Abstract
Background:
Moderate sedation is required for out-patient transesophageal echocardiography (TEE). Our objective was to compare the effect of Ketofol and dexmedetomidine for outpatient procedural sedation in diagnostic TEE with a hypothesis that Ketofol would be as effective as dexmedetomidine.
Patients and Methods:
Fifty adult patients of age group 18-60 years with atrial septal defect, rheumatic valvular heart disease undergoing diagnostic TEE in the outpatient echocardiography laboratory were randomized into two groups, group D and group KF. GROUP D: Dexmedetomidine infusion -200 μg in 20 ml normal saline. GROUP KF: Ketofol infusion: (ketamine: propofol, 1mg: 3 mg in 20 ml syringe). Loading dose of drug at 1ml/kg/hour IV till Ramsay sedation score (RSS) ≥ 3 achieved followed by maintenance infusion at 0.05 ml/kg/hour till end of procedure.
Results:
The primary outcome - time to achieve Ramsay sedation score ≥ 3 was significantly lesser with Ketofol as compared to Dexmedetomidine 260[69] seconds vs 460 [137], (p value<0.05).
Conclusion:
In out-patient setting, ketofol is favourable over dexmedetomidine for sedation regimen for diagnostic TEE as lesser time is taken to achieve optimal sedation with lesser hemodynamic perturbations, post procedure complications and better cardiologist satisfaction.
Keywords: Dexmedetomidine, echocardiography, ketamine, ketofol, outpatient, propofol, transesophageal
Introduction
Moderate sedation is required for transesophageal echocardiography (TEE) as it is a semi-invasive procedure, to ease discomfort while introducing the TEE probe into the patient's esophagus, reduce gag reflex, and minimize hemodynamic perturbations in cardiac patients.[1] Various agents including benzodiazepines, opioids are currently being used in echocardiography (ECHO) laboratories for sedation. However, it has been shown that these drugs cause respiratory depression putting the patient at risk for aspiration and hypoxemia.[2] Other agents which have been used for outpatient procedures such as endoscopic retrograde cholangiopancreatography (ERCP), electroconvulsive therapy (ECT), orthopedic, ophthalmic, and gynecological procedures requiring moderate sedation are dexmedetomidine, propofol, ketamine, and combination of benzodiazepines with opioids such as alfentanil and remifentanil.[3,4,5]
Dexmedetomidine is a selective alpha 2 adrenergic receptor agonist that has sedative, analgesic, and anxiolytic properties.[6,7] It has been used in sedation in TEE and ERCP, and it was found that dexmedetomidine provides effective sedation with better maintenance of hemodynamic profile during the procedure.[3,4]
Ketofol is a combination of ketamine and propofol. Ketamine is an N-methyl D-aspartate receptor antagonist with the properties of sedation, analgesia, and amnesia without causing respiratory depression. Its drawbacks are vomiting and recovery agitation. Propofol has rapid onset and fast recovery time from sedation but is inadequate as a sole agent in semi-invasive procedures as it lacks analgesic properties. It induces cardiovascular depression and hypotension in a dose-dependent manner and also causes apnea.[8] The untoward effects of ketamine and propofol are reduced and balanced by each other as a combination (ketofol) producing synergistic, smoother sedation with a favorable hemodynamic profile.[8,9] Ketofol has been used in proportions of 1:1–1:10 (ketamine:propofol), and it has been shown that 1:3 has a better advantage in procedural sedation.[10] Ketofol has been used in ECT, pediatric cardiac catheterization, ERCP, dressings for burns, short procedural sedation for lumbar puncture, and bone marrow aspiration in various combinations and has shown adequate sedation with balanced hemodynamic parameters.[4,5,9,11]
Dexmedetomidine has been used in diagnostic TEE in a previous study.[3] Although ketofol was used in cardiac catheterization, a thorough literature search failed to show any study using ketofol for TEE. Diagnostic TEE is an outpatient procedure (mean time: 15 min) and requires optimal sedation and as cardiac patients are at greater risk for decompensation with administration of sedatives, it is aimed to minimize hemodynamic perturbations. Therefore, in the present study, we propose to compare the effects of dexmedetomidine and ketofol in outpatient sedation for TEE. We hypothesize that ketofol would be as effective as dexmedetomidine for outpatient procedural sedation in diagnostic TEE. The primary aim of the study was the time to achieve Ramsay sedation score (RSS) ≥3 and the secondary objectives were the hemodynamic parameters, need for rescue sedation, postprocedure complications, and patient and cardiologist satisfaction score.
Materials and Methods
This study was a prospective, randomized, double-blinded study. It was conducted in ECHO laboratory of Advanced Cardiac Centre, Postgraduate Institute of Medical Education and Research, Chandigarh. This study was conducted between July 2015 and June 2016 after approval by the Institute Ethics committee (INT/IEC/2015/817), clinical trials registration (ID: NCT02867930), and patient's written informed consent. Fifty adults of age group 18–60 years with atrial septal defect, rheumatic heart disease (mitral stenosis, mitral regurgitation, aortic stenosis, and aortic regurgitation) were included in the study. Those with atrial fibrillation with fast ventricular rate, symptomatic bradycardia, congestive cardiac failure, body mass index >30, esophageal stricture or tumor or laceration, history of gastrointestinal surgery, or history of dysphagia, active esophagitis, and peptic ulcer disease were excluded from the study.
