Abstract
The npq1 Arabidopsis mutant is deficient in the violaxanthin de-epoxidase enzyme that converts violaxanthin to zeaxanthin in excess light (xanthophyll cycle). We have compared the behavior of mature leaves (ML) and developing leaves of the mutant and the wild type in various light environments. Thermoluminescence measurements indicated that high photon flux densities (>500 μmol m−2 s−1) promoted oxidative stress in the chloroplasts of npq1 ML, which was associated with a loss of chlorophyll and an inhibition of the photochemical activity. Illuminating leaf discs in the presence of eosin, a generator of singlet oxygen, brought about pronounced lipid peroxidation in npq1 ML but not in wild-type leaves. No such effects were seen in young leaves (YL) of npq1, which were quite tolerant to strong light and eosin-induced singlet oxygen. Non-photochemical energy quenching was strongly inhibited in npq1 YL and ML and was not improved with high-light acclimation. Our results confirm that the xanthophyll cycle protects chloroplasts from photooxidation by a mechanism distinct from non-photochemical energy quenching and they reveal that the absence of xanthophyll cycle can be compensated by other protective mechanisms. npq1 YL were observed to accumulate considerable amounts of vitamin E during photoacclimation, suggesting that this lipophilic antioxidant could be involved in the high phototolerance of those leaves.
Violaxanthin (V) de-epoxidase is a chloroplastic enzyme that is localized in the lumen of the thylakoids and converts the diepoxide xanthophyll V to the monoepoxide antheraxanthin (A) and the epoxide-free zeaxanthin (Z) at an optimum pH of about 5 (Rockholm and Yamamoto, 1996; Eskling et al., 1997; Bugos et al., 1998). In vivo, the de-epoxidation reaction is triggered by an increase in the proton gradient across the thylakoid membranes in plants exposed to excessive light. Upon return to limiting light conditions, Z is epoxidized back to V by a Z epoxidase enzyme localized on the stromal side of the thylakoid membranes. These stoichiometric and cyclic conversions of V, A, and Z are called the xanthophyll (or V) cycle and play a major role in controlling the efficiency of light harvesting in plants (Demmig-Adams and Adams, 1996; Horton et al., 1996). In limiting light conditions, the presence of V in the light-harvesting complexes (LHCs) of photosystem II (PSII) is associated with maximum efficiency of light harvesting, whereas the synthesis of Z is correlated with down-regulation of the light-harvesting efficiency caused by an increased dissipation of excitation energy as heat. The latter process, which is measured as non-photochemical quenching (NPQ) of chlorophyll (Chl) fluorescence, is potentially important for the protection of the photosynthetic apparatus from photodamage; it can protect the photosensitive PSII reaction center from overexcitation, and it also can lower the formation of harmful reactive molecules in the LHCs such as triplet Chl and singlet oxygen (1O2). Accordingly, full conversion of V to Z prior to light stress at chilling temperatures has been observed to prevent irreversible photoinhibition of PSII in the mangrove Rhizophora mangle (Demmig-Adams et al., 1989).
Several mutants of Arabidopsis affected in the NPQ process have been isolated by Niyogi et al. (1998). Among those mutants, npq1 has been shown to be defective in the gene encoding V de-epoxidase. Leaves of this mutant have no functional V de-epoxidase, are unable to convert V to Z, and exhibit strongly inhibited NPQ. However, the npq1 mutation did not affect the efficiency of electron transport either in low light or in strong, saturating light (Niyogi et al., 1998; Havaux and Niyogi, 1999). Although short-term light stress was observed to damage PSII more severely in the mutant compared to the wild type, this differential PSII-photoinhibition was attenuated in long-term experiments (Niyogi et al., 1998; Havaux and Niyogi, 1999), suggesting that photoacclimation of PSII reduced the requirement for an active V cycle. However, the npq1 mutant was found to suffer from lipid peroxidative damage during prolonged exposure to high light, indicating increased susceptibility to photooxidation in the absence of the V cycle (Havaux and Niyogi, 1999). It was suggested that the antioxidant effect of the V cycle on thylakoid lipids supplements that of the lipophilic antioxidant vitamin E (α-tocopherol). In the present study, we examined further the photosynthetic behavior of npq1 in different light environments. Different aspects of the photochemical apparatus of the chloroplasts (pigments, photochemical activity, and lipid peroxidation) were analyzed in plants exposed to different photosynthetically active photon flux densities (PPFDs) up to 1,500 μmol m−2 s−1 and also in leaf discs exposed to 1O2. The results confirm that the V cycle protects the chloroplasts against photooxidation and that this protection directly involves Z in a mechanism distinct from NPQ. Leaf age was found to strongly influence the photoprotection exerted by the V cycle, with young leaves (YL) of npq1 being apparently unaffected by the lack of V cycle when exposed to PPFDs that photodamaged mature leaves (ML).
RESULTS
Photosynthetic Pigments and Tocopherols in Wild-Type and npq1 Leaves under Low- and High-Light Conditions
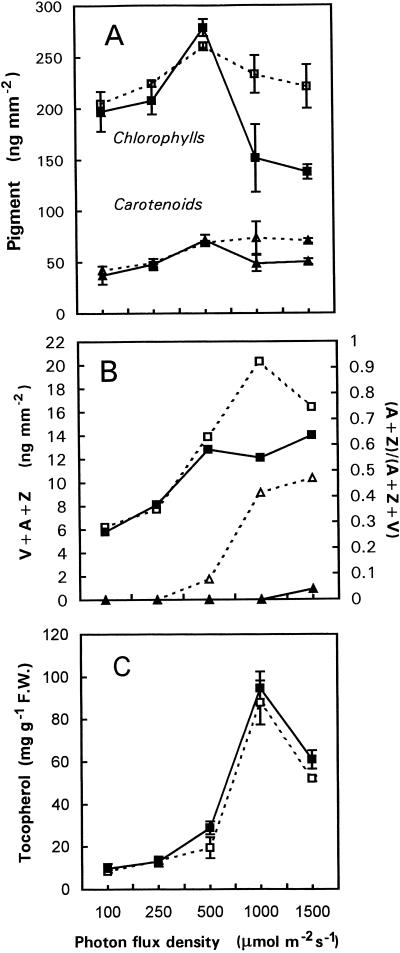
Figure 1A shows the total Chl and carotenoid concentrations in ML of the wild type and the npq1 mutant of Arabidopsis grown for 10 or 20 d under a wide range of PPFDs from 100 to 1,500 μmol m−2 s−1. When the PPFD was increased up to 500 μmol m−2 s−1, the levels of both Chls and carotenoids increased in wild-type leaves. Above 500 μmol m−2 s−1, the carotenoid content was stable, whereas the Chl level decreased slightly (by approximately 10%). The npq1 mutant behaved like the wild type in the low/moderate PPFD range (<500 μmol m−2 s−1), but the Chl content strongly decreased at high PPFDs. For instance, in npq1 plants grown at 1,500 μmol m−2 s−1, the Chl level fell to about 60% of the level measured in wild-type leaves. The carotenoid concentration also decreased in npq1 leaves grown at high PPFDs but to a smaller extent compared to the Chl content. However, this decrease was not found for the V-cycle carotenoids, which increased with PPFDs in both genotypes (Fig. 1B). The V-cycle pigments were partially de-epoxidized in wild-type plants at PPFDs higher than 500 μmol m−2 s−1 (Fig. 1B). At the highest PPFD, almost 50% of the V pool was de-epoxidized in the wild type. As expected, no significant synthesis of A and Z was found in the npq1 mutant.
