Abstract
Introduction:
Peri-operative monitoring of coagulation is important to diagnose potential cause of hemorrhage, to manage coagulopathy and guide treatment with blood products in patients undergoing cardiac surgery with cardiopulmonary bypass. This study was done to evaluate usefulness of Thromboelastography (TEG) and routine coagulation tests (RCT) in assessing hemostatic changes and predicting postoperative bleeding in patients undergoing cardiac surgery with cardiopulmonary bypass.
Methods:
Fifty adult patients undergoing cardiac surgery with cardiopulmonary bypass were enrolled in this prospective study. Preoperative and post-operative samples were collected for routine coagulation tests and TEG. Regression analysis and test of significance using Pearson's correlation coefficient was performed to assess correlation between routine coagulation tests and corresponding TEG parameters. Regression analysis was done to study relation between blood loss at 24 hours and various coagulation parameters.
Results:
The Routine coagulation test i.e. PT, INR, APTT showed no significant correlation with corresponding TEG parameters in pre-operative samples. However platelet count significantly correlated (p = 0.004) with MA values in postoperative samples. A significant correlation (p = 0.001) was seen between fibrinogen levels and alpha angles as well as with MA in both baseline preoperative and postoperative samples. TEG parameters R time and MA in postoperative samples were the only parameters that predicted bleeders with fair accuracy.
Conclusion:
Though the techniques of RCT and TEG are different, a few RCT e.g. platelet count and fibrinogen correlated with corresponding TEG parameters i.e. MA and Alpha angle. TEG parameters (R time and MA in postoperative samples) were able to predict blood loss better than RCT.
Keywords: Cardiopulmonary bypass, routine coagulation tests, thromboelastography
Introduction
Coagulation and fibrinolysis processes in cardiac surgery with cardiopulmonary bypass (CPB) are monitored by conventional plasma-based assays including prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen in addition to platelet count. Thromboelastography (TEG), a point-of-care method, is also increasingly used for assessment of hemostatic abnormalities during CPB. These two methods are fundamentally different; the conventional coagulation tests measure the various components of the hemostasis in isolation. TEG measures the various components of hemostasis as they interact with one another in vivo. Perioperative monitoring of coagulