishing among mucinosus and non-mucinous neoplasms. However, intracystic molecular marker analysis, by identifying mutations in DNA of particular genes as KRAS,GNAS,VHL, CDKN2A and others constitute the most relevant advancement of last years and will contribute in the next future to a better management of CPL. The role of EUS-FNA according to international guidelines is still controversial. While 2012 Fukuoka guidelines are restrictive in their indications AGA 2015 guidelines support it when high risk features are present, enhancing the role of the cytological evaluation in taking decisions.
Keywords: Aspiration, biopsy, cystic pancreatic tumors, EUS
INTRODUCTION
Considering the prevalence of cystic pancreatic lesions (CPLs) in asymptomatic people, these lesions emerge as an important public health problem of developed countries. Due to the widespread use of improved imaging techniques in an aging population more and more incidentally CPL are detected, as is reflected in recent studies, posing a serious problem to the physicians who are attending these patients.[1]
CPLs may be classified simply into two main classes such as nonneoplastic and neoplastic cysts [Figure 1]. Neoplastic cysts are more commonly defined as pancreatic cystic neoplasms (PCNs). It is important to distinguish nonneoplastic cysts from neoplastic or nonmucinous from mucinous cysts because the latter are considered being premalignant lesions.[2]
Figure 1.
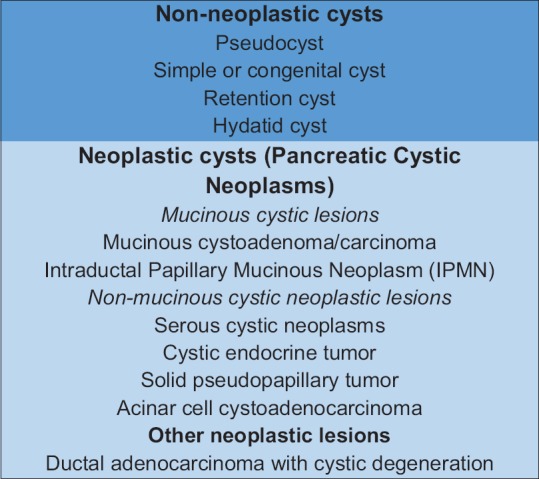
Classification of cystic pancreatic lesions
EUS and magnetic resonance imaging with magnetic resonance cholangiopancreatography are considered the most accurate techniques for detecting mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN)[3] [Figure 2a and b]. EUS is particularly valuable in assessing diagnostic features and potential predictors of malignancy, including size, shape, number of cysts, septa, nodules, solid masses associated with the cyst, pancreatic duct diameter, communication with pancreatic duct, and lymph nodes. Certain CPL have some characteristic appearance: Serous cystadenoma (SCA) is often depicted as a microcystic and honeycomb pattern although recently some authors have proposed new endosonographic criteria,[4] a CPL without septations and solid component in a parenchyma with features of chronic pancreatitis suggests a pseudocyst with high certainty.[5] MCN can be usually diagnosed when there is a cyst with septation of variable thickness, a visible wall and a peripheral calcification. Some predictors of malignancy can also be assessed by EUS, as the presence of mural nodules and its differentiation from mucus. Mucin globules are hypoechoic, have smooth edges and hyperechoic rims, and move when patients are repositioned while mural nodule is isoechoic or hyperechoic and has irregular margins.[6,7] The use of contrast enhancement is of great value in these circumstances, as the mural nodule must enhance after the administration of the contrast,[8] while mucin, as an avascular structure, must not.
Figure 2.