Figure 1.
Total Chl (squares) and carotenoid (triangles) contents (A), xanthophyll-cycle pigments pool (V + A + Z, squares) and de-epoxidation status of the xanthophyll cycle (A + Z)/(V + A + Z) (triangles; B), and α-tocopherol content of ML of Arabidopsis plants (white symbols, wild type; black symbols, npq1; C) grown at PPFDs from 100 to 1,500 μmol m−2 s−1. Duration of the light treatments was 20 d except for the highest PPFD (10 d). Leaves were taken in the morning, approximately 1 h after the beginning of the light phase. Data are the means of four separate experiments ± sd.
We have also examined the level of α-tocopherol, the major antioxidant present in the thylakoid membrane lipid matrix (Fryer, 1992). Increasing PPFD caused a marked increase in the concentration of this compound, with maximal accumulation being found in plants grown at 1,000 μmol m−2 s−1 (Fig. 1C). Both genotypes behaved similarly, although npq1 seemed to contain slightly more α-tocopherol than the wild type.
Table I presents a detailed analysis of the pigment and tocopherol content in wild-type and npq1 plants at low and high PPFD. As observed in Figure 1 for longer treatments, exposure of npq1 plants to a PPFD of 1,500 μmol m−2 s−1 for 3 d (photoperiod, 15 h) caused a marked decrease in Chl; Chl b decreased by 30% and Chl a decreased by 15% in well-developed ML of the npq1 mutant (Table I). In contrast, the carotenoid concentrations remained stable (neoxanthin, lutein, and β-carotene) or increased (V + A + Z, +75%). In the wild type, all pigments except Chl b increased during the strong-light treatment, particularly the V + A + Z pool, which increased by 160%.
Table I.
Effects of high-light stress (3 d at 1,500 μmol m−2 s−1) on the pigment content (ng mm−2) and the tocopherol content (μg g−1 fresh wt) of YL and ML of wild type and npq1 Arabidopsis grown at a PPFD of 250 μmol m−2 s−1
| Compounds | 250 μmol m−2 s−1
|
1,500 μmol m−2 s−1
|
||||
|---|---|---|---|---|---|---|
| Wild-type ML | npq1 ML | WT
|
npq1
|
|||
| ML | YL | ML | YL | |||
| Photosynthetic pigments | ||||||
| Neoxanthin | 5.2 ± 0.1 | 5.0 ± 0.1 | 6.4 ± 0.7 | 5.2 ± 0.2 | 4.5 ± 0.7 | 5.7 ± 0.4 |
| V + A + Z | 7.7 ± 0.3 | 8.1 ± 1.0 | 20.0 ± 3.5 | 22.9 ± 0.8 | 13.9 ± 1.3 | 23.5 ± 2.2 |
| Lutein | 24.1 ± 0.5 | 23.6 ± 1.9 | 35.4 ± 1.8 | 33.3 ± 1.7 | 26.5 ± 3.4 | 35.9 ± 1.4 |
| β-Carotene | 12.5 ± 4.8 | 11.1 ± 2.7 | 14.9 ± 2.1 | 14.2 ± 2.2 | 13.9 ± 1.3 | 14.6 ± 1.7 |
| Chl b | 74.6 ± 3.1 | 67.7 ± 4.9 | 74.2 ± 7.0 | 59.6 ± 3.2 | 48.1 ± 9.6 | 56.4 ± 3.1 |
| Chla | 149.6 ± 5.8 | 139.8 ± 10.8 | 167.6 ± 19.1 | 141.4 ± 4.5 | 116.6 ± 22.7 | 138.5 ± 9.9 |
| Chl a/Chl b | 2.01 | 2.06 | 2.25 | 2.37 | 2.42 | 2.45 |
| Vitamin E | ||||||
| α-Tocopherol | 23.5 ± 1.7 | 19.8 ± 0.9 | 57.2 ± 2.1 | 78.7 ± 5.1 | 43.8 ± 3.9 | 91.9 ± 3.7 |
| γ-Tocopherol | 0.5 ± 0.3 | 0 | 15.5 ± 1.2 | 12.6 ± 0.1 | 12.4 ± 2.2 | 19.1 ± 2.6 |
Data are mean values of a minimum of three separate experiments ± sd. For the low-light growth conditions, only data of ML are given because there was no significant difference between YL and ML (wild type and npq1) with respect to their pigment and tocopherol content.
When developing npq1 YL in the center of the leaf rosette were examined (Table I), a completely different picture was observed. The strong-light treatment did not provoke loss of pigments as was observed in ML. npq1 YL were very similar to wild-type YL with respect to the pigment concentration (excluding, of course, A and Z), both in low light and in high light. In low light there was no significant difference between YL and ML of wild type and npq1 (data not shown). No synthesis of Z or A took place during high-light treatment of npq1 YL (data not shown). In the wild type, the steady-state epoxidation status of the xanthophyll cycle was roughly similar in YL and ML ([A + Z]/[V + A + Z] ≈ 0.45) in the experiment (Table I).
The 3-d light treatment caused a pronounced accumulation of α-tocopherol in both wild-type and npq1 leaves (Table I). This accumulation was particularly marked in npq1 YL (92 μg g−1 fresh weight versus 79 μg g−1 in wild-type YL), whereas npq1 ML accumulated less α-tocopherol than all other types of leaves (44 μg g−1). γ-Tocopherol was also found in high-light-treated leaves, whereas only traces of this compound were detected in leaves grown at moderate PPFD. Again, accumulation of γ-tocopherol was the most pronounced in npq1 YL.