Patient preparation
Diagnostic TEE was done on outpatient basis. Preanesthetic evaluation was done to assess patient's demographics, present and past medical history, general physical examination, and laboratory investigations. Patient was informed about the procedure and the intervention to be done in the study and written informed consent was taken. All patients were kept fasting, 8 h for solid foods and 2 h for clear liquids. Patients were randomized into two groups as Group D (n = 25) (Dexmedetomidine group) and Group KF (n = 25) (ketofol group) using computer-generated numbers and blinding was done with sealed opaque envelope technique. The patient, the primary investigator, and the cardiologist performing the TEE were blinded to the study drug.
Plan of intervention
The area was equipped with pipeline oxygen supply, airway and resuscitation devices, and suction equipment. Monitoring was done with spectra slim clarity monitor (Clarity Medical Private Limited, India) and electrocardiogram, heart rate (HR), pulse oximetry, noninvasive blood pressure, end-tidal carbon dioxide were measured. End-tidal carbon dioxide was measured through sampling line attached to nasal prongs. 20-G intravenous (IV) access was obtained for drug and fluid administration. Drug to be administered was prepared by assistant who was not involved in the direct clinical management of the patient in our study. The syringe (Dispo Van 20cc, Hindustan Syringes and Medical Devices Limited, India), high-pressure tubing, and infusion line were covered with silver paper. Both bolus and maintenance doses were administered through syringe infusion pump (Injectomat Agilia infusion pump, Fresenius Kabi, France).
Group D: dexmedetomidine (Dextomid, Neon Laboratories, India) was prepared as 200 μg in 20 ml syringe (10 μg/ml).
Group KF: ketofol was prepared in a proportion of ketamine: propofol, 1:3 with 1.3 ml of ketamine (Aneket, Neon laboratories, India) (50 mg/ml) and 19 ml of propofol (1%) (Nirfol 1%, Nirlife, India) in 20 ml syringe (final concentration of ketamine 3.2 mg + propofol 9.5 mg/ml).
Monitors were attached and baseline HR, systolic (SBP) and diastolic blood pressure (DBP), oxygen saturation, and end-tidal carbon dioxide values were recorded by anesthetist blinded to the drug administered. Oxygen was administered through nasal prongs at 4 L/min; sampling port of end-tidal carbon dioxide was attached to the nasal prongs at the nostril. Ringer's lactate drip was started at 10 ml/kg/h. After loading dose of 1 ml/kg/h, IV drug was administered, patients were assessed every 30 s using RSS.[12] When a sedation score of ≥3 was achieved, it was considered adequate for TEE probe insertion. Once adequate sedation was obtained with the bolus, infusion rate at 0.05 ml/kg/h was maintained at a constant rate till end of procedure. Wong-Baker facial pain score (FPS)[13] was used during the procedure to assess analgesia as there would be difficulty in communicating with the patient to ask about pain score during sedation after TEE probe insertion. RSS, HR, SBP and DBP, end-tidal carbon dioxide, oxygen saturation, and pain score were noted at six stages: T0 - presedation baseline, T1 - immediately before probe insertion, T2 - at probe insertion, T3-10 min after probe insertion, T4 - end of procedure, and T5 - postprocedure at recovery area. If patient or cardiologist was not satisfied with adequacy of sedation or FPS ≥4, rescue sedation was given with 25 μg fentanyl (Verfen, Verve Healthcare Limited, India) boluses. The other variables noted during the procedure were total drug dose administered, total procedure time which was the time after the patient has achieved RSS ≥3 to the completion of TEE (not including the time taken to achieve RSS ≥3.), any coughing/gagging, complications-desaturation, apnea, hypotension, and need for vasopressor/bradycardia/assisted ventilation, myoclonus, and pain on injection during procedure.
Hypotension was defined as 20% below baseline BP and was to be managed by fluid bolus/injection mephentermine 0.1 mg/kg IV. Apnea was defined as the cessation of respiration and absence of ETCO2 trace for >20 s. Desaturation was defined as SpO2 <90%. Bradycardia was defined as HR <50beats/min and was to be managed with injection atropine 0.02 mg/kg. Assisted ventilation was defined as need for mask ventilation/airway devices. At the end of procedure, the drug infusion was stopped and these variables were noted: total recovery time taken as time from when infusion was stopped to time to achieve modified Aldrete score (MAS) ≥9[14] and postprocedure incidence of nausea, vomiting, psychomimetic effects, pain, recovery agitation, and patients’ and cardiologists’ satisfaction scores[3] [Annexure 1].
