Abstract
Background and Objectives:
Isolated pancreatic tuberculosis (PTB) is extremely rare worldwide. The purpose of this multicenter retrospective study is to analyze imaging features of histologically confirmed isolated PTB in order to determine the diagnostic features of the new methods contrast enhanced ultrasound (CEUS), ultrasound elastography and contrast enhanced endoscopic ultrasound (CE-EUS).
Patients and Methods:
We report on a retrospective data collection of 12 cases of PTB confirmed by histology or cytology. All examinations were interpreted by two independent readers in consensus. CEUS, CE-EUS and ultrasound elastography were performed according to the European Federation of Societies for Ultrasound in Medicine and Biology guidelines.
Results:
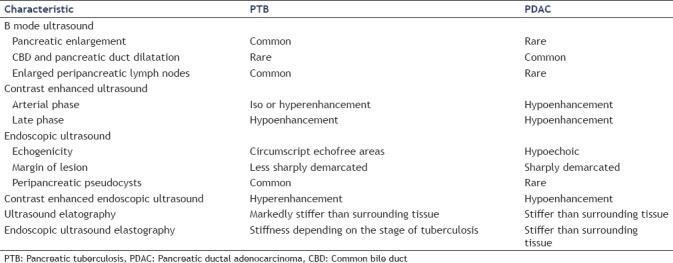
In PTB patients the common bile duct was never dilated. Multiple retroperitoneal lymph nodes are the second important B-mode ultrasound feature detected in 75% of PTB patients. CE-EUS was performed in three PTB patients demonstrating hyperenhancement. On elastography, all PTB lesions were markedly stiffer than surrounding pancreatic parenchyma.
Conclusions:
Here we report the first time on CEUS and elastography features of PTB. PTB had some typical imaging features with iso- or hyperenhancement on CE(E) US. PTB is markedly stiffer on elastography. If clinicians are aware of clinical features of PTB and conduct appropriate investigations with multiple modalities including B-mode ultrasound, CEUS, and EUS guided fine needle aspiration, diagnosis of PTB without laparotomy is possible and the disease can be effectively treated with anti-tuberculous drugs.
Keywords: Contrast enhanced endoscopic ultrasound, contrast enhanced ultrasound, elastography, guidelines, pancreatic tuberculosis
INTRODUCTION
Extra-pulmonary tuberculosis (TB) is an emerging clinical problem worldwide, including dissemination to the gastrointestinal tract,[1,2,3] liver,[4] spleen and mesenteric lymph nodes.[5,6,7,8] Pancreatic TB (PTB) is an extremely rare disease, which usually occurs as a complication of miliary TB in immunodeficiency individuals.[9,10] PTB has been little reported in the literature over the past few decades and its clinical characteristics remain unclear.[11,12,13,14]
The accurate preoperative diagnosis of PTB is clinically challenging because it is difficult to differentiate from other tumorous and non-tumorous lesions. Isolated PTB is extremely rare worldwide. It can mimic carcinoma, lymphoma, cystic neoplasia, retroperitoneal tumors, pancreatitis or pseudocysts.[15] Due to the paralleling clinical presentations and imaging findings in pancreatic TB and pancreatic neoplasm, and patients exhibiting a wide spectrum of non-specific complaints, the majority of the cases are diagnosed after surgical exploration for presumed pancreatic malignancy.[16,17] Accurate diagnosis may avoid delays in treatment and unnecessary surgery.[18]
Solitary pancreatic involvement of TB may present with variable radiological manifestations.[19,20] The imaging picture of this chronic infection may be revealed as variable pancreatic lesions, including pancreatic cystic neoplasm, such as serous cystadenoma.[21] It is often difficult to differentiate PTB mimicking cystic neoplasms from benign or malignant pancreatic cystic neoplasms.[19,22,23] Until now, non-invasive imaging studies such as B-mode ultrasound (BMUS), computed tomography (CT) and magnetic resonance imaging (MRI) can only localize a focal lesion in the pancreas. However non-surgical imaging diagnosis of PTB continues to be a challenge.
Contrast enhanced ultrasound (CEUS) is a safe and accurate imaging method to evaluate the vascularity of pancreatic lesions, which improves the characterization of pancreatic masses.[24] As a first line examination, CEUS performed immediately after the detection of a pancreatic mass at BMUS, providing a significant improvement in diagnostic accuracy.[24,25] The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines and recommendations on the clinical practice of CEUS updated 2011 on non-hepatic applications included the use of CEUS in focal pancreatic lesions.[26] With the review of literature, there is no report about CEUS features of pancreatic TB.