Photooxidative Damage and Photosynthesis in YL and ML of npq1 and Wild Type
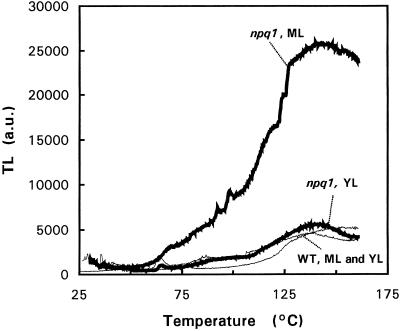
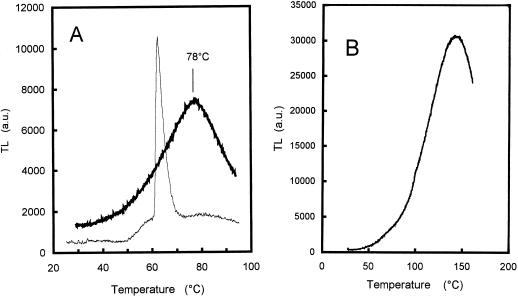
Accumulation of the lipophilic antioxidants, α-tocopherol and γ-tocopherol, at high PPFDs suggests the formation of 1O2 in the chloroplasts (Logan et al., 1998). We have previously shown that the absence of xanthophyll cycle is associated with an increased susceptibility to photooxidative damage of the chloroplasts (Havaux and Niyogi, 1999), and this phenomenon is reflected here by the loss of pigments in ML of high-light-grown npq1 plants. The unsuccessful adaptation of npq1 to photooxidative conditions was checked by thermoluminescence (TL) measurements (Fig. 2). The high-temperature TL bands emitted by Chl-containing material at temperatures higher than 70°C are independent of pre-illumination and are attributed to lipid peroxidation products (Vavilin and Ducruet, 1998). Light emission is believed to result from interaction of compounds such as oxy- and peroxy-radicals or dioxetanes leading to generation of triplet carbonyls (Cadenas, 1984), which can interact with Chl a molecules (Sharov et al., 1996), resulting in luminescence emission. The amplitude of the high-temperature TL bands, usually peaking at 70°C to 90°C and 115°C to 135°C, is well correlated with the amount of lipid peroxides present in the thylakoid membranes, providing a good indicator of the lipid peroxidation status of thylakoid membranes (Hideg and Vass, 1993; Stallaert et al., 1995; Marder et al., 1998; Vavilin and Ducruet, 1998; Havaux and Niyogi, 1999). The relative amplitude of the two lipid peroxide-related TL bands has been shown to depend on the water content of the leaf samples and on the rate of dehydration during TL heating (Ducruet and Vavilin, 1999; see also below). The main advantages of the TL method are that the level of lipid peroxidation is measured in situ and that the signal reflects peroxidative damage of the thylakoid membranes only.
Figure 2.
TL curves of Arabidopsis ML and YL (wild type and npq1 mutant) treated for 3 d at 1,500 μmol m−2 s−1. Thick lines, npq1; thin lines, wild type.
Figure 2 shows the TL curves of YL and ML from wild-type and npq1 plants treated at 1,500 μmol m−2 s−1 for 3 d. As described previously (Havaux and Niyogi, 1999), npq1 ML exhibited a very strong luminescence band at 135°C, indicating accumulation of lipid hydroperoxides and peroxidative degradation of thylakoid membrane lipids in the absence of the V cycle. However, npq1 YL had a very low TL signal in the 25°C to 150°C region that was indistinguishable from both YL and ML leaves of the wild type.
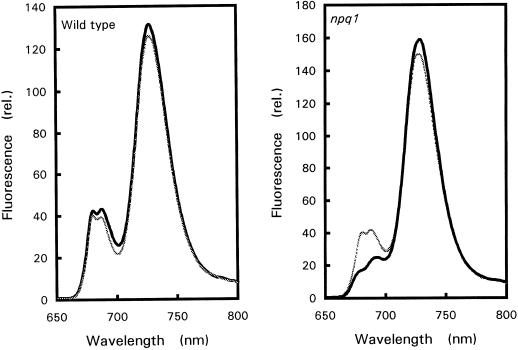
Figure 3 shows the 77 K Chl-fluorescence emission spectra of high-light-treated YL and ML. The fluorescence bands at approximately 680 and 690 nm are attributed to PSII, whereas the 730-nm band is due to the photosystem I (PSI) LHC (Krause and Weis, 1984; Govindjee, 1995). npq1 ML noticeably differed from wild-type YL and ML and from npq1 YL; the PSII bands were substantially reduced compared to the PSI band. This confirms that the pigment system of npq1 ML was more sensitive to photodestruction than that of npq1 YL or wild-type leaves and suggests that pigment destruction in npq1 leaves preferentially affected PSII.
Figure 3.
77 K Chl fluorescence emission spectra of Arabidopsis leaves (YL, thin gray lines; ML, thick black lines) treated for 3 d at a PPFD of 1,500 μmol m−2 s−1.
The results from room-temperature Chl-fluorescence and photoacoustic measurements (Table II) showed that strong-light stress had little or no effect on wild-type leaves and npq1 YL. In contrast, photochemistry was inhibited in npq1 ML, as indicated by the lowering of the quantum yield of PSII-mediated electron transport (ΦPSII), the quantum yield of oxygen evolution, and the efficiency of photochemical energy storage. Under control light conditions (250 μmol m−2 s−1), the photosynthetic characteristics of YL and ML were identical in both genotypes.
Table II.
Photosynthetic characteristics of YL and ML of wild type and npq1 Arabidopsis determined by Chl fluorometry and photoacoustics before and after high-light stress (3 d at 1,500 μmol m−2 s−1)
| Treatment | Fluroescence (ΦPSII) | Photoacoustics
|
|
|---|---|---|---|
| ΦO2a | ESb | ||
| Control | |||
| WT ML | 0.69 ± 0.01 | 2.39 ± 0 | 20 ± 3 |
| WT YL | 0.69 ± 0.01 | 2.31 ± 0.15 | 17 ± 4 |
| npq1 ML | 0.68 ± 0.02 | 2.59 ± 0.12 | 22 ± 5 |
| npq1 YL | 0.68 ± 0.02 | 1.87 ± 0.25 | 18 ± 2 |
| Light treated | |||
| WT ML | 0.60 ± 0.09 | 2.09 ± 0.41 | 16 ± 5 |
| WT YL | 0.65 ± 0.02 | 2.55 ± 0.25 | 24 ± 3 |
| npq1 ML | 0.36 ± 0.18 | 1.24 ± 0.33 | 10 ± 4 |
| npq1 YL | 0.61 ± 0.07 | 2.83 ± 0.45 | 21 ± 10 |
Data are mean values of more than three separate experiments ± sd. Measurements were performed in white light at a PPFD of 100 μmol m−2 s−1.
ΦO2, Quantum yield of oxygen evolution.
ES, Photochemical energy storage.
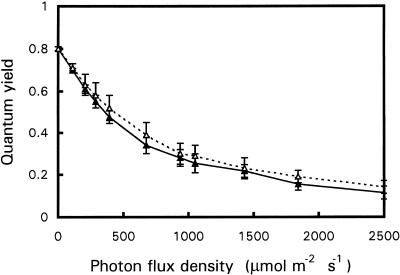
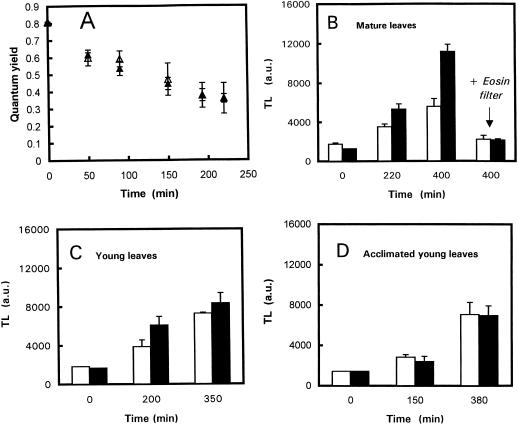
The ability of npq1 YL to tolerate high light was not due to an intrinsically higher efficiency of PSII electron transport or an improved capacity for NPQ. Figure 4 shows that ΦPSII in npq1 YL (grown in moderate light) was not significantly different from that of ML when exposed to a wide range of PPFDs. Although the absence of the V cycle in npq1 resulted in a marked reduction of the NPQ of Chl fluorescence, as previously demonstrated (Niyogi et al., 1998), Table III shows that acclimation to strong white light did not restore NPQ to the wild-type level even in developing npq1 YL. However, measurements of 77 K Chl fluorescence showed that acclimated npq1 YL had a slightly different distribution of light energy between PSII and PSI, in favor of PSI (Fig. 3). The ratio of the Chl fluorescence emission at 730 nm to the Chl fluorescence emission at 680 nm (measured at 77 K) was 3.83 ± 0.36 and 4.31 ± 0.45 in wild-type ML and YL, respectively. In acclimated npq1 YL, the ratio was 4.79 ± 0.65, and it increased to 8.66 ± 2.21 in stressed npq1 ML.