Statistical analysis
Sample size was estimated based on a previous study on ERCP[4] that, in two groups, the difference in time to achieve RSS was 0.8 min (In Group D, 12.4 and Group KF 13.2 with standard deviation of 0.8). ERCP has a similar level of semi-invasive procedure with endoscope insertion, and hence it was used as the reference for sample size calculation. The sample size came out to be 22 participants per group at a power of 90% and confidence interval of 95%. For possible dropouts, it was decided to include 10% extra participants, so finally it was decided to include 25 participants per group. Normality of quantitative data was checked by Kolmogorov–Smirnov tests of normality. Student's t-test was applied for comparison of two groups. Mann–Whitney U-test was used for statistical analysis of skewed continuous variables or ordered categorical data. Time to achieve RSS ≥3, total procedure time, recovery time, and the total drug administered were analyzed by Mann–Whitney test. Proportions were compared using Pearson's Chi-square test. For comparison (time-related variables) of hemodynamics, repeated measure ANOVA was applied. All statistical tests were two-sided and were performed at a significance level of α = 0.05. Analysis was conducted using SPSS for Windows (version 22.0; SPSS Inc., Chicago, IL, USA).
Results
Fifty patients were enrolled and randomized into two groups: Group D – dexmedetomidine group and Group KF – ketofol group of 25 patients in each group. There was no loss to follow-up and 25 patients in each group were analyzed. The demographic data in both the groups were comparable and well matched [Table 1]. The primary outcome - time to achieve RSS ≥3 was significantly less in Group KF 260[69] seconds compared to Group D 460[137] seconds [Figure 1]. There was a significant decrease in HR from baseline in Group D. In Group KF, there was no significant change in HR trend from baseline. On comparing the groups, there was a significant difference at time points T2, T3, T4, and T5 between Group D and Group KF with lower HR in Group D [Figure 2]. In Group D, there was a significant decrease in SBP and DBP from baseline at T3 and T4, but there was no significant change in trend from baseline in Group KF [Figure 3]. In both groups, there was no significant change in trend of oxygen saturation or end-tidal carbon dioxide from baseline [Table 2]. There FPSs were comparable in both groups. The total drug administered in Group D - 6.95 ml (69 ± 29 μg of dexmedetomidine) was significantly more than Group KF - 4.72 ml (45 ± 10.7 mg propofol + 15 ± 3.6 mg ketamine). There was no significant difference in the total procedure time and recovery time in both groups [Table 3]. There was no need for rescue sedation in either of the groups. There was no significant difference in the complications such as coughing/gagging, desaturation, apnea, hypotension, and need for vasopressor/bradycardia/assisted ventilation, myoclonus, and pain on injection during procedure. The incidence of bradycardia in Group D was 2/25 (8%) [Table 4]. The patient satisfaction score in the both groups was comparable but the cardiologist satisfaction score was significantly better in Group KF compared to Group D [Figure 4].
Table 1.
Demographic
| Variable | Group D | Group KF |
|---|---|---|
| Age (years) | 32.16±10.8 | 32.28±9.83 |
| Weight (kg) | 55.84±11.53 | 58.28±13.82 |
| Height (cms) | 158.16±6.58 | 158.44±8.01 |
| BMI | 22.15±3.32 | 23.01±4.22 |
| Sex (male:female) | 11:14 | 11:14 |
| ASA status II: III | 23:2 | 22:3 |
| Comorbidities | 3/25 (chronic smoker-1, hypertension-2) | 3/25 (DM-1, hypertension-1, hypothyroidism-1) |
| Diagnosis ASD/MS/MR/AS/AR/others | 11/9/3/2 (others: VHD + VSD-1, VHD + IE-1) | 12/7/4/2 (others: VHD + IE-1, VHD + DORV -1) |
Variables expressed in mean±SD, ratios, numbers. Student’s t-test was applied. ASA: American Society of Anaesthesiologists, DM: Diabetes mellitus, VHD: Valvular heart disease, VSD: Ventricular septal defect, IE: Infective endocarditis, DORV: Double outlet right ventricle, ASD/MS/MR/AS/AR: Atrial septal defect/mitral stenosis/mitral regurgitation/aortic stenosis/aortic regurgitation, BMI: Body mass index, Group D: Dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3, SD: Standard deviation
Figure 1.
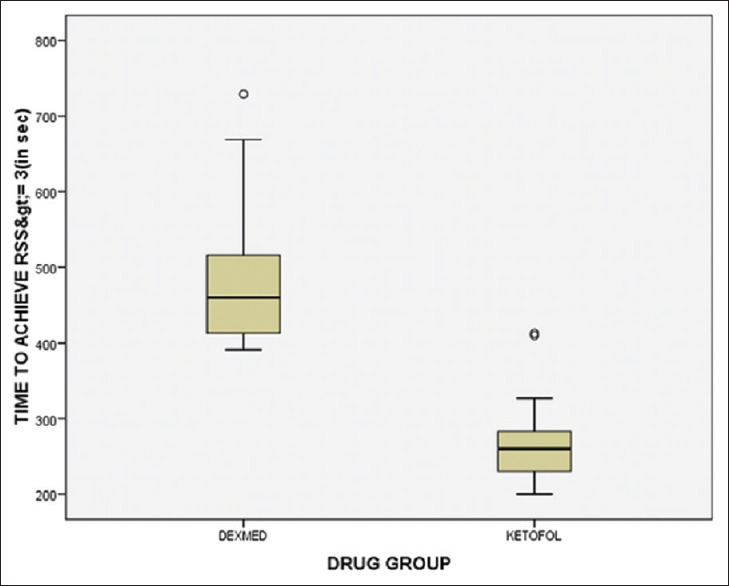
Time to achieve Ramsay sedation score ≥3. Values expressed as median (inter-quartile range) Mann–Whitney test applied. *P ≤ 0.05 and considered statistically significant. Group D: 460[137], Group KF: 260[69], P = 0.00* Group D: Dexmedetomidine (10 μg/ml). GROUP KF: Ketofol with proportion of ketamine: propofol, 1:3
Figure 2.