The purpose of this multicenter retrospective study is to analyze imaging features of histologically confirmed PTB in order to describe diagnostic features of CEUS, ultrasound elastography and contrast enhanced endoscopic ultrasound (CE-EUS).
PATIENTS AND METHODS
Patients
We report on a retrospective data collection of 12 cases of PTB confirmed by histology or cytology. The average age at diagnosis was 54 ± 11 years (37–70 years). Six patients were male and six were female [Table 1].
Table 1.
Baseline characteristics of pancreatic tuberculosis and pancreatic ductal adenocarcinoma patients
Based on traditional imaging modalities (CT, MRI and conventional BMUS), the pre-EUS diagnoses included autoimmune pancreatitis in two patients, cystadenoma in two patients, pancreatic adenocarcinomas (PDAC) in five patients, neuroendocrine tumor in one patient, metastasis lesion in one patient and lymphoma in one patient. All lesions were histologically confirmed as PTB by surgical biopsies or via EUS - fine needle aspiration (FNA).
Since PDAC is the most important differential diagnosis, twenty-two patients with histopathologically proven PDAC were analyzed as peer group.
Examination technique
Conventional ultrasound and CEUS were performed in all patients with one of six ultrasound systems: Philips iU22 unit (Philips Bothell, WA, USA; C5-1 convex array probes, 1–5 MHz), or LOGIQ E9 (GE Healthcare, Milwaukee, WI, USA; C1-5 convex array probes, 1–5 MHz) or Hitachi (Hi vision EUB-6500, Preirus, Ascendus; C715 convex array probes, 1–5 MHz), or SIEMENS (Acuson Sequoia or S2000™), or Toshiba (Aplio platinum 500; Aplio CV, convex array probes 3–6 MHz). CEUS was performed using contrast harmonic real-time imaging at a low MI 0.05–0.30. The ultrasound contrast agent SonoVue was injected at a dose of 2.4 mL, immediately followed by an injection of 10 mL sodium chloride solution. Images were recorded for 3 min after contrast agent injection.
CE-EUS and EUS elastography were performed as recently described using longitudinal echoendoscopes EG-3870 UTK and Hitachi platforms (HI vision EUB-6500, Preirus, Ascendus).[27]
Three patients were assessed by the acoustic radiation force impulse (ARFI) technology using Acuson S2000™ equipment with Virtual Touch™ Quantification software (VTQ) for the assessment of pancreatic lesions’ stiffness by obtaining the shear wave velocity (SWV). Ten measurements of ARFI VTQ were made in each suspected pancreatic lesion. The SWV was expressed in m/s. The depth of measurement ranged from 4.5 cm to 5.5 cm from skin.
Imaging Evaluation (contrast enhanced ultrasound, endoscopic ultrasound, elastography)
All examinations were interpreted by two independent readers (15 and 20 years of experience with pancreatic CEUS imaging) who were blinded to the clinical and pathologic data. CEUS enhancement pattern of PTB was evaluated according to the 2011 EFSUMB guidelines.[26,28] The CEUS features of isolated PTB lesions were compared to the surrounding normal pancreatic parenchyma.
The VTQ values of SWV measurements were presented as the mean, median, standard deviation range.
Final diagnoses, treatment and clinical follow up
The final diagnosis of nine PTB patients was achieved by core needle biopsy (18-gauge 20-cm single-use biopsy needles; Temno, Germany, or BioPince, Pflugbeil, Germany) as part of workup for a preoperative confirmation of pancreatic carcinoma. In another three PTB patients, fine needle biopsy was performed during EUS.
In all patients with PTB, clinical follow-up to 12 months confirmed the diagnosis.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA). Continuous parameters such as the mean ± standard deviation and the Chi-square test and Fisher's exact test were used to compare categorical parameters between groups. P < 0.05 was considered statistically significant.
Institutional board approval
Institutional Board Approval was obtained.
RESULTS
Conventional ultrasound
On conventional BMUS, all 12 cases of PTB were detected as isolated hypoechoic focal pancreatic lesions. All PDAC lesions were detected as hypoechoic focal pancreatic lesions in the pancreatic head [Figure 1].
Figure 1.
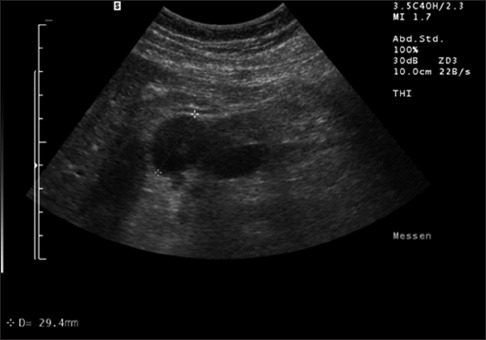
Circumscript pancreatic tuberculosis using transcutaneous B-mode
A pathologically dilated common bile duct (CBD) was common in PDAC lesions (86.3%), but not in PTB lesions (P < 0.05).