Figure 4.
ΦPSII in YL (▵) and ML (▴) of npq1 (grown at 250 μmol m−2 s−1) at different PPFDs. Data are mean values of four separate experiments ± sd.
Table III.
NPQ in wild-type and npq1 Arabidopsis leaves before and after 3 d at a PPFD of 1,500 μmol m−2 s−1
| Treatment | NPQ |
|---|---|
| Control | |
| Wild type | |
| YL | 2.36 ± 0.24 |
| ML | 2.40 ± 0.03 |
| Npq1 | |
| YL | 0.74 ± 0.08 |
| ML | 0.49 ± 0.08 |
| Light acclimated | |
| Wild type | |
| YL | 3.01 ± 0.24 |
| ML | 1.94 ± 0.44 |
| Npq1 | |
| YL | 0.69 ± 0.07 |
| ML | 0.41 ± 0.11 |
NPQ was measured in the steady state after 15 min of illumination with a white light at a PPFD of 1,600 μmol m−2 s−1. Data are mean values of four separate experiments ± sd.
Leaf Infiltration with Eosin
Arabidopsis YL and ML were exposed to 1O2 toxicity by infiltrating leaf discs with eosin, a well-known generator of 1O2 in the light (Knox and Dodge, 1985). Leaf discs floating on the aqueous solution of eosin were illuminated with white light at a PPFD of 500 μmol m−2 s−1. This treatment resulted in a marked lipid peroxidation as reflected by the appearance of a strong TL band at 80°C (Fig. 5A). The 135°C TL band (observed in high-light-treated plants, Fig. 2) was attenuated in this experiment because of the high water content of the leaf discs floating on the aqueous solution of eosin. Indeed Ducruet and Vavilin (1999) have shown that the 80°C/90°C TL band is actually a pseudoband resulting from a competition between thermolysis of peroxides (corresponding to the rising edge of the 130°C/140°C TL band) and a non-radiative hydrolysis below 100°C in wet samples. Consequently, the amount of water retained within the sample during TL warming is a prominent factor governing the TL emission at high temperature. The water content of leaf discs floating on eosin for 5 h was observed to be higher than that of control leaves (92.9% versus 91.4%). When the water content of eosin-treated leaf discs was reduced by leaving the discs on the laboratory bench for 1 h in the dark (resulting in a leaf water content of 86.8%), the 80°C TL band was converted into a 135°/140°C band (Fig. 5B), thus confirming the prominent role of water in the relative amplitude of the two TL bands. The 80°C TL band shown in Figure 5A and the 135°C TL band shown in Figure 2 reflect then similar photooxidative damage in the leaf tissues.
Figure 5.
Eosin-induced lipid peroxidation in Arabidopsis leaf discs. A, TL curve of a wild-type Arabidopsis leaf disc before (thin line) and after (thick line) illumination (500 μmol m−2 s−1, 375 min) in the presence of eosin. The sharp 65°C TL band in control leaves is typical of Arabidopsis (and was not observed in other plant species such as tobacco, potato, or barley). The origin of this band is unknown; it is not related to lipid peroxidation and could be due to thermolysis of a (yet unidentified) volatile compound (Ducruet and Vavilin, 1998). B, TL was measured in a leaf disc kept for 1 h on filter paper in the dark after light stress (500 μmol m−2 s−1, 375 min) in the presence of eosin. The leaf water content decreased from 92.8% to 86.8% during this treatment.
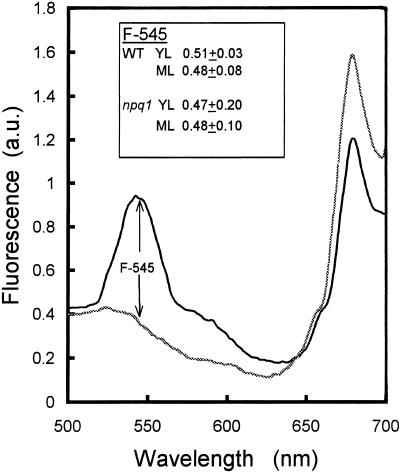
The different types of leaves used in this study took up the same amount of eosin. Eosin-infiltrated leaves illuminated at 390 nm exhibited a strong fluorescence band in the green spectral region peaking at approximately 545 nm, which was not found in control leaves (Fig. 6). The latter wavelength is very close to the maximal fluorescence wavelength of eosin in solution (approximately 543 nm). In addition, the excitation spectrum of this band corresponded to the absorbance spectrum of eosin characterized by a maximum at approximately 390 nm and a shoulder at approximately 430 nm (data not shown). There was no significant difference in the amplitude of the 545-nm band between YL and ML or between wild type and npq1 leaves (Fig. 6, inset), indicating comparable concentrations of eosin in the leaf tissues.
Figure 6.
In vivo fluorescence emission of Arabidopsis leaves (wild type) excited at 390 nm. Thick black line, Leaf disc infiltrated with eosin for 5 h; thin gray line, control leaf disc. Inset, Amplitude of eosin fluorescence at 545 nm in wild-type and npq1 leaf discs. The excitation spectrum of the fluorescence at 545 nm (not shown) exhibited a peak at approximately 390 nm and a shoulder at around 430 nm.
As expected from the similar pigment content of npq1 and wild type (Table I), the rate of eosin excitation in the blue region did not differ between the different samples. This was determined by measuring light absorption in the blue spectral region (400–490 nm) using photoacoustics. The amplitude of the light-saturated photothermal signal (Apt) is proportional to the light absorption of the sample (Malkin and Canaani, 1994) and can thus be used as a measure of leaf absorbance. Average values of Apt (in arbitrary units) were similar in all leaves examined: 6.12 ± 0.75 and 6.18 ± 0.25 in wild-type ML and YL, respectively, and 6.03 ± 0.19 and 6.35 ± 0.14 in npq1 ML and YL, respectively. From the latter results and the control experiments shown in Figures 5 and 6, the rate of 1O2 formation from eosin excitation appeared to be similar in the different types of leaves examined in this study. Therefore, different responses to the eosin treatment can be interpreted in terms of differential tolerance to 1O2 toxicity.