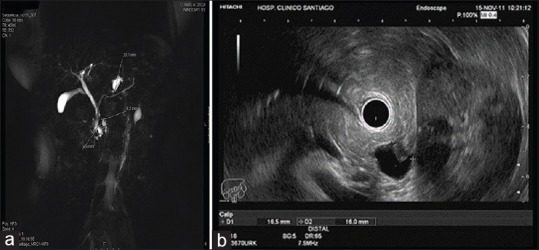
(a) Small cystic lesion of 16 mm × 16 mm placed in the tail of the pancreas with a small solid component, concordant with a branch-duct intraductal papillary mucinous neoplasm. (b) Magnetic resonance cholangiopancreatography of a multifocal branch-duct intraductal papillary mucinous neoplasm
However, despite the utility of EUS imaging in diagnostic evaluation and estimating malignant potential of CPL, EUS alone is not adequate for the diagnosis of pancreatic cysts.[9] When surgical histology is used as a reference standard, the diagnostic accuracy of EUS imaging ranges from 40% to 96%. A single prospective study demonstrated that the sensitivity (56%) and specificity (45%) of EUS morphology alone for differentiating mucinous cysts from nonmucinous cysts were low, resulting in poor overall accuracy (51%).[10] Furthermore, EUS performance is highly operator dependent and the interobserver agreement of morphological features of EUS when evaluating a CPL leaves much to be desired, even when the endoscopist performing the EUS is an expert.[11] In this scenario, when EUS-FNA is often performed.
In this review, we will try to explain first which is the starting point of EUS-FNA, how can we optimize its diagnostic yield and finally which place EUS-FNA occupy according to the international consensus guidelines.
WHEN AND HOW TO PERFORM AN EUS-FNA AND WHAT INFORMATION IS USUALLY OBTAINED?
There is not an specific minimum limit on size for performing an EUS-FNA. As explained below, there are different recommendations according to guidelines about when performing FNA regarding size, but at this point, no solid statement based on a high level of evidence can be established.
EUS-FNA of CPL is a well-established procedure that is commonly performed using different size standard needles. Depending of the size, the location and the presumed difficulty of perforating the lesion is possible to choose among a 19G needle (mainly for lesions placed in the body or tail of the pancreas) and 19G flexible or 22G for lesions placed in the head or uncinate process. 25G-EUS needles should only be used in those cases with a clear solid component as the viscosity of the fluid when aspirated may preclude to obtain material from the cyst with needles of small calibers (a thicker needle means better aspiration).
One major concern when doing an EUS-FNA in this setting is the development of complications. Infection is the most well documented and that is the reason why is recommended (without any kind of evidence) the administration of antibiotics periprocedure. Bleeding must be also keep in mind and so an EUS-FNA should neither be performed without normal coagulation parameters nor in a patient taken oral anticoagulants or ADP antagonists (clopidogrel, prasugrel, and ticagrelor). Even aspirin intake or nonsteroidal anti-inflammatory drugs in this scenario are considered as a contraindication by some guidelines.[12]
By performing an EUS-FNA in a CPL is possible to do a macroscopic subjective evaluation of the fluid aspirated from the cyst. Liquid from MCNs tend to be clear and high viscosity while a thin, muddy-brown fluid is usually obtained from a pseudocyst. Finally, an hematic sample is commonly observed in rich vascularized tumors as SCA. However, only by the macroscopic evaluation of the fluid is not possible obtain in the majority of the cases a final reliable diagnosis. An interesting approach to evaluate the viscosity of the sample is the “string sign.” This is a very single and cheap test that consists on placing a drop of the fluid aspirated between two fingers and spreading them apart. The test is considered to be positive when the string that is formed has a length of more than one cm or lasts more than 1 s before disruption. This string sign positive have demonstrated to be very specific for the diagnostic of mucinous lesions, but with a poor sensitivity of 58%.[13]