Multiple retroperitoneal lymph nodes were detected in 75% PTB patients, but not in PDAC patients [Table 2].
Table 2.
Comparison of B-mode ultrasound findings between pancreatic tuberculosis and pancreatic ductal adenocarcinoma lesions
Contrast enhanced ultrasound
In six patients, CEUS was performed. After contrast agent injection as well as during the late phase, all six PTB lesions (100%) and most of the PDAC lesions (86.3%) were hypoenhancing (P > 0.05) on CEUS. In the arterial phase iso- or hyperenhancement was seen in six patients comparing to the surrounding tissue.
Contrast enhanced endoscopic ultrasound
CE-EUS was performed in three patients with PTB demonstrating hyperenhancement in all cases. All 22 cases of PDAC were hypoenhancing in CE-EUS.
Elastography
EUS elastography was performed in five PTB lesions, all of them were markedly stiffer than surrounding pancreatic parenchyma [Figure 2].
Figure 2.
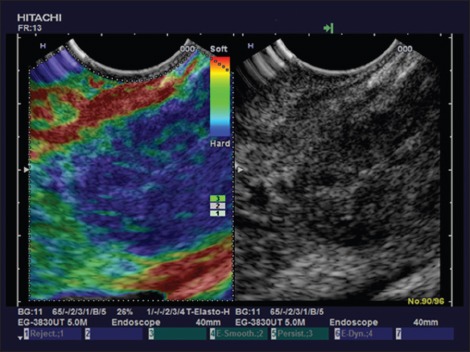
Pancreatic tuberculosis using endoscopic ultrasound elastography with stiff tissue diffusely infiltrating the pancreas
VTQ were performed in three PTB lesions, mean SWV were 3.66 ± 1.21 m/s, which were stiffer than the surrounding pancreas parenchyma (1.08 ± 0.70 m/s) [Table 3].
Table 3.
Typical imaging findings of pancreatic tuberculosis and pancreatic ductal adenocarcinoma lesions
DISCUSSION
Epidemiology
TB of the abdomen is seen in 12% of patients with miliary TB.[29,30] PTB, which was first reported by Auerbach in 1944, is extremely rare.[31] The incidence of pancreatic TB is reported to be <4.7% worldwide. Pancreatic TB most often occurs as a complication of miliary TB. The low frequency of pancreatic TB may be partly due to the biological resistance of the pancreas to tubercular infection. Pancreatic enzymes, including lipases and deoxyribonucleases, have antimycobacterial effects.[32]
Pancreatic TB is a common opportunistic infection in human immunodeficiency virus (HIV)-positive patients and is considered to be an acquired immunodeficiency syndrome defining illness.[15] The proposed mechanism for the spread of pancreatic TB are as follows: the ingestion of mycobacterial organisms, the spread from the GI tract to the lymphatics, and then directly to the adjacent abdominal organs;[33] or the hematogenous or lymphatic dissemination from a remote focus.[32]
Clinical features
The clinical presentation of pancreatic TB is insidious and non-specific. Common symptoms and signs include abdominal pain, weight loss, nausea/vomiting, fever, abdominal mass[9,33] and obstructive jaundice.[1,19]
Intra-abdominal TB frequently involve abdominal lymph nodes, the spleen, the peritoneum, the liver, and the gastrointestinal tract.[15] However, even in HIV infected patients, TB of the pancreas is relatively rare with an incidence of 0.46% based on ultrasound findings.[20] Insolated primary pancreatic TB is particularly rare.
Xia et al.[9] have summarized characteristic features of PTB as follows: (1) mostly occurs in young people, especially female; (2) have past history of TB or come from endemic zone of TB; (3) often present with epigastric pain, fever, and weight loss; and (4) ultrasound or CT scan show pancreatic mass and peri-pancreatic nodules, some with focal calcification.