Oxidative Damage in YL and ML of npq1 and Wild Type Exposed to Eosin-Generated 1O2
Figure 7A shows that 1O2 generated by eosin caused PSII photoinhibition. However, the degree of photoinhibition was similar in the wild type and the npq1 mutant. In contrast Figure 7B shows that lipid peroxidation was more pronounced in npq1 ML samples than in wild-type samples. After 400 min in the light, the amplitude of the 80°C TL band was 2-fold higher in npq1 compared to wild type. When leaves were irradiated through a filter of eosin to prevent excitation of the dye, no lipid peroxidation was observed in npq1 or wild-type leaves, indicating that lipid peroxidation resulted from 1O2 generation by eosin and not via Chl excitation. Consequently, results shown in Figure 7B show that npq1 chloroplasts are more vulnerable to 1O2-induced damage than wild-type chloroplasts. The same phenomenon was observed with YL (Fig. 7C), although the difference between npq1 and the wild type was less marked than that observed with ML. When YL acclimated to the strong white light (3 d at 1,500 μmol m−2 s−1) were illuminated with white light at a PPFD of 500 μmol m−2 s−1 in the presence of eosin (Fig. 7D), lipid peroxidation in npq1 was reduced compared with nonacclimated npq1 leaves (compare with Fig. 7C), and no difference was found between npq1 and the wild type. Qualitatively similar results were obtained when light-acclimated leaves were exposed to a greater light stress (1,500 μmol m−2 s−1). One can conclude that npq1 YL became resistant to 1O2 during light acclimation.
Figure 7.
A, PSII photoinhibition (estimated by the decrease in the maximal quantum yield of PSII photochemistry Fv/Fm) in mature Arabidopsis leaf discs (wild type, white symbols; npq1, black symbols) during light stress (500 μmol m−2 s−1) in the presence of eosin. B through E, Change in the amplitude of the 80°C TL band in wild-type (white bars) and npq1 (black bars) Arabidopsis leaves illuminated in the presence of eosin. B, ML exposed to a PFD of 500 μmol m−2 s−1 (white light or light filtered through eosin) + eosin. (A + Z)/(A + Z + V) = 0 for npq1 and 0.50 for wild type. C, YL exposed to 500 μmol m−2 s−1 + eosin. D, YL taken from plants treated for 3 d at 1,500 μmol m−2 s−1 illuminated in the presence of eosin with a white light of PPFD 500 μmol photons m−2 s−1. (A + Z)/(A + Z + V) = 0.04 for npq1 and 0.71 for wild type. Data are the means of a minimum of three separate experiments ± sd.
DISCUSSION
The photosensitivity of the photosynthetic apparatus of Arabidopsis plants increases markedly in the absence of the xanthophyll cycle. Indeed we observed a substantial loss of photosynthetic pigments (Fig. 1; Table I) and an inhibition of the photochemical activity of the chloroplasts (Table II) when npq1 plants were transferred to high PPFD in the range 1,000 to 1,500 μmol m−2 s−1. Both phenomena, which were not observed in wild-type plants, probably resulted from photooxidative damage as revealed by in vivo TL measurements. The strong 135°C TL band recorded in npq1 ML pre-exposed to high light (Fig. 2) indicated peroxidative damage of thylakoid membrane lipids (Vavilin and Ducruet, 1998). Thus, the presence of Z and A during strong illumination noticeably reinforced the tolerance of the photosynthetic membranes to photooxidation, in agreement with previous work (Havaux and Niyogi, 1999). In this study, we observed that a mutant of Arabidopsis (npq4) characterized by a strongly diminished NPQ (but normal V cycle; see Li et al., 2000) was much less photosensitive than npq1 (with both NPQ and V cycle inhibited), indicating that photoprotection requires not only NPQ but also the physical presence of de-epoxidized xanthophylls.
The photoprotective action of the V cycle seems to be particularly important to preserve the PSII function in high light (Fig. 3; Table II). Loss of PSII activity has been reported to be closely related to thylakoid membrane lipid photodestruction under certain light stress conditions (Sharma and Singhal, 1992; Hideg et al., 1994). Oxidative stress induced by exogenous compounds, such as eosin or the fungal elicitor cryptogein, was also reported to cause a preferential destruction of PSII (Knox and Dodge, 1985; Stallaert et al., 1995). However, it is difficult to decide from our results whether PSII destruction occurred after lipid peroxidation or if lipid peroxidation was induced by PSII photoinhibition.
Experiments with the photosensitizing dye eosin (Fig. 7) showed that photooxidation of npq1 ML could result from an increase in the intrinsic sensitivity of the thylakoid membranes to active forms of oxygen. Generation of 1O2 during illumination of the dye caused lipid peroxidation, as expected (Knox and Dodge, 1985), and this phenomenon was significantly amplified in npq1 leaf discs deficient in A and Z compared to wild-type leaf samples containing high levels of Z (Fig. 7, B and C). Thus, the absence of de-epoxidized xanthophylls formed in the V cycle was associated with an increased sensitivity of thylakoid membrane lipids toward the toxicity of 1O2 and perhaps of other active forms of oxygen. This cannot be attributed to different NPQ activities since, in our experiments, 1O2 was generated by direct excitation of eosin. When excitation of the dye was prevented by an appropriate light filter, no lipid peroxidation was noticed, although the PPFD of the (red) light reaching the leaf samples and the level of photoinduced NPQ were unchanged (Fig. 7B). Moreover, the enhanced lipid peroxidation in eosin-treated npq1 leaves was not accompanied by an increase in PSII inhibition relative to wild-type leaves (Fig. 7A).
Chl excitation can result in 1O2 formation in the LHCs or PSII reaction centers, whereas exogenously added eosin is expected to generate 1O2 throughout the thylakoid membrane. Because the npq1 mutant was more susceptible to eosin-induced lipid peroxidation, it seems that the protective effect of Z and A is not restricted to the LHCs but may also involve Z and A that are located in the thylakoid lipid matrix (Havaux, 1998a). This does not exclude, of course, that the V-cycle-dependent NPQ also protects thylakoid from photodestruction by deactivating singlet-excited Chl and by reducing the probability of triplet Chl and 1O2 formation in the LHCs.
Because of their higher number of conjugated double bonds, Z and A are better photoprotectors than V, with a higher efficiency for de-exciting 1O2 (Mathews-Roth et al., 1974) and, consequently, the appearance of Z in the LHCs and/or in their immediate surroundings can enhance the resistance of the photosystems to oxygen toxicity. Carotenoids can also trap various types of free radicals and, when incorporated into artificial lipid membranes, they protect them from being oxidized (Krinsky, 1979). The latter effect was very obvious in liposomes incorporated with Z (Lim et al., 1992; Sielewiesiuk et al., 1997; Woodall et al., 1997). This high protection efficiency of Z could be related to the location of the xanthophyll molecules in the lipid bilayer (Oshima et al., 1993); Z is oriented to the parallel to the hydrocarbon chain of lipids (Gruszecki and Sielewiesiuk, 1990) whereas V, particularly in the cis configuration (Yamamoto and Bangham, 1978; Gruszecki et al., 1999), and non-polar carotenes (such as β-carotene; Van de Ven et al., 1984; Gabrielska and Gruszecki, 1996) orient their long isoprenoid chain perpendicular to the lipid acyl group. Several authors have provided data suggesting that the vertical orientation of Z is very favorable for protection against oxidation at all depths in the hydrophobic lipid phase (Subczynski et al., 1991; Woodall et al., 1997; Berglund et al., 1999).