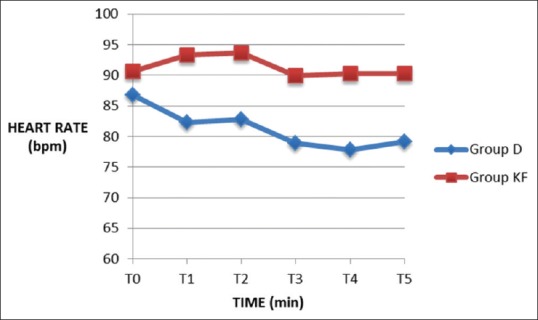
Heart rate in beats per minute. Values expressed as mean ± standard deviation, Student's t-test was applied. *P ≤ 0.05 and considered statistically significant. Group D: Dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3. T0 - presedation baseline, T1 - immediately before probe insertion, T2 - at probe insertion, T3-10 min after probe insertion, T4 - end of procedure, T5 - postprocedure at recovery area
Figure 3.
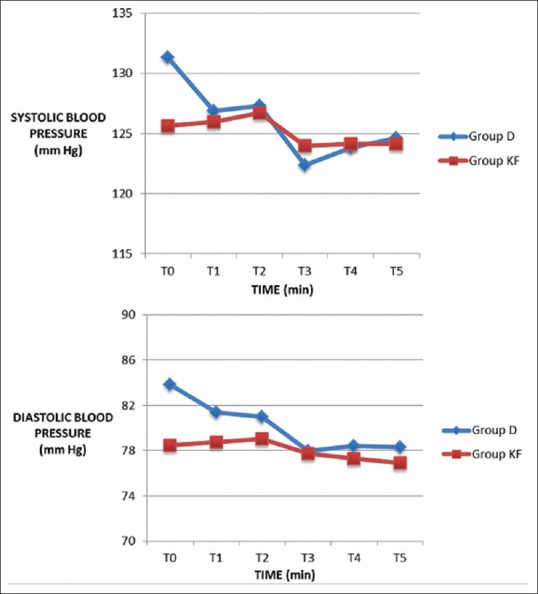
Systolic blood pressure and diastolic blood pressure at time points T0–T5. Values expressed as mean ± standard deviation, Student's t-test was applied. *P ≤ 0.05 and considered statistically significant. Group D: dexmedetomidine (10 μg/ml), Group KF: ketofol with proportion of ketamine: propofol, 1:3. In Group D, there was a significant decrease in systolic blood pressure and diastolic blood pressure from baseline at T3 and T4 but there was no significant change in trend from baseline in group KF. T0 - presedation baseline, T1 - immediately before probe insertion, T2 - at probe insertion, T3-10 min after probe insertion, T4 - end of procedure, T5 - postprocedure at recovery area
Table 2.
End-tidal carbon dioxide (mmHg) at T0–T5
| Time | Group D | P-value from T0 within group D | Group KF | P-value from T0 within group KF | P-value between groups |
|---|---|---|---|---|---|
| T0 | 34.08±2.91 | 34.48±1.35 | 0.53 | ||
| T1 | 33.64±2.19 | 1.0 | 34.52±1.78 | 1.0 | 0.13 |
| T2 | 33.92±1.73 | 1.0 | 34.12±1.96 | 1.0 | 0.70 |
| T3 | 33.88±1.56 | 1.0 | 33.56±1.80 | 0.07 | 0.51 |
| T4 | 33.96±1.67 | 1.0 | 33.6±1.63 | 0.19 | 0.44 |
| T5 | 33.8±1.87 | 1.0 | 33.44±1.44 | 0.07 | 0.45 |
Values expressed as mean±SD, Student’s t-test was applied. Group D: Dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3. In both the groups, there was no significant change in trend of end-tidal carbon dioxide from baseline. T0: Presedation baseline, T1: Immediately before probe insertion, T2: At probe insertion, T3: 10 min after probe insertion, T4: End of procedure, T5: Post procedure at recovery area, SD: Standard deviation
Table 3.