Imaging features
Pancreatic TB can be classified radiologically as follows: The most common form is mass-forming (with or without diffuse pancreatic enlargement) and accounts for 94.4% of cases. Additionally, there is a diffuse and a small, nodular type. The masses can be radiographically similar to pancreatic tumors, autoimmune pancreatitis, abscesses, lymphomas, or pseudocysts.[5,15,27,34,35,36,37,38,39,40]
B-mode ultrasound
Conventional ultrasound usually depicts a focal hypoechoic lesions at or adjacent to the pancreas,[11,14,41,42] heterogeneously hypo-isoechoic lesions (primarily in the head of the pancreas), diffuse enlargement of the pancreas and enlarged peripancreatic and other abdominal lymph nodes.[43]
An important image finding in PTB is the normal appearing CBD and the pancreatic duct, even if the mass is located centrally in the head of the pancreas. This is in contrast to pancreatic adenocarcinoma, where the pancreatic duct is dilated in centrally located tumors.[16] Pancreatic TB should be suspected in patients with associated lymphadenopathy in the portohepatic and para-aortic regions.[29]
The diffuse form of PTB is characterized by pancreatic enlargement with narrowing of the main pancreatic duct and heterogeneous enhancement.[44]
Endoscopic ultrasound
PTB usually manifests as circumscript echoless areas with a solid impressing or semiliquid but echoic inner content. In contrast to pancreatitis the surrounding reactive changes are less prominent. Peripancreatic pseudocysts may be another manifestation of PTB mimicking complicated chronic pancreatitis.
Contrast-enhanced ultrasound
Here we described for the first time the imaging features of CEUS. CEUS reveals heterogeneous enhancement pattern. Non-enhancement in the necrotic zones and hyperenhancement in the most active zones. In the arterial phase PTB showed typically iso- or hyperenhancement comparing to the surrounding tissue, which is the most important feature to differentiate PDAC [Figure 3].
Figure 3.
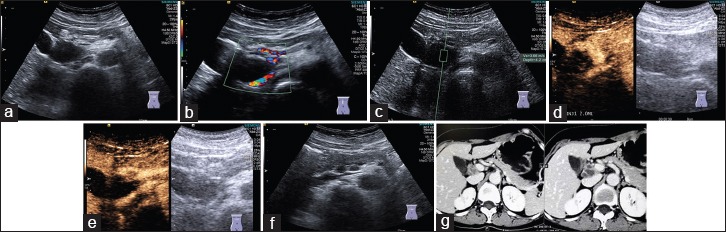
Pancreatic tuberculosis in B_mode (a), color Doppler flow imaging (b) and ultrasound elastography with Virtual Touch™ Quantification measurement (c). On contrast enhanced ultrasound arterial phase (d) and portal venous phase (e), the focal pancreatic lesion was partially septa enhanced. Lymphadenopathies were detected both on B-mode ultrasound (f) and computed tomography (g)
Endoscopic ultrasound using elastography
Here we described for the first time the imaging features of endoscopic elastography. Depending on the stage diffuse infiltration of TB of the pancreas reveals stiffer tissue compared to the surrounding healthy pancreatic parenchyma if the infiltration is circumscript. In contrast to ductal adenocarcinoma the focal pancreatic lesion is less sharply demarcated. In addition, lymphoma may show less stiff tissue. In the case of diffuse tuberculous infiltration of the whole organ this shows stiff tissue a finding which is similar to autoimmune pancreatitis.[5,6,7,8,45,46,47,48,49] The lymph node findings are similar to the lung.[50]
Computed tomography and magnetic resonance imaging
In cases of PTB, contrast enhanced CT may show a sharply delineated mass in the pancreatic head with irregular margins and peripheral enhancement. However these features are not specific for PTB.[51]
MRI demonstrated multinodular masses which were low signal intensity in T1 weighted image and high signal in T2, with non-enhanced internal necrotic foci and peripheral enhancement.[18]
On both CT and MRI, it is most commonly described as a heterogeneous, lobulated, and multicystic mass. Pancreatic TB is appearing with hypodensity on CT, and low signal intensity on T1-weighted images; it varies from hypo to hyper signal intensity on T2-weighted images.[19,44] Pancreatic TB typically has a thick rim enhancement and internal septa enhancement on postcontrast images.[52] Such image characteristics on CT and MRI are similar, if not identical, to those of pancreatic neoplasia, making the radiologic diagnosis of pancreatic TB to be a challenge.
Positron emission tomography - computed tomography
An increase in F-18 fludeoxyglucose (FDG) uptake is seen in malignancy as well as in inflammation. In the case of pancreatic TB, the lesions are composed of cells with an elevated level of glucose metabolism-epithelioid cells, Langhans giant cells and lymphocytes– consecutively resulting in a high F-18 FDG uptake.[17]
Enhanced ultrasound guided fine needle aspiration
The diagnosis of pancreatic TB has traditionally been based on biopsy samples taken during surgery. More recently, studies employing EUS-guided FNA for preoperative diagnosis showed some promising results.[18,29,53,54,55] Recommendations have been reported in the EFSUMB guidelines.[56,57,58,59,60,61,62,63,64,65]
EUS offers a superior tissue contrast and a higher local regional resolution compared to CT or MRI. In contrast to percutaneous FNA, EUS-guided FNA has a higher diagnostic accuracy of 76.2% for pancreatic TB, which is comparable to that for pancreatic cancer.[53] The diagnostic accuracy of EUS-FNA was reported to be 76%–95% for pancreatic cancer and 46% for focal inflammation.[66] EUS guided FNA may be useful in the diagnosis of an undetermined pancreatic lesion. Resulting, a laparotomy can be avoided when TB is diagnosed.