Although the exact localization and orientation of the xanthophyll-cycle pigments in native thylakoid membranes are not known yet, there is support for a dynamic equilibrium between protein complex-associated xanthophylls and lipid matrix-localized xanthophylls, at least during strong light treatment (Hager and Holocher, 1994; Rockholm and Yamamoto, 1996; Tardy and Havaux, 1997; Bugos et al., 1998; Havaux, 1998a; Ruban et al., 1999; Gruszecki et al., 1999). If this is the case (and considering the in vitro data reported above), the transient presence of Z in the thylakoid membrane lipid matrix could be an efficient system for protecting membrane lipids from destruction by active forms of oxygen generated in strong light (or via eosin).
Another important observation of this study is that developing npq1 YL were much less affected by the absence of the V cycle than well-developed ML. Previous studies of npq mutants did not take into account the influence of leaf age on the responses of the photosynthetic apparatus to light stress (Niyogi et al., 1998; Havaux and Niyogi, 1999). Eosin caused less damage to YL (Fig. 7, B and C), and high-light stress of whole npq1 plants did not lead to significant photosynthetic perturbations and photooxidation in YL in the center of the leaf rosette, in contrast to ML, which were severely damaged (Figs. 2 and 3; Tables I and II). Very recently, Bugos et al. (1999) reported that V de-epoxidase mRNA, protein, and activity levels are developmentally regulated in tobacco. V de-epoxidase protein and activity were very low in YL, but the levels were much higher in ML. This could indicate that YL have some special features that decrease the need for an active V cycle and, if this is the case in Arabidopsis too, this could be a possible reason why YL of npq1 were quite similar to wild-type YL. However, in this study, the steady-state epoxidation status of the V-cycle pigments did not differ much between wild-type YL and ML, although no extensive analysis of the characteristics of the V cycle (rate of V photoconversion, light dependence of Z accumulation, etc.) was performed.
It was also observed that acclimation to high light for 3 d increased the phototolerance of npq1 YL to the wild-type level (Fig. 7D). Again, the differential phototolerance of npq1 YL and ML cannot be explained by different NPQ activities; NPQ was drastically inhibited in both npq1 YL and ML, and growth of npq1 plants in strong light was not associated with an increase in NPQ in either YL or ML (Table III). Moreover, it is unlikely that the higher phototolerance of npq1 YL compared to ML is due to a higher photosynthetic activity, as shown by the results of Figure 4. Developing leaves were able to acclimate to high-light irradiance in such a way that the V cycle was not required to prevent photooxidative damage, and this acclimation did not involve NPQ. Thus, photoprotection by the xanthophyll cycle seems to be relevant mainly to environmental situations where rapid changes in PFD can occur and leaves can be transiently exposed to bright light.
Acclimation of npq1 YL to excess light may involve a lowering of the rate of generation of active oxygen species in the chloroplasts and/or a stimulation of photoprotective and repair mechanisms (Melis, 1991; Demmig-Adams and Adams, 1992). Photosynthetic adaptation to high-light irradiance usually involves a selective decrease in the Chl b-containing LHCs of PSII, and this probably occurred in our Arabidopsis plants as indicated by the increase in the Chl a to Chl b ratio (Table I). However, the latter ratio did not differ significantly between YL of light-acclimated npq1 and wild-type plants. We also observed that acclimated npq1 YL had a slightly higher ratio of the Chl fluorescence emission at 730 nm to the Chl fluorescence emission at 680 nm (measured at 77K; Fig. 3), suggesting a change in the light distribution between the two photosystems in favor of PSI. Growth of Arabidopsis plants at high-light intensities also induced accumulation of carotenoids, particularly those involved in the V cycle (Table I), but npq1 YL did not differ significantly from wild-type leaves with respect to the carotenoid level.
In contrast npq1 YL exposed to strong light accumulated considerable amounts of vitamin E (α- and γ-tocopherol) to a level significantly higher than that found in wild-type leaves. This increase in vitamin E may represent an acclimative response of the npq1 mutant to compensate for the lack of Z and A in the photosynthetic membranes. Both α- and γ-tocopherol have an antioxidant and stabilizing action in biomembranes (Di Mascio et al., 1990; Fryer, 1992). Accumulation of vitamin E in npq1 leaves indicates that membrane lipids are one of the chloroplast components that are the most affected by the lack of V cycle, since vitamin E is located exclusively in the thylakoid membrane lipid matrix. One should note, however, that nonacclimated npq1 YL were observed to be more tolerant to 1O2 toxicity than older leaves (Fig. 7), although their tocopherol content was identical (approximately 19 μg g−1 fresh weight for both types of leaves), indicating that factors different from the vitamin E content are also involved in the differential phototolerance of npq1 YL and ML. It is known that the lipid composition of leaves can change substantially with maturity (Liljenberg and Von Arnold, 1987) and, consequently, one cannot exclude that chloroplast lipids are less sensitive to photooxidative damage when leaves are in an early stage of development. Carlsson et al. (1996) reported that pea and wheat YL were less sensitive to oxidation by ozone than older leaves, with marked changes in lipid composition being observed in the latter leaves.
In summary, this study has shown that the protective action of the V cycle against photodestruction of the chloroplast was obvious only in well-developed ML of Arabidopsis. Loss of the V cycle had no such effect in developing YL due to some compensatory mechanisms or some special features of the leaves. Therefore, the absence of an active V cycle does not necessarily result in leaf photooxidative damage at high-light intensities. This study has also confirmed that part of the photoprotective action of the V cycle involves a site of action that is distinct from the LHCs where NPQ occurs: The presence of Z itself in the thylakoid membranes enhances the tolerance of thylakoids to lipid peroxidation. The molecular mechanism by which Z exerts its “direct” antioxidant action in the thylakoid membrane remains to be established.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The npq1-2 mutant of Arabidopsis, affected in the structural gene of V de-epoxidase (Niyogi et al., 1998), and the wild type (ecotype Columbia) were grown under controlled conditions of temperature (22°C/18°C, day/night), air humidity (60%), and light (100, 250, 500, 1,000, and 1,500 μmol m−2 s−1). Plants were first grown from seeds for 4 weeks at a PPFD of 250 μmol photons m−2 s−1 and were subsequently transferred to the final PPFD where they were kept for 3, 10, or 21 d, depending on the experiments. Photoperiod was 8 h except when plants were exposed to the highest PPFD (1,500 μmol m−2 s−1) for 3 d (photoperiod, 15 h). PPFDs were measured with a LI-COR quantum meter (Li-185B/Li-190SB, LI-COR, Lincoln, NE).
Two types of leaves were examined in this study: fully developed ML and developing YL. The so-called YL were the leaves in the center of the leaf rosette with 1.5- to 2-cm length. The average length of the ML in the rosette was 4.1 ± 0.3 cm. We did not characterize the metabolic status of the leaves, but the transition from sink to source leaves in Arabidopsis is known to occur at a relatively early stage of development (Truernit and Sauer, 1995). Little growth occurred during light stress (3 d at 1,500 μmol m−2 s−1).