Although performed since many years, the role of the cytological evaluation of CPL by EUS-FNA is still controversial. When doing a cytological evaluation of a CPL, is possible to obtain relevant information about the type of lesion being evaluated: inflammatory cells, as histiocytes, macrophages or neutrophils, are commonly seen in the context of a pseudocyst; cuboidal cells which stain for glycogen, are typical of the SCA, while epithelial mucinous cells or extracellular mucin, are considered diagnostic of a mucinous lesion. Although this approach identifies tumors with close to 100% of specificity, it has a low level of sensitivity, as is reflected in recent metanalysis which establish a rate of 54%[14] Other authors reported a diagnostic accuracy of only the 31% for the cytological evaluation in a prospective cohort of 128 CPL[15] The low sensitivity results from factors such as the low yield of lesion cells from the aspirate, insufficient simple volume, and contamination of samples with gastrointestinal wall cells.[16]
An important point that must be highlighted about the role of cytology in EUS-FNA of CPL is the fact that the majority of studies report low rates of sensitivity mainly based on just aspirating the fluid of the cyst (of poor cellularity). Other studies have, besides sending the aspirated cyst fluid for cytology, also performed FNA of cyst wall or its solid component (mural nodule or mass lesion), leading to a significant increase in diagnostic yield. Lim et al.[17] showed that in the absence of solid component, the cytology yield was 28.8% while if the mural nodule was sampled with FNA, the yield was 78%. Similarly, Shirley et al.[18] showed diagnostic cytology findings in 89.6% of patients with suspicious imaging findings (mural nodules/masses, etc.) while no diagnostic cytology finding in any case without such imaging findings (i.e., when only cyst fluid was aspirated for cytology). For all this reasons, at present, the real role of the cytological evaluation by EUS-FNA in CPL remains, at least, controversial, with some guidelines supporting its role[19] as others being very strict in their indications.[20]
Another possibility available when doing an EUS-FNA is performing an intracystic marker analysis of the fluid aspirated. Excluding the role of the intracystic molecular markers (explained below), this topic is focused on different common markers (used in other typical cancers) that help us to distinguish among different kinds of CPL. Among them, Ca 19.9, Ca 125, CEA, amylase, Ca 72.4, and Ca 15.3 has been used in several trials, but by far the most common evaluated is the CEA. Since many years, the cut-off point of CEA intracystic level commonly considered to differentiate between mucinous lesions from nonmucinous lesions was 192 ng/mL, according to the cooperative study performed by Brugge et al.[10] However, over the years, this level has been declining, and some recent trials provide new cut-off levels of 105 ng/mL, 50 ng/mL, and even 7 ng/mL for distinguishing mucinous of nonmucinous lesions.[21,22,23] Therefore, it is possible to conclude that CEA alone is a suboptimal marker for this task.
HOW CAN WE OPTIMIZE THE ROLE OF EUS-FNA, WHAT IS EXPECTED FOR THE NEAR FUTURE?
Considering the low diagnostic yield of cytologic evaluation and the lack of specificity of intracystic common markers for achieving a final preoperative diagnosis, of at least for doing a differentiation among mucinous or nonmucinous lesions, some devices and technologies have emerged trying to overcome this problems.
A device through the EUS needle cytology brush, the EchoBrush (ECHO-19-CB; Cook Medical, Bloomington, Ind) has been introduced to the market a few years ago. It allows direct sampling of cystic pancreatic epithelium under EUS guidance [Figure 3]. Previous trials reported increases of diagnostic sensitivity using this brush in the differential diagnosis of CPL.[24,25,26] Some of this studies only reflects the experience of using this technique, others are retrospective and all of them are nonrandomized, hampering a real comparison with conventional EUS-FNA. Even more, this brush can only be introduced inside a 19G-EUS needle, hindering the puncture of lesions placed in the head or uncinate process; in fact, the rigidity of the tip of the brush makes difficult the exit of the needle in certain positions. Our group have demonstrated in a randomized and multicenter trial that the use of this device did not improve neither the diagnostic adequacy simple nor the diagnostic yield in this setting.[27]
Figure 3.