Definitive diagnosis of PTB is made by demonstrating caseating granulomas, acid fast bacilli on Ziehl–Nelson staining or the presence of Mycobacterium tuberculosis DNA by a polymerase chain reaction in the tissue obtained from the lesion.[9]
Differential diagnoses
Pancreatic TB most commonly presents as a solitary lesion with multiple cystic components. The main differential diagnoses of pancreatic solid-cystic masses include cystic neuroendocrine tumor, mucinous cystadenocarcinoma, serous cystadenoma, solid pseudopapillary neoplasm and cystic degeneration of pancreatic adenocarcinoma. Pancreatic TB should also be considered in the differential diagnosis of pancreatic solid-cystic mass lesions.[29] From clinical reviews, between 60% and 100% cases of PTB were initially diagnosed as having pancreatic cancer[9,12] and between 45% and 86% required surgery to confirm the diagnosis.[13,22]
Interestingly, though commonly seen in pancreatic cancer, pancreatic ductal dilatation is seldomly found in pancreatic TB. Nagar et al.[23] recommended to take TB of the pancreas as a differential diagnosis for pancreatic masses associated with peripancreatic lymphadenopathy.
On CEUS, ductal adenocarcinoma is reported to be hypovascular in a percentage from 73% to 93%.[67,68] A multicenter study named PAMUS reported the high accuracy of CEUS in characterizing pancreatic masses, declaring that solid pancreatic lesions were correctly characterized in respect to pathology with an accuracy of 91.7% and pancreatic ductal adenocarcinoma was correctly characterized with an accuracy of 87.8%.[69] A specificity of 100% for trans-abdominal CEUS diagnosis of ductal adenocarcinoma with a sensitivity of 90% is reported.[70,71]
Management of pancreatic tuberculosis
Management of pancreatic TB is based on anti-tubercular treatment for 6–12 months, with complete clinical and radiologic response.[30]
No definitive guidelines are available in literature regarding the role of reimaging. Singh et al.[51] have suggested radiological follow-up to document regression of the mass but time intervals are not exactly specified. They have suggested that if radiological improvement does not occur even after anti-tubercular treatment, the mass has to be resected for histological confirmation. However, there is no data available on co-existing pancreatic carcinoma with TB. Xia et al.[9] have done a follow-up CT scan in 11 of 16 patients and found complete resolution in all, though time was variable between 78 and 186 days with a mean of 132 days. Therefore, we conclude that a repeat imaging at the end of anti-tubercular treatment and thereafter at regular intervals will help us to monitor and understand the natural process of the disease.
If the diagnosis is delayed, pancreatic TB can be fatal; the disease has a 10.8% mortality rate (which is comparable to the mortality rate of 9.1% in immune-competent patients).[15] However, pancreatic TB responds well to standard ATDs.
Weaknesses of our study
Weaknesses of our retrospective and non-comparative study include the retrospective data acquisition and the non-comparative character of reported imaging features.
CONCLUSIONS
TB of the pancreas is rare, with a wide range of non-specific clinical presentation and image features overlapping with those seen in pancreatic neoplasia. To our best knowledge, our paper had a comparatively large number of histopathological-proven PTB patients partially examined with new technologies so far not reported in the literature. Imaging diagnosis of PTB represents a diagnostic challenge. On BMUS PTB most commonly presents as a solitary lesion with multiple enlarged peripancreatic lymph nodes and without dilatation of the CBD. In the arterial phase of CE(E) US PTB showed iso- or hyperenhancement comparing to the surrounding tissue, which is the most important feature to differentiate PDAC. Additionally, PTB was markedly stiffer than the surrounding pancreatic tissues on (endoscopic) ultrasound elastography.