Eosin Treatments
Eosin was used to generate 1O2 in the light (Knox and Dodge, 1985). Leaf discs of 1-cm diameter were floated on eosin (aqueous solution of eosin Y 5% [w/v], Sigma, St. Louis) and were illuminated with white light at a PPFD of 500 μmol m−2 s−1 produced by a 150-W metal halide lamp (Osram, Munich, Germany). The temperature of the eosin solution was maintained constant at 22°C. In one experiment, a 1-cm thickness of eosin was used as a filter to allow irradiation of the samples without excitation of the dye. A PPFD of 500 μmol m−2 s−1 was transmitted by this filter. We checked that white light and the filtered light at a PPFD of 500 μmol m−2 s−1 induced the same level of NPQ. Eosin concentration in the leaf discs was estimated from the in vivo fluorescence emission of eosin (see below).
Photosynthetic Pigments and Tocopherols
Photosynthetic pigments were extracted in methanol. After centrifugation and filtration, pigments were separated and quantified by HPLC as previously described (Havaux and Tardy, 1996). Tocopherols were extracted in N,N-dimethylformamide and were quantified by HPLC as described elsewhere (Wildi and Lütz, 1996), except that the HPLC system was from Thermo Separations (Egelsbach, Germany). Tocopherols alternatively were extracted in methanol and were quantified by HPLC using a liquid chromatograph (model 5000, Varian, Palo Alto, CA) with a C-18 column (Alltima Peek, 5 μm, Alltech, Deerfield, IL) in methanol. Tocopherol was detected by fluorescence (excitation at 290 nm; emission at 330 nm) with an RF-530 system (Shimadzu, Tokyo).
Chl Thermoluminometry
The TL emitted by leaf discs (five discs of 6-mm diameter) was measured with a laboratory-built apparatus (Havaux, 1998b). The samples were slowly heated at a rate of 6°C min−1 from 25°C to 150°C. Temperature was monitored with a K-type thermocouple. The luminescence emission was recorded with a R376 photomultiplier tube (Hamamatsu Photonics, Hamamatsu City, Japan). The current from the photomultiplier was amplified by a 70710 transimpedance preamplifier (Oriel, Stratford, CT) and was recorded by a computer equipped with a DAQPad-1200 acquisition card (National Instruments, Austin, TX) and software written by J.-M. Ducruet (Commissariat à l'Energie Atomique/Saclay, France).
Chl Fluorometry
In vivo Chl fluorescence from the upper surface of the leaves was measured with a PAM-101 fluorometer (Walz, Effeltrich, Germany; Bolhar-Nordenkampf et al., 1989), as previously described (Havaux and Tardy, 1996). The initial level Fo of Chl fluorescence was excited by a dim red light modulated at 1.6 kHz. The maximal level of Chl fluorescence was induced by an 800-ms pulse of intense white light (4,500 μmol photons m−2 s−1). From the maximal fluorescence level (Fm′) and the steady-state fluorescence level (Fs) emitted by illuminated leaves, the actual ΦPSII was calculated as (Fm′ − Fs)/Fm′. The maximal quantum yield of PSII photochemistry was measured in dark-adapted leaves as (Fm − Fo)/Fm = Fv/Fm, where Fm is the maximal fluorescence level in the dark. NPQ was determined as (Fm/Fm′) − 1 (Bilger and Björkman, 1994). Chl fluorescence emission spectra were measured in liquid nitrogen (77K) using an LS50B luminescence spectrometer (Perkin-Elmer, Beaconsfield, UK) equipped with fiber optics. Fluorescence was excited at 440 nm. Fluorescence of eosin infiltrated in leaf discs (for 5 h) was measured at room temperature with a 390-nm exciting light. Leaf discs were carefully rinsed with distilled water before fluorescence measurements.
Photoacoustic Spectroscopy
The photoacoustic signals generated by leaf discs of 1-cm diameter were measured with a laboratory-built photoacoustic spectrometer that has been described (Havaux and Tardy, 1996). The leaf sample placed in the hermetically closed photoacoustic cell was illuminated with white light (<700 nm, 100 μmol m−2 s−1) modulated at 19 Hz. Photochemistry was saturated with a strong background light of 4,500 μmol m−2 s−1. The photobaric signal (due to modulated oxygen evolution; amplitude, Aox) was separated from the photothermal signal using a well-documented procedure involving light saturation of photosynthesis and phase adjustment in the lock-in amplifier (Malkin and Canaani, 1994). The relative difference between the actual photothermal signal (Apt′) and the maximal thermal signal (recorded in the presence of the strong background light; amplitude, Apt) was estimated at a high frequency of 370 Hz, where the photobaric signal is completely damped out. Photochemical energy storage was calculated from the actual and maximal levels of the high-frequency photothermal signal (Malkin and Canaani, 1994) as follows: (Apt − Apt′)/Apt. The quantum yield of oxygen evolution was estimated (in relative values) by the ratio of Aox to Apt, taking into account the energy storage (Malkin and Canaani, 1994). Apt was also measured in modulated blue light (19 Hz, 400–490 nm, 6 μmol m−2 s−1) using an Oriel 57530 interference filter.
ACKNOWLEDGMENTS
Many thanks to J.-M. Ducruet (Commissariat à l'Energie Atomique/Saclay, France) for help with Chl TL and to R. Strasser (University of Geneva) for the loan of fluorescence accessories and helpful discussion.