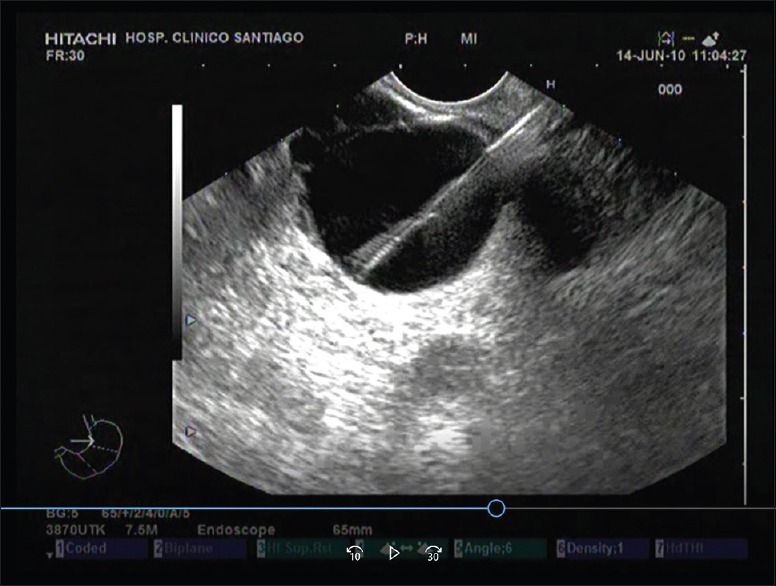
Cytologic brush (Echobrush®) used added to EUS-FNA. Look at the hyperechogenic distal tip of the brush targeting the inner wall of the cyst
Another interesting possibility is the use of a small biopsy forceps inside the 19G working channel of EUS needle for obtaining material for a histological evaluation. This method has been recently evaluated in solid pancreatic lesions with success,[28] but the experience in the setting of CPL is scarce and limited to small series and case reports.[29,30,31] A well-designed study with more amount of patients is needed to check the reproducibility, safety, and usefulness of this approach with these new models of mini-biopsy forceps [Figure 4].
Figure 4.
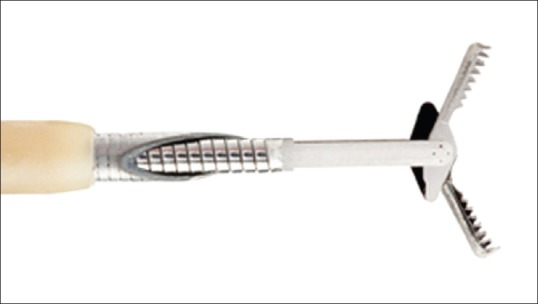
Mini biopsy forceps used inside the EUS-needle working channel (US endoscopy®)
Nevertheless, the most developed new techniques associated with EUS-FNA in CPL in the last years are the cystoscopy and the needle confocal laser endomicroscopy (nCLE). The cystoscopy provides the opportunity of exploring, with a fiberoptic probe introduced through the 19G-EUS needle, the inner part of the cyst, looking for the presence of mucin, commonly seen in mucinous lesions, and also the inner epithelial surface of the cyst trying to identify villous structures that may correspond to a mural nodule, also typical from mucinous neoplasms. The results of the DETECT study,[32] which evaluated the role of cystoscopy and nCLE in a cohort of 30 patients (18 of them with proven histological diagnosis) show good rates of sensitivity and negative predictive value for the diagnosis of mucinous lesions, that even reach 88% and 87%, respectively, when combine with nCLE. Introducing a specific probe of Endomicroscopy through a 19G- or 22G-EUS needle (nCLE) enables to perform a microscopic in vivo observation of the internal epithelial surface of the cyst. Three main trials have evaluated the role of nCLE in the diagnosis of CPL. The DETECT, above mentioned, and the INSPECT,[33] focused mainly in detecting mucinous neoplasms and the French CONTACT[34] study. The majority of the papers about nCLE highlight the good specificity of some “endomicroscopic features” (as the observation of papillary projections or a dark ring) for identifying IPMNs while demonstrating a poor sensitivity. In the study of Napoleon et al. (CONTACT),[34] the sequences of nCLE enables the identification of a particular pattern for the SCA. The pattern was present independently of the morphological presentation of the SCA (i.e., uni- or multilocular) and was described as a densely woven network of tortuous blood vessels with intense and dynamic circulation of blood cells visualized. This characteristic vascular organizational structure of SCA was consensually identified as the superficial vascular network [Figure 5]. Compared with the final diagnosis, the overall accuracy, sensitivity, specificity, PPV, and NPV of the superficial vascular network criterion for the SCA were 87%, 69%, 100%, 100%, and 82%, respectively. Nevertheless, it is important to mention that nCLE is an expensive and time-consuming technique which requires high degree of experience, so its usage, currently, is still limited to select tertiary referral centers.
Figure 5.
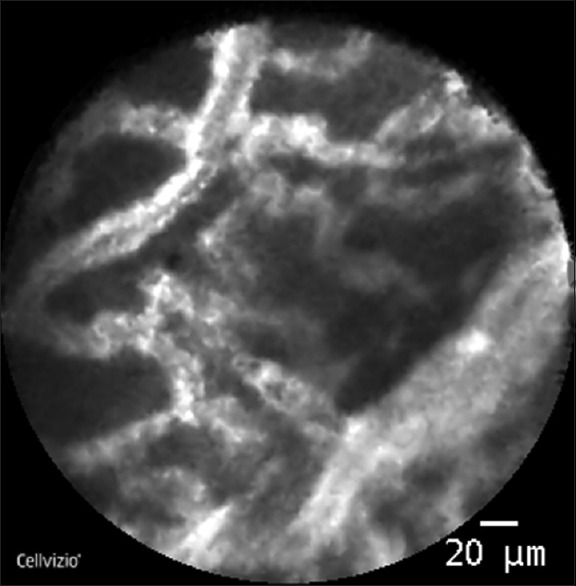
Needle confocal LASER endomicroscopy of a cystic pancreatic lesion: Serous cystadenoma (courtesy of Dr. Dabizzi (Pancreato-Biliary Endoscopy and Endosonography Division, San Raffaele Scientific Institute IRCCS, Vita Salute San Raffaele University, Milan, Italy)
But what undoubtedly means a complete breakthrough in the diagnosis and characterization of the different CPL is the intracystic molecular marker analysis. This means identifying concrete DNA mutations, in particular genes associated with an specific type of lesion. K-RAS and GNAS genes are the most evaluated but others as VHL, TP53, SMAD4, and CDKN2A have also been studied. Springer et al.[35] evaluated retrospectively 130 patients with resected PCNs. The molecular marker panel correctly identified 67 of the 74 patients who did not require surgery and could, therefore, reduce the number of unnecessary operations by 91%. In the same way, Al-Haddad et al.,[36] used the combination of clinical features of EUS with CEA level and cytological evaluation with molecular marker analysis of KRAS and GNAS in an integrated molecular pathology (IMP) analysis. In a retrospective cohort of 492 patients, the IMP analysis was compared to Fukuoka 2012 guidelines[20] for the detection of pre/malignant lesions. IMP more accurately determined the malignant potential of pancreatic cysts than Fukuoka 2012 guideline management criteria model. This results are also supported by recent trial[37] in which a novel algorithmic pathway using molecular testing of pancreatic cyst fluid detected advanced neoplasia better than AGA guidelines[19] criteria. As conclusion, the ability of EUS-FNA to provide samples for doing a molecular marker analysis makes it a very useful tool for identifying preoperatively those CPL with a malignant potential that would benefit for surgical resection. The main drawback of this approach is that this technology is expensive and not widely available to all centers, but in the opinion of the author, molecular marker analysis will become soon as first-line diagnostic test for the characterization of CPL.
WHICH IS THE ROLE OF EUS-FNA ACCORDING TO THE GUIDELINES?
To help physicians to take decisions when they are facing with a CPL, many guidelines have been developed in last years. However, the specific role of EUS-FNA is sometimes unclear, with some guidelines being critical with it while others supporting and enhancing its use.