However, if clinicians are aware of its clinical and imaging features and conduct appropriate investigations with multiple modalities including BMUS, CEUS, and EUS guided FNA, diagnosis of PTB without laparotomy is possible and the disease can be effectively treated with anti-tuberculous drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Barreiros AP, Braden B, Schieferstein-Knauer C, et al. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol. 2008;43:1224–31. doi: 10.1080/00365520802158606. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich CF, Lembcke B, Jenssen C, et al. Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med. 2014;35:400–21. doi: 10.1055/s-0034-1385154. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Lembcke B, Jenssen C, et al. Intestinal ultrasound in rare gastrointestinal diseases, update, part 2. Ultraschall Med. 2015;36:428–56. doi: 10.1055/s-0034-1399730. [DOI] [PubMed] [Google Scholar]
- 4.Barreiros AP, Chiorean L, Braden B, et al. Ultrasound in rare diffuse liver disease. Z Gastroenterol. 2014;52:1247–56. doi: 10.1055/s-0034-1384996. [DOI] [PubMed] [Google Scholar]
- 5.Cui XW, Chang JM, Kan QC, et al. Endoscopic ultrasound elastography: Current status and future perspectives. World J Gastroenterol. 2015;21:13212–24. doi: 10.3748/wjg.v21.i47.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorean L, Barr RG, Braden B, et al. Transcutaneous ultrasound: Elastographic lymph node evaluation. Current clinical applications and literature review. Ultrasound Med Biol. 2016;42:16–30. doi: 10.1016/j.ultrasmedbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176–90. doi: 10.4103/2303-9027.162995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich CF, Hocke M, Jenssen C. Ultrasound for abdominal lymphadenopathy. Dtsch Med Wochenschr. 2013;138:1001–18. doi: 10.1055/s-0032-1333027. [DOI] [PubMed] [Google Scholar]
- 9.Xia F, Poon RT, Wang SG, et al. Tuberculosis of pancreas and peripancreatic lymph nodes in immunocompetent patients: Experience from China. World J Gastroenterol. 2003;9:1361–4. doi: 10.3748/wjg.v9.i6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacharia GS, Antony R, Kolassery S, et al. Isolated pancreatic tuberculosis masquerading as pancreatic cancer. Gastroenterol Rep (Oxf) 2014;2:154–7. doi: 10.1093/gastro/gou017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng J, Tadi K, Halpern M, et al. Pancreatic tuberculosis in a human immunodeficiency virus positive patient: A case report. World J Gastroenterol. 2008;14:939–40. doi: 10.3748/wjg.14.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho SB. Pancreatic tuberculosis presenting with pancreatic cystic tumor: A case report and review of the literature. Korean J Gastroenterol. 2009;53:324–8. doi: 10.4166/kjg.2009.53.5.324. [DOI] [PubMed] [Google Scholar]
- 13.Demir K, Kaymakoglu S, Besisik F, et al. Solitary pancreatic tuberculosis in immunocompetent patients mimicking pancreatic carcinoma. J Gastroenterol Hepatol. 2001;16:1071–4. doi: 10.1046/j.1440-1746.2001.02467.x. [DOI] [PubMed] [Google Scholar]
- 14.Eyal AS, Karusseit VO. Tuberculosis of the pancreas mimicking carcinoma. Int J Infect Dis. 2008;12:108–10. doi: 10.1016/j.ijid.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Meesiri S. Pancreatic tuberculosis with acquired immunodeficiency syndrome: A case report and systematic review. World J Gastroenterol. 2012;18:720–6. doi: 10.3748/wjg.v18.i7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonthalia N, Ray S, Pal P, et al. Fine needle aspiration diagnosis of isolated pancreatic tuberculosis: A case report. World J Clin Cases. 2013;1:181–6. doi: 10.12998/wjcc.v1.i5.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santhosh S, Bhattacharya A, Rana SS, et al. Pancreatic tuberculosis: Evaluation of therapeutic response using F-18 fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Indian J Nucl Med. 2014;29:257–9. doi: 10.4103/0972-3919.142635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JB, Lee SS, Kim SH, et al. Peripancreatic tuberculous lymphadenopathy masquerading as pancreatic malignancy: A single-center experience. J Gastroenterol Hepatol. 2014;29:409–16. doi: 10.1111/jgh.12410. [DOI] [PubMed] [Google Scholar]
- 19.Huang CT, Lo CY, Lee TH. Isolated peripancreatic tuberculous lymphadenopathy: A rare manifestation of abdominal tuberculosis mimicking pancreatic cystic neoplasm. J Dig Dis. 2013;14:105–8. doi: 10.1111/1751-2980.12011. [DOI] [PubMed] [Google Scholar]
- 20.Maniar JK, Kamath RR, Mandalia S, et al. HIV and tuberculosis: Partners in crime. Indian J Dermatol Venereol Leprol. 2006;72:276–82. doi: 10.4103/0378-6323.26723. [DOI] [PubMed] [Google Scholar]