Total drug administered in ml, total procedure time in seconds, and recovery time in seconds (modified aldrete score ≥9)
| Parameter | Group D | Group KF | P |
|---|---|---|---|
| Total drug administered (ml) | 6.95 (2.99) | 4.72 (1.13) | 0.00* |
| Total procedure time (sec) | 1080 (181) | 950 (300) | 0.11 |
| Recovery time (sec) | 161 (93) | 160 (58) | 0.49 |
Values expressed as median (interquartile range) Mann–Whitney test applied. *P≤0.05 and considered statistically significant. There was no significant difference in the total procedure time and recovery time in both groups. There was no need for rescue sedation in either of the groups. Group D: Dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3
Table 4.
Secondary outcome variables
| Complication | Group D | Group KF | P |
|---|---|---|---|
| Coughing | 2/25 | 2/25 | 1.0 |
| Gagging | 1/25 | 3/25 | 0.29 |
| Hypotension | - | - | |
| Desaturation/apnea | - | - | |
| Bradycardia | 2/25 | 0/25 | 0.15 |
| Need for assisted ventilation | - | - | |
| Myoclonus | - | - | |
| Pain on injection | 0/25 | 3/25 | 0.07 |
| Nausea/vomiting | - | - | |
| Recovery agitation | - | - | |
| Psychomimetic effects | - | - |
(Values expressed as proportions), Compared using Pearson’s Chi-square test. Group D: Dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3. The incidence of bradycardia in Group D was 2/25 (8%)
Figure 4.
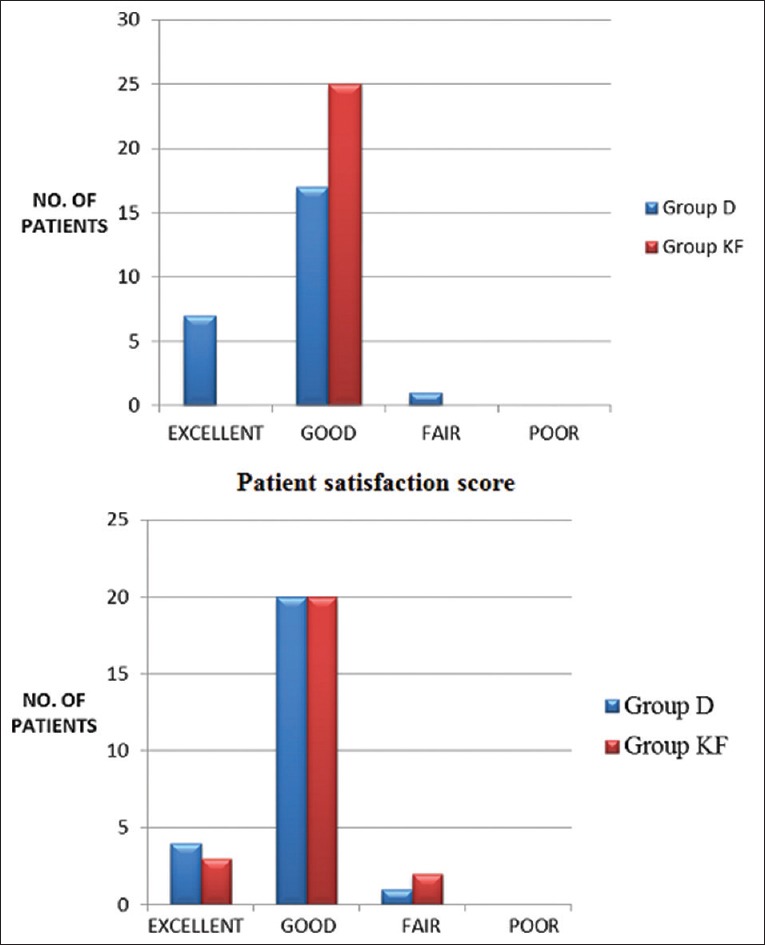
Satisfaction scores. Expressed as percentage, *P ≤ 0.05 and considered statistically significant. Group D: dexmedetomidine (10 μg/ml), Group KF: Ketofol with proportion of ketamine: propofol, 1:3
Discussion
Time to achieve Ramsay sedation score ≥3
The primary outcome of the study was the time to achieve (RSS) ≥3 with dexmedetomidine and ketofol (1:3) in diagnostic TEE. The results in our study have shown that the time to achieve RSS ≥3 is significantly lesser in Group KF compared to Group D. This may be attributed to the slower onset of action of dexmedetomidine. Yaǧan et al. showed that ketofol - 1:2 took lesser time (8.93 ± 1.72 min) to achieve sedation compared to dexmedetomidine (16.1 ± 2.67 min) in patients undergoing cataract surgery.[15] Hassan compared dexmedetomidine and ketofol (1:1) in ERCP and found the time to achieve RSS ≥3 with dexmedetomidine (12.4 ± 1.1 min) and ketofol (13.2 ± 0.5 min) to be comparable.[4] These differences in results are because of various ratios of ketamine and propofol combination used. Larger part of propofol in ketofol leads to faster onset and offset of sedative effect.