The international guidelines for the management of IPMNs and mucinous cystadenoma,[20] that are the result of a consensus conference that took place in the city of Fukuoka in 2012, established very strict criteria for the use of EUS-FNA. In fact, they consider the fluid analysis as “still investigational” and that must be performed in centers with expertise in EUS-FNA and cytological interpretation. Fukuoka guidelines consider two scenarios in which EUS-FNA may be useful: (1) Small branch-duct IPMN without worrisome features and (2) for the distinction of a small oligocystic SCA from a small branch duct IPMN; sometimes this differentiation is challenging and may require EUS-FNA with CEA determination. It is important to highlight that Japanese investigators do not recommend cystic fluid analysis routinely and believe that a cyst of any size with “worrisome features” should not be aspirated, because it may cause leakage of the fluid content, possibly leading to peritoneal dissemination.[38] However, the results of the PIPE study,[39] in which 175 resected IPMNs with a preoperative EUS-FNA were analyzed and compared with 68 patients with no preoperative tissue sampling, demonstrated that there are no difference of peritoneal seeding by preoperative sampling or not a CPL.
When to perform an EUS-FNA is something that is much more clear according to the American Gastroenterological Association (AGA) Guidelines.[19] These guidelines only consider when evaluating a CPL 3 high-risk features: dilation of main pancreatic duct (MPD), size more than 3 cm, and the presence of a solid component associated with the cyst. When 2 out of these 3 features are present is when an EUS-FNA must be performed. If as a result of the cytologic evaluation of the cyst fluid, a “concerning cytology” (suspicious or positive for malignancy) is found, we must send the patient to surgery. AGA guidelines have been widely criticized in many aspects and one of them is the enhanced role they give to cytological evaluation, taking into account the low diagnostic performance it has in this scenario.
Finally, in 2016, the American Society of Gastrointestinal Endoscopy (ASGE) published a specific document about the role of endoscopy in cystic pancreatic neoplasms.[40] The main recommendations about when to puncture a cyst is summarized in: (1) EUS-FNA is recommended in cystic lesions >3 cm or in the presence of mural nodules, MPD dilation or associated mass (moderate level of evidence); (2) EUS-FNA is optional in asymptomatic patients with cysts <3 cm and without mural nodule/associated mass nor MPD dilation (low level of evidence); (3) it is recommended an initial evaluation of intracystic fluid with CEA, amylase, and cytology (moderate level of evidence); and (4) it may be considered a molecular marker analysis when initial evaluation with CEA and cytology is not conclusive (low level of evidence).
According to the abovementioned guidelines, important conceptual differences can be detected: Fukuoka guidelines[20] aim to directly subject the high-risk lesions to surgery (to avoid neoplastic dissemination while performing EUS-FNA, although the results of the PIPE study[39] does not support this idea) and to perform EUS-FNA in apparently low-risk lesions (to confirm they are really benign and hence can be followed-up). On the other hand, American guidelines (both AGA and ASGE),[19,40] recommend EUS-FNA in apparently high-risk lesions, so that if they have high-risk cytology, they can be subjected to surgery, while apparently low-risk lesions can just be followed up (without performing EUS-FNA). Which of these two approaches about the use of EUS-FNA is better is something that must be demonstrated in well-designed validation studies in the future.
Considering the opinions of the different guidelines on when to perform an EUS-FNA, making a decision on this topic may be difficult in clinical practice. It is author's opinion that perhaps no cystic lesion below 2 cm deserve an EUS-FNA, unless it has a clear solid component inside it or a surgical procedure is considered. We do also believe that a CPL >2 cm or with high-risk features may be taken into account for the EUS-FNA even when surgical resection seems to be clear. When evaluating the correlation among the presumptive preoperative diagnosis with the histology of the surgical specimens is possible to detect a not insignificant rate of misdiagnosis.[41,42,43] It should be underscored that, even following the guidelines, we are sending to surgery some patients that did not really need it leading to an important morbidity and clinical consequences because of the pancreatic surgery.[44]