LITERATURE CITED
- Berglund AH, Nilsson R, Liljenberg C. Permeability of large unilamellar digalactosyldiacylglycerol vesicles for protons and glucose: influence of α-tocopherol, β-carotene, zeaxanthin and cholesterol. Plant Physiol Biochem. 1999;37:179–186. [Google Scholar]
- Bilger W, Björkman O. Relationships among violaxanthin deepoxidation, thylakoid membrane conformation, and nonphotochemical chlorophyll fluorescence quenching in leaves of cotton (Gossypium hirsutum L.) Planta. 1994;193:238–246. [Google Scholar]
- Bolhar-Nordenkampf HR, Long SP, Baker NR, Öquist G, Schreiber U, Lechner EG. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Funct Ecol. 1989;3:497–514. [Google Scholar]
- Bugos RC, Chang S-H, Yamamoto HY. Developmental expression of violaxanthin de-epoxidase in leaves of tobacco growing under high and low light. Plant Physiol. 1999;121:207–213. doi: 10.1104/pp.121.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugos RC, Hieber AD, Yamamoto HY. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem. 1998;273:15321–15324. doi: 10.1074/jbc.273.25.15321. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biological chemiluminescence. Photochem Photobiol. 1984;40:823–830. doi: 10.1111/j.1751-1097.1984.tb04657.x. [DOI] [PubMed] [Google Scholar]
- Carlsson AS, Wallin G, Sandelius AS. Species- and age-dependent sensitivity to ozone in young plants of pea, wheat and spinach: effects in acyl lipid and pigment content and metabolism. Physiol Plant. 1996;98:271–280. [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Demmig-Adams B, Winter K, Krüger A, Czygan F-C. Zeaxanthin synthesis, energy dissipation, and photoprotection of photosystem II at chilling temperatures. Plant Physiol. 1989;90:894–898. doi: 10.1104/pp.90.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio P, Devasagayam TPA, Kaiser S, Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- Ducruet J-M, Vavilin D. Proceedings of the Winter Meeting of the Society of Free Radical Research, Granada, Spain, December 1998. 1998. Experimental conditions for using high-temperature chlorophyll thermoluminescence as an indicator of oxidative stress. [Google Scholar]
- Ducruet J-M, Vavilin D. Chlorophyll high-temperature thermoluminescence emission as an indicator of oxidative stress: perturbating effects of oxygen and leaf water. Free Radic Res. 1999;31:187–192. doi: 10.1080/10715769900301491. [DOI] [PubMed] [Google Scholar]
- Eskling M, Arvidsson P-O, Akerlund HE. The xanthophyll cycle, its regulation and components. Physiol Plant. 1997;100:806–816. [Google Scholar]
- Fryer MJ. The antioxidant effects of thylakoid vitamin E (α-tocopherol) Plant Cell Environ. 1992;15:381–392. [Google Scholar]
- Gabrielska J, Gruszecki WI. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim Biophys Acta. 1996;1285:167–174. doi: 10.1016/s0005-2736(96)00152-6. [DOI] [PubMed] [Google Scholar]
- Govindjee Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol. 1995;22:131–160. [Google Scholar]
- Gruszecki WI, Grudzinski W, Banaszek-Glos A, Matula M, Kernen P, Krupa Z, Sielewiesiuk J. Xanthophyll pigments in light-harvesting complex II in monomolecular layers: localization, energy transfer and orientation. Biochim Biophys Acta. 1999;1412:173–183. doi: 10.1016/s0005-2728(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Gruszecki WI, Sielewiesiuk J. Galactolipid multilayers modified with xanthophylls: orientation and diffractometric studies. Biochim Biophys Acta. 1991;1069:21–26. doi: 10.1016/0005-2736(91)90099-t. [DOI] [PubMed] [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998a;3:147–151. [Google Scholar]
- Havaux M. Probing electron transport through and around photosystem II in vivo by the combined use of photoacoustic spectroscopy and chlorophyll fluorometry. Isr J Chem. 1998b;38:247–256. [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photo-oxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Tardy F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta. 1996;198:324–333. [Google Scholar]
- Hideg E, Spetea C, Vass I. Singlet oxygen and free radical production during acceptor- and donor-side-induced photoinhibition: studies with spin trapping EPR spectroscopy. Biochim Biophys Acta. 1994;1186:143–152. [Google Scholar]
- Hideg E, Vass I. The 75°C thermoluminescence band of green tissues: chemiluminescence from membrane-chlorophyll interaction. Photochem Photobiol. 1993;58:280–283. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Knox JP, Dodge AD. The photodynamic action of eosin, a singlet-oxygen generator. Planta. 1985;164:22–34. doi: 10.1007/BF00391021. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence as a tool in plant physiology: II. Interpretation of fluorescence signals. Photosynth Res. 1984;5:139–157. doi: 10.1007/BF00028527. [DOI] [PubMed] [Google Scholar]
- Krinsky NI. Carotenoid protection against oxidation. Pure Appl Chem. 1979;51:649–660. [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- Liljenberg C, Von Arnold S. Effects of physiological and ontogenetical aging on membrane lipid levels in pea leaves (Pisum sativum) J Plant Physiol. 1987;130:255–265. [Google Scholar]
- Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1120:178–184. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- Logan BA, Demmig-Adams B, Adams WW, III, Grace SC. Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDS in the field. J Exp Bot. 1998;49:1869–1879. [Google Scholar]
- Malkin S, Canaani O. The photoacoustic method and its characteristics in use for the study of photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:493–526. [Google Scholar]
- Marder JB, Droppa M, Caspi V, Raskin VI, Horvath G. Light-independent thermoluminescence from thylakoids of greening barley leaves: evidence for involvement of oxygen radicals and free chlorophyll. Physiol Plant. 1998;104:713–719. [Google Scholar]
- Mathews-Roth MM, Wilson T, Fujimori E, Krinsky NI. Carotenoid chromophore length and protection against photosensitization. Photochem Photobiol. 1974;19:217–222. doi: 10.1111/j.1751-1097.1974.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima S, Ojima F, Sakamoto H, Ishiguro Y, Terao J. Inhibitory effect of β-carotene and astaxanthin on photosensitized oxidation of phospholipid bilayer. J Nutr Sci Vitaminol. 1993;39:607–615. doi: 10.3177/jnsv.39.607. [DOI] [PubMed] [Google Scholar]
- Rockholm DC, Yamamoto HY. Violaxanthin de-epoxidase: purification of a 43-kilodalton lumenal protein from lettuce by lipid-affinity precipitation with monogalactosyldiacylglyceride. Plant Physiol. 1996;110:697–703. doi: 10.1104/pp.110.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J Biol Chem. 1999;274:10458–10465. doi: 10.1074/jbc.274.15.10458. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Singhal GS. The role of thylakoid lipids in the photodamage of photosynthetic activity. Physiol Plant. 1992;86:623–629. [Google Scholar]
- Sharov VS, Briviba K, Sies H. Assessment of the C-525 laser dye as a chemiluminescence sensitizer for lipid peroxidation in biological membranes: a comparison with chlorophyll-a. Free Radic Biol Med. 1996;21:833–843. doi: 10.1016/0891-5849(96)00236-5. [DOI] [PubMed] [Google Scholar]
- Sielewiesiuk J, Matula M, Gruszecki WI. Photo-oxidation of chlorophyll a in digalactosyldiacylglycerol liposomes containing xanthophyll pigments: indication of a special photoprotective ability of zeaxanthin. Cell Mol Biol Lett. 1997;2:59–68. [Google Scholar]
- Stallaert VM, Ducruet J-M, Tavernier E, Blein J-P. Lipid peroxidation in tobacco leaves treated with the elicitor cryptogein: evaluation by high-temperature thermoluminescence emisison and chlorophyll fluorescence. Biochim Biophys Acta. 1995;1229:290–295. [Google Scholar]
- Subczynski WK, Markowska E, Sielewiesiuk J. Effects of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. Biochim Biophys Acta. 1991;1068:68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- Tardy F, Havaux M. Thylakoid membrane fluidity and thermostability during the operation of the xanthophyll cycle in higher-plant chloroplasts. Biochim Biophys Acta. 1997;1330:179–193. doi: 10.1016/s0005-2736(97)00168-5. [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta. 1995;196:564–570. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- Van de Ven M, Kattenberg M, Van Ginkel G, Levine YK. Study of the orientation ordering of carotenoids in lipid bilayers by resonance-Raman spectroscopy. Biophys J. 1984;45:1203–1210. doi: 10.1016/S0006-3495(84)84269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilin DV, Ducruet JM. The origin of 115–130°C thermoluminescence bands in chlorophyll containing material. Photochem Photobiol. 1998;68:191–198. [Google Scholar]
- Wildi B, Lütz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–146. [Google Scholar]
- Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta. 1997;1336:575–686. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY, Bangham AD. Carotenoid organization in membranes: thermal transition and spectral properties of carotenoid-containing liposomes. Biochim Biophys Acta. 1978;507:119–127. doi: 10.1016/0005-2736(78)90379-6. [DOI] [PubMed] [Google Scholar]