Heart rate, systolic and diastolic blood pressure
Our study showed that patients in Group D showed lower HR. Hassan had also found lower HR with dexmedetomidine infusion but no change with ketofol infusion (1:1) in ERCP,[4] and our results were consistent with their finding. This reduction in HR is due to increase in vagal tone, reduced levels of circulating norepinephrine,[6,16] with dexmedetomidine. McCutcheon et al. compared the effects of dexmedetomidine infusion to midazolam and fentanyl in 56 patients undergoing carotid endarterectomy under cervical plexus block and found that dexmedetomidine was associated with lesser need of interventions to treat tachycardia and hypertension associated with the procedure.[17] The effect of dexmedetomidine on HR may prove more suitable for a few specific procedures such as carotid endarterectomy more than procedural sedation in patients with cardiac disease where these hemodynamic perturbations are best avoided as decreasing the HR may prove deleterious in cardiac disease. Patients in ketofol group showed more stability in HR which is due to the countered actions of sympathomimetic effect of ketamine and hemodynamic depression of propofol. In our study, Group D showed a trend of reduction in SBP and DBP as compared to baseline, whereas Group KF, there was no significant difference from baseline. Hypotension was the most common adverse event with dexmedetomidine infusion as seen in patients undergoing a range of surgical and diagnostic procedures under regional anesthesia such as orthopedic, vascular stents, breast biopsy under sedation, and mechanical ventilation.[18,19] This finding is due to modulation of catecholamine release resulting in sympatholytic effect. Hypertension with initial infusion of dexmedetomidine occurs due to α-mediated vasoconstriction,[20] but it was not seen in our study. In pediatric cardiac catheterization, lesser hypotension was found with ketofol (3:1) in comparison to propofol.[21] Hassan showed lower mean arterial pressure with dexmedetomidine compared to ketofol (1:1) in ERCP.[4] These findings suggest that addition of ketamine to propofol causes better hemodynamic stability and lesser incidence of hypotension. These findings suggest that ketofol may have a clinical advantage over dexmedetomidine in controlling hemodynamics.
Respiratory parameters
The advantage of dexmedetomidine in moderate sedation is the absence of respiratory depression. Ketamine-induced sympathoadrenal activation also leads to improved ventilation. Patient getting propofol sedation for extracorporeal shock wave lithotripsy had significantly slower respiratory rate compared to dexmedetomidine.[22] Badrinath et al. studied the effect of ketofol on hundred patients undergoing breast biopsy and showed that small dose ketamine combined with propofol improves ventilation during sedation.[23] Whereas Daabis et al. compared ketofol 1:1 and 4:1 concentrations in hundred patients undergoing short procedural sedation and a slight increase in end-tidal carbon dioxide was seen in both the groups pointing toward mild respiratory depression with ketofol.[24] In our study, there was no incidence of desaturation; all the patients had an oxygen saturation of ≥97% throughout the procedure in both the groups. The end-tidal carbon dioxide values were within normal range and showed no significant difference between the groups. Hence, as regards to respiratory stability, both dexmedetomidine and ketofol (1:3) fare equal in our study.
Facial pain score and recovery time
Wong-Baker FPS was comparable in both the groups with no additional requirement of rescue sedation (fentanyl bolus) during the procedure suggesting adequate analgesia by both the drugs. Analgesic-sparing properties of dexmedetomidine have been observed in patients undergoing elective surgery under regional anesthesia getting intraoperative sedation with dexmedetomidine, and it was observed that they had significantly lesser opioid requirement for postoperative pain compared to propofol group.[25] Similarly, ketofol sedation in emergency department showed adequate procedural sedation and analgesia with low-dose ketamine added to propofol.[26]
In our study, the recovery time - time to achieve (MAS) ≥9 was comparable in both the groups which was approximately 2.6 min. A dosing simulation study by Coulter et al. showed faster recovery time with a dosing combination of 1:3 - ketamine: propofol with predicted recovery of 13 min with infusion regimen.[10] Wang et al. showed a recovery time of 8 min with ketofol (1:3) for medical termination of pregnancy and Khutia et al. showed a recovery time of 13 min with IV ketofol infusion (1:2) regimen in emergency short procedures in children.[27,28] Hassan showed no difference in recovery times between dexmedetomidine infusion and ketofol (1:1) infusion in patients undergoing ERCP with modified Aldrete score ≥9 achieved in 11.4 ± 0.5 min in dexmedetomidine group and 12.5 ± 1.8 min in ketofol group.[4] The faster recovery time in our study may be attributed to lesser dose of ketamine used as compared to other studies. The initial higher MAS within 5 min could be due to the stimulus of TEE probe removal. The exact values of MAS were not measured at different time points after probe removal in our study; hence, we cannot comment on the drug prolonged effect. In a study of propofol sedation for TEE, the MAS was <9 in propofol group up to 30 min.[29]
Satisfaction scores and complications