Finally, it is important to point out, that the clinical conditions of the patient should seriously be considered (age and comorbidities), individualizing case by case when making concrete decisions. This point is not only exclusive of the EUS-FNA but also when referring a patient to surgery or establish a particular surveillance, taking into account that many patients will die of other concomitant pathologies and not by their cystic tumor.[45]
CONCLUSIONS
Despite the wide use of EUS-FNA in CPL over the years, at present, its role remains controversial. Cytological evaluation and intracystic marker analysis (CEA) have been criticized and supported to the same extent in the literature. New devices as cystoscopy and mainly nCLE seem promising, specifically for some kinds of CPL as SCA. Intracystic molecular marker analysis, by detecting concrete gene mutations, will probably allow, in the near future, not only just the identification of the CPL being faced but also its risk of developing malignancy, becoming a crucial tool to take decisions with our patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10:e0121317. doi: 10.1371/journal.pone.0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375–88. doi: 10.3978/j.issn.2078-6891.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jong K, van Hooft JE, Nio CY, et al. Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions. Scand J Gastroenterol. 2012;47:1056–63. doi: 10.3109/00365521.2012.674970. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Linghu E, Chai N, et al. New criteria to differentiate between mucinous cystic neoplasm and serous cystic neoplasm in pancreas by endoscopic ultrasound: A preliminarily confirmed outcome of 41 patients. Endosc Ultrasound. 2017;6:116–22. doi: 10.4103/eus.eus_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song MH, Lee SK, Kim MH, et al. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891–6. doi: 10.1016/s0016-5107(03)70026-1. [DOI] [PubMed] [Google Scholar]
- 6.Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: Management and unanswered questions. Gastroenterology. 2013;144:1303–15. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 7.Zhong N, Zhang L, Takahashi N, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192–8. doi: 10.1016/j.cgh.2011.09.029. 198e1-2. [DOI] [PubMed] [Google Scholar]
- 8.Serrani M, Lisotti A, Caletti G, et al. Role of contrast harmonic-endoscopic ultrasound in pancreatic cystic lesions. Endosc Ultrasound. 2017;6:25–30. doi: 10.4103/2303-9027.190931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World J Gastrointest Endosc. 2015;7:213–23. doi: 10.4253/wjge.v7.i3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.de Jong K, Verlaan T, Dijkgraaf MG, et al. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy. 2011;43:579–84. doi: 10.1055/s-0030-1256434. [DOI] [PubMed] [Google Scholar]
- 12.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 13.Bick BL, Enders FT, Levy MJ, et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy. 2015;47:626–31. doi: 10.1055/s-0034-1391484. [DOI] [PubMed] [Google Scholar]
- 14.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 15.de Jong K, Poley JW, van Hooft JE, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy. 2011;43:585–90. doi: 10.1055/s-0030-1256440. [DOI] [PubMed] [Google Scholar]
- 16.Genevay M, Mino-Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977–83. doi: 10.1097/SLA.0b013e3182383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim LG, Lakhtakia S, Ang TL, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicentre asian study. Dig Dis Sci. 2013;58:1751–7. doi: 10.1007/s10620-012-2528-2. [DOI] [PubMed] [Google Scholar]
- 18.Shirley LA, Walker J, Krishna S, et al. Routine cyst fluid cytology is not indicated in the evaluation of pancreatic cystic lesions. J Gastrointest Surg. 2016;20:1581–5. doi: 10.1007/s11605-016-3175-2. [DOI] [PubMed] [Google Scholar]
- 19.Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–4. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Gaddam S, Ge PS, Keach JW, et al. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: Results of a large multicenter study. Gastrointest Endosc. 2015;82:1060–9. doi: 10.1016/j.gie.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Oh HC, Kang H, Brugge WR. Cyst fluid amylase and CEA levels in the differential diagnosis of pancreatic cysts: A single-center experience with histologically proven cysts. Dig Dis Sci. 2014;59:3111–6. doi: 10.1007/s10620-014-3254-8. [DOI] [PubMed] [Google Scholar]