- 21.Hong SG, Kim JS, Joo MK, et al. Pancreatic tuberculosis masquerading as pancreatic serous cystadenoma. World J Gastroenterol. 2009;15:1010–3. doi: 10.3748/wjg.15.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foo FJ, Verbeke CS, Guthrie JA, et al. Pancreatic and peripancreatic tuberculosis mimicking malignancy. JOP. 2007;8:201–5. [PubMed] [Google Scholar]
- 23.Nagar AM, Raut AA, Morani AC, et al. Pancreatic tuberculosis: A clinical and imaging review of 32 cases. J Comput Assist Tomogr. 2009;33:136–41. doi: 10.1097/RCT.0b013e31816c82bc. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio M, Canestrini S, De Robertis R, et al. CEUS of the pancreas: Still research or the standard of care. Eur J Radiol. 2015;84:1644–9. doi: 10.1016/j.ejrad.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 25.D’Onofrio M, Zamboni GA, Malagò R, et al. Resectable pancreatic adenocarcinoma: Is the enhancement pattern at contrast-enhanced ultrasonography a pre-operative prognostic factor? Ultrasound Med Biol. 2009;35:1929–37. doi: 10.1016/j.ultrasmedbio.2009.06.1100. [DOI] [PubMed] [Google Scholar]
- 26.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich CF, Sahai AV, D’Onofrio M, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933–40. doi: 10.1016/j.gie.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 28.Sugumar A, Chari ST. Autoimmune pancreatitis. J Gastroenterol Hepatol. 2011;26:1368–73. doi: 10.1111/j.1440-1746.2011.06843.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohamadnejad M, Sotoudeh M, Malekzadeh R. Education and imaging. Gastrointestinal: Pancreatic tuberculosis masquerading as malignancy. J Gastroenterol Hepatol. 2014;29:418. doi: 10.1111/jgh.12527. [DOI] [PubMed] [Google Scholar]
- 30.Pramesh CS, Heroor AA, Gupta SG, et al. Pancreatic tuberculosis: An elusive diagnosis. HPB (Oxford) 2003;5:43–5. doi: 10.1080/13651820310000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auerbach O. Acute generalized miliary tuberculosis. Am J Pathol. 1944;20:121–36. [PMC free article] [PubMed] [Google Scholar]
- 32.Franco-Paredes C, Leonard M, Jurado R, et al. Tuberculosis of the pancreas: Report of two cases and review of the literature. Am J Med Sci. 2002;323:54–8. doi: 10.1097/00000441-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Weiss ES, Klein WM, Yeo CJ. Peripancreatic tuberculosis mimicking pancreatic neoplasia. J Gastrointest Surg. 2005;9:254–62. doi: 10.1016/j.gassur.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 34.De Molo C, Cui XW, Pirri C, et al. Pancreas mobile. Z Gastroenterol. 2013;51:1165–70. doi: 10.1055/s-0033-1335185. [DOI] [PubMed] [Google Scholar]
- 35.Pirri C, Cui XW, De Molo C, et al. The pancreatic head is larger than often assumed. Z Gastroenterol. 2013;51:390–4. doi: 10.1055/s-0033-1335175. [DOI] [PubMed] [Google Scholar]
- 36.Hocke M, Cui XW, Domagk D, et al. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc Ultrasound. 2014;3:123–30. doi: 10.4103/2303-9027.131040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietrich CF, Chichakli M, Hirche TO, et al. Sonographic findings of the hepatobiliary-pancreatic system in adult patients with cystic fibrosis. J Ultrasound Med. 2002;21:409–16. doi: 10.7863/jum.2002.21.4.409. [DOI] [PubMed] [Google Scholar]
- 38.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–7. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich CF, Hirche TO, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–20. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 40.Hocke M, Ignee A, Dietrich CF. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy. 2011;43:163–5. doi: 10.1055/s-0030-1256022. [DOI] [PubMed] [Google Scholar]
- 41.Rezeig MA, Fashir BM, Al-Suhaibani H, et al. Pancreatic tuberculosis mimicking pancreatic carcinoma: Four case reports and review of the literature. Dig Dis Sci. 1998;43:329–31. doi: 10.1023/a:1018854305652. [DOI] [PubMed] [Google Scholar]
- 42.Tan KK, Chen K, Liau KH, et al. Pancreatic tuberculosis mimicking pancreatic carcinoma: Series of three cases. Eur J Gastroenterol Hepatol. 2009;21:1317–9. doi: 10.1097/MEG.0b013e32832bab73. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Kapoor D, Singh J, et al. Isolated tuberculosis of the pancreas: A report of two cases and review of the literature. Trop Gastroenterol. 2003;24:76–8. [PubMed] [Google Scholar]
- 44.De Backer AI, Mortelé KJ, Bomans P, et al. Tuberculosis of the pancreas: MRI features. AJR Am J Roentgenol. 2005;184:50–4. doi: 10.2214/ajr.184.1.01840050. [DOI] [PubMed] [Google Scholar]