In the present study, we observed that the patient satisfaction score in both the groups was comparable and there was better cardiologist satisfaction in ketofol group compared to dexmedetomidine. This is similar to the study by Hassan which showed greater patient and endoscopist satisfaction with ketofol compared to dexmedetomidine in ERCP.[4] Different studies have shown similar or better patient satisfaction scores with dexmedetomidine as compared to propofol (nasotracheal fiber-optic intubation) or midazolam (TEE) but recall of the endoscopy was more in the dexmedetomidine group.[3,30] Higher patient and physician satisfaction was seen with ketofol sedation compared to ketamine-midazolam for endobronchial ultrasound-guided biopsy or to propofol in procedural sedation in emergency department with no complications of emergence and hypersalivation.[31,32,33]
In our study, there was no need for assisted ventilation, no incidence of desaturation, or apnea in both the groups. There were no significant differences in complications such as gagging, coughing, hypotension requiring vasopressor administration, pain on drug administration, nausea, and vomiting. Mortero et al. showed that a small dose of ketamine added to propofol can attenuate propofol-induced hypoventilation when used for day-care and ambulatory surgeries.[34] Akin et al. showed no incidence of apnea in ketofol group compared to propofol group in pediatric cardiac catheterization.[21] Frey et al. compared propofol and ketofol (1:3) as bolus in sedation for retrobulbar block procedures and found the need for airway interventions was 18 in propofol group versus 9 in ketofol group.[35] The incidence of nausea and vomiting in adults with ketamine is 5%–15%. However, the antiemetic effects of propofol effectively counterbalance this adverse effect. A 20 min procedural sedation with ketofol dosing regimen of 1:3 and 1:4 was associated with longest antiemetic duration of 65–70 min.[10]
There was no incidence of psychomimetic effects and recovery agitation in both the groups in our study. Children undergoing lumbar puncture or bone marrow aspiration biopsy using 1:2 and 1:3 ketofol concentrations showed that the combination of 1:3 had lower psychomimetic effects and was associated with a shorter recovery time.[11] The incidence of recovery agitation with ketamine in adults is 10%–20%, but in a study with ketofol for emergency procedural sedation, the incidence was 3.6% only.[26] Martin et al. conducted a study on postsurgical intensive care unit sedation with dexmedetomidine and found the incidence of bradycardia to be 9% compared to placebo (2%).[36] This is comparable to our study where the incidence of bradycardia was 8% in dexmedetomidine group (2/25). The comparison of antisialogogue property of dexmedetomidine and the effect of excessive salivation with ketamine which may cause desaturation as complication cannot be exactly determined as insertion of TEE probe itself causes an increase in salivation which may be a confounding variable for the response to the drugs.
Limitations
In our study, there was no control group of standard of care of sedation such as midazolam, propofol, or only local anesthetic spray comparison to dexmedetomidine and ketofol. End-tidal carbon dioxide measurement through sampling line attached to nasal prongs might not give an accurate value as there was no definitive airway used. BIS was not measured in our study, so a quantitative analysis of the depth of sedation was not known. Future scope of the study is to compare different proportions of ketofol in sedation in diagnostic TEE.
Conclusion
Ketofol infusion (1:3) is a better sedation regimen for outpatient diagnostic TEE compared to dexmedetomidine as lesser time is taken to achieve optimal sedation, faster awakening with no hemodynamic perturbations, postprocedure complications, and better patient and cardiologist satisfaction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Annexure 1: Cardiologist Satisfaction Questionnaire
Reference: Self made
Was the level of sedation adequate at the time of probe insertion?
Was the level of sedation adequate at the time of imaging?
Was any part of the procedure worse for imaging than the rest of the procedure?
Would you recommend this sedation for this procedure to anyone else?
Would you accept the same kind of sedation for your patient when you perform it the next time?
Satisfaction score
| Score | Response |
|---|---|
| 1 | Excellent |
| 2 | Good |
| 3 | Fair |
| 4 | Poor |
References
- 1.Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921–64. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH, et al. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Cooper L, Candiotti K, Gallagher C, Grenier E, Arheart KL, Barron ME, et al. A randomized, controlled trial on dexmedetomidine for providing adequate sedation and hemodynamic control for awake, diagnostic transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2011;25:233–7. doi: 10.1053/j.jvca.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Hassan HI. Dexmedetomidine versus ketofol for moderate sedation in Endoscopic Retrograde Cholangiopancreatography (ERCP) comparative study. Egypt J Anesth. 2015;31:15–21. [Google Scholar]
- 5.Kogan A, Efrat R, Katz J, Vidne BA. Propofol-ketamine mixture for anesthesia in pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2003;17:691–3. doi: 10.1053/j.jvca.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence CJ, Prinzen FW, de Lange S. The effect of dexmedetomidine on the balance of myocardial energy requirement and oxygen supply and demand. Anesth Analg. 1996;82:544–50. doi: 10.1097/00000539-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Aydogan H, Aydogan T, Uyanıkoglu A, Kucuk A, Yuce HH, Karahan MA, et al. Propofol-ketamine combination has shorterrecovery times with similar hemodynamicscompared to propofol alone in uppergastrointestinal endoscopy in adults. A randomized trial. Acta Med Mediterr. 2013;29:259–64. [Google Scholar]