- 23.Oppong KW, Dawwas MF, Charnley RM, et al. EUS and EUS-FNA diagnosis of suspected pancreatic cystic neoplasms: Is the sum of the parts greater than the CEA? Pancreatology. 2015;15:531–7. doi: 10.1016/j.pan.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Sendino O, Fernández-Esparrach G, Solé M, et al. Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis. 2010;42:877–81. doi: 10.1016/j.dld.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Al-Haddad M, Raimondo M, Woodward T, et al. Safety and efficacy of cytology brushings versus standard FNA in evaluating cystic lesions of the pancreas: A pilot study. Gastrointest Endosc. 2007;65:894–8. doi: 10.1016/j.gie.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 26.Lozano MD, Subtil JC, Miravalles TL, et al. EchoBrush may be superior to standard EUS-guided FNA in the evaluation of cystic lesions of the pancreas: Preliminary experience. Cancer Cytopathol. 2011;119:209–14. doi: 10.1002/cncy.20133. [DOI] [PubMed] [Google Scholar]
- 27.Lariño-Noia J, Iglesias-García J, Macías M, et al. A multicenter, prospective, comparative, randomized open-trial of endoscopic ultrasound cytologic brushing vs fine needle aspiration (FNA) for the pathological diagnosis of cystic pancreatic lesions 44st European Pancreatic Club (EPC) Meeting AB S16-1. Pancreatology. 2012:S16–1. AB. [Google Scholar]
- 28.Nakai Y, Isayama H, Chang KJ, et al. Apilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016;84:158–62. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 29.Shakhatreh MH, Naini SR, Brijbassie AA, et al. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc Int Open. 2016;4:E439–42. doi: 10.1055/s-0042-101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coman RM, Schlachterman A, Esnakula AK, et al. EUS-guided, through-the-needle forceps: Clenching down the diagnosis. Gastrointest Endosc. 2016;84:372–3. doi: 10.1016/j.gie.2016.03.785. [DOI] [PubMed] [Google Scholar]
- 31.Aparicio JR, Martínez J, Niveiro M, et al. Direct intracystic biopsy and pancreatic cystoscopy through a 19-gauge needle EUS (with videos) Gastrointest Endosc. 2010;72:1285–8. doi: 10.1016/j.gie.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–14. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Konda VJ, Meining A, Jamil LH, et al. Apilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 34.Napoléon B, Lemaistre AI, Pujol B, et al. Anovel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 35.Springer S, Wang Y, Dal Molin M, et al. Acombination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–10. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136–42. doi: 10.1055/s-0034-1390742. [DOI] [PubMed] [Google Scholar]
- 37.Singhi AD, Zeh HJ, Brand RE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: A clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc. 2016;83:1107–700. doi: 10.1016/j.gie.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: A multi-institutional study of the Japan Pancreas Society. Pancreas. 2011;40:67–71. doi: 10.1097/MPA.0b013e3181f749d3. [DOI] [PubMed] [Google Scholar]
- 39.Yoon WJ, Daglilar ES, Fernández-del Castillo C, et al. Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: The PIPE study. Endoscopy. 2014;46:382–7. doi: 10.1055/s-0034-1364937. [DOI] [PubMed] [Google Scholar]
- 40.Muthusamy VR, Chandrasekhara V, Acosta RD, et al. ASGE Standards of Practice Committee. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc. 2016;84:1–9. doi: 10.1016/j.gie.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Birnbaum DJ, Gaujoux S, Berbis J, et al. Surgery for pancreatic neoplasms: How accurate are our surgical indications? Surgery. 2017;162:112–9. doi: 10.1016/j.surg.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Correa-Gallego C, Ferrone CR, Thayer SP, et al. Incidental pancreatic cysts: Do we really know what we are watching? Pancreatology. 2010;10:144–50. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Chiaro M, Segersvärd R, Pozzi Mucelli R, et al. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol. 2014;21:1539–44. doi: 10.1245/s10434-013-3465-9. [DOI] [PubMed] [Google Scholar]
- 44.Ma GK, Goldberg DS, Thiruvengadam N, et al. Comparing American Gastroenterological Association Pancreatic Cyst Management Guidelines with Fukuoka consensus guidelines as predictors of advanced neoplasia in patients with suspected pancreatic cystic neoplasms. J Am Coll Surg. 2016;223:729–30. doi: 10.1016/j.jamcollsurg.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Sahora K, Ferrone CR, Brugge WR, et al. Effects of comorbidities on outcomes of patients with intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2015;13:1816–23. doi: 10.1016/j.cgh.2015.04.177. [DOI] [PubMed] [Google Scholar]