- 45.Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850–60. doi: 10.3748/wjg.v19.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2014;83:403–4. doi: 10.1016/j.ejrad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 48.Cui XW, Hocke M, Jenssen C, et al. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;52:212–21. doi: 10.1055/s-0033-1356153. [DOI] [PubMed] [Google Scholar]
- 49.Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–47. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound. 2016;5:233–8. doi: 10.4103/2303-9027.187866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh DK, Haider A, Tatke M, et al. Primary pancreatic tuberculosis masquerading as a pancreatic tumor leading to Whipple's pancreaticoduodenectomy. A case report and review of the literature. JOP. 2009;10:451–6. [PubMed] [Google Scholar]
- 52.Ozden I, Emre A, Demir K, et al. Solitary pancreatic tuberculosis mimicking advanced pancreatic carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:279–83. doi: 10.1007/s005340170029. [DOI] [PubMed] [Google Scholar]
- 53.Song TJ, Lee SS, Park DH, et al. Yield of EUS-guided FNA on the diagnosis of pancreatic/peripancreatic tuberculosis. Gastrointest Endosc. 2009;69(3 Pt 1):484–91. doi: 10.1016/j.gie.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Puri R, Thandassery RB, Eloubeidi MA, et al. Diagnosis of isolated pancreatic tuberculosis: The role of EUS-guided FNA cytology. Gastrointest Endosc. 2012;75:900–4. doi: 10.1016/j.gie.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part IV – EUS-guided Interventions: General aspects and EUS-guided sampling (long version) Ultraschall Med. 2016;37:E33–76. doi: 10.1055/s-0035-1553785. [DOI] [PubMed] [Google Scholar]
- 57.Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part IV – EUS-guided interventions: General aspects and EUS-guided sampling (short version) Ultraschall Med. 2016;37:157–69. doi: 10.1055/s-0035-1553788. [DOI] [PubMed] [Google Scholar]
- 58.Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part V – EUS-guided therapeutic interventions (short version) Ultraschall Med. 2016;37:412–20. doi: 10.1055/s-0035-1553742. [DOI] [PubMed] [Google Scholar]
- 59.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part III – Abdominal treatment procedures (long version) Ultraschall Med. 2016;37:E1–32. doi: 10.1055/s-0035-1553917. [DOI] [PubMed] [Google Scholar]
- 60.Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part III – Abdominal treatment procedures (short version) Ultraschall Med. 2016;37:27–45. doi: 10.1055/s-0035-1553965. [DOI] [PubMed] [Google Scholar]
- 61.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part I. General aspects (long version) Ultraschall Med. 2015;36:E1–14. doi: 10.1055/s-0035-1553593. [DOI] [PubMed] [Google Scholar]
- 62.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part I. General aspects (short version) Ultraschall Med. 2015;36:464–72. doi: 10.1055/s-0035-1553601. [DOI] [PubMed] [Google Scholar]
- 63.Sidhu PS, Brabrand K, Cantisani V, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part II. Diagnostic Ultrasound-guided interventional procedures (long version) Ultraschall Med. 2015;36:E15–35. doi: 10.1055/s-0035-1554036. [DOI] [PubMed] [Google Scholar]
- 64.Sidhu PS, Brabrand K, Cantisani V, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part II. Diagnostic ultrasound-guided interventional procedures (short version) Ultraschall Med. 2015;36:566–80. doi: 10.1055/s-0035-1566760. [DOI] [PubMed] [Google Scholar]
- 65.Dietrich CF, Fusaroli P, Jenssen C. European federation of societies for ultrasound in medicine and biology guidelines 2015 on interventional endoscopic ultrasound. Endosc Ultrasound. 2016;5:143–8. doi: 10.4103/2303-9027.183968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 67.D’Onofrio M, Zamboni G, Tognolini A, et al. Mass-forming pancreatitis: Value of contrast-enhanced ultrasonography. World J Gastroenterol. 2006;12:4181–4. doi: 10.3748/wjg.v12.i26.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitano M, Kudo M, Maekawa K, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–9. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Onofrio M, Barbi E, Dietrich CF, et al. Pancreatic multicenter ultrasound study (PAMUS) Eur J Radiol. 2012;81:630–8. doi: 10.1016/j.ejrad.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 70.Dietrich CF, Braden B, Hocke M, et al. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J Cancer Res Clin Oncol. 2008;134:635–43. doi: 10.1007/s00432-007-0326-6. [DOI] [PubMed] [Google Scholar]
- 71.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]