- 9.Yalcin S, Aydoǧan H, Selek S, Kucuk A, Yuce HH, Karababa F, et al. Ketofol in electroconvulsive therapy anesthesia: Two stones for one bird. J Anesth. 2012;26:562–7. doi: 10.1007/s00540-012-1378-6. [DOI] [PubMed] [Google Scholar]
- 10.Coulter FL, Hannam JA, Anderson BJ. Ketofol dosing simulations for procedural sedation. Pediatr Emerg Care. 2014;30:621–30. doi: 10.1097/PEC.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 11.Ghadami Yazdi A, Ayatollahi V, Hashemi A, Behdad Sh, Ghadami Yazdi E. Effect of two different concentrations of propofol and ketamine combinations (Ketofol) in pediatric patients under lumbar puncture or bone marrow aspiration. Iran J Ped Hematol Oncol. 2013;3:187–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong DL, Baker CM. Smiling faces as anchor for pain intensity scales. Pain. 2001;89:295–300. doi: 10.1016/s0304-3959(00)00375-4. [DOI] [PubMed] [Google Scholar]
- 14.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 15.Yaǧan Ö, Karakahya RH, Taş N, Küçük A. Comparison of dexmedetomidine versus ketamine-propofol combination for sedation in cataract surgery. Turk J Anaesthesiol Reanim. 2015;43:84–90. doi: 10.5152/TJAR.2014.45220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert T, Maze M. Dexmedetomidine: Another arrow for the clinician's quiver. Anesthesiology. 2004;101:568–70. doi: 10.1097/00000542-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 17.McCutcheon CA, Orme RM, Scott DA, Davies MJ, McGlade DP. A comparison of dexmedetomidine versus conventional therapy for sedation and hemodynamic control during carotid endarterectomy performed under regional anesthesia. Anesth Analg. 2006;102:668–75. doi: 10.1213/01.ane.0000197777.62397.d5. [DOI] [PubMed] [Google Scholar]
- 18.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY, et al. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 19.Hoy SM, Keating GM. Dexmedetomidine: A review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71:1481–501. doi: 10.2165/11207190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Alhashemi JA. Dexmedetomidine vs midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]
- 21.Akin A, Esmaoglu A, Guler G, Demircioglu R, Narin N, Boyaci A, et al. Propofol and propofol-ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–7. doi: 10.1007/s00246-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 22.Kaygusuz K, Gokce G, Gursoy S, Ayan S, Mimaroglu C, Gultekin Y, et al. A comparison of sedation with dexmedetomidine or propofol during shockwave lithotripsy: A randomized controlled trial. Anesth Analg. 2008;106:114–9. doi: 10.1213/01.ane.0000296453.75494.64. [DOI] [PubMed] [Google Scholar]
- 23.Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovich AD. The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg. 2000;90:858–62. doi: 10.1097/00000539-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Daabiss M, Elsherbiny M, Al Otaibi R. Assessment of different concentrations ofketofol in procedural operations. Br J Med Pract. 2009;2:27–31. [Google Scholar]
- 25.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 26.Andolfatto G, Willman E. A prospective case series of single-syringe ketamine-propofol (Ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18:237–45. doi: 10.1111/j.1553-2712.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Jiang X, Pang L, Dong S, Feng Y, Prajapati SS, et al. A randomized double-blind controlled study of the efficacy of ketofol with propofol-fentanyl and propofol alone in termination of pregnancy. Afr J Pharm Pharmacol. 2012;6:2510–4. [Google Scholar]
- 28.Khutia SK, Mandal MC, Das S, Basu SR. Intravenous infusion of ketamine-propofol can be an alternative to intravenous infusion of fentanyl-propofol for deep sedation and analgesia in paediatric patients undergoing emergency short surgical procedures. Indian J Anaesth. 2012;56:145–50. doi: 10.4103/0019-5049.96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toman H, Erkılınc A, Kocak T, Guzelmeric F, Savluk OF, Dogukan M, et al. Sedation for transesophageal echocardiography: Comparison of propofol, midazolam and midazolam-alfentanil combination. Med Glas (Zenica) 2016;13:18–24. doi: 10.17392/825-16. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC, et al. Acomparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010;65:254–9. doi: 10.1111/j.1365-2044.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 31.Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care. 2010;55:702–6. [PubMed] [Google Scholar]
- 32.Dal T, Sazak H, Tunç M, Sahin S, Yılmaz A. A comparison of ketamine-midazolam and ketamine-propofol combinations used for sedation in the endobronchial ultrasound-guided transbronchial needle aspiration: A prospective, single-blind, randomized study. J Thorac Dis. 2014;6:742–51. doi: 10.3978/j.issn.2072-1439.2014.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David H, Shipp J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med. 2011;57:435–41. doi: 10.1016/j.annemergmed.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA, et al. The effects of small-dose ketamine on propofol sedation: Respiration, postoperative mood, perception, cognition, and pain. Anesth Analg. 2001;92:1465–9. doi: 10.1097/00000539-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Frey K, Sukhani R, Pawlowski J, Pappas AL, Mikat-Stevens M, Slogoff S, et al. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: Comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89:317–21. doi: 10.1097/00000539-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]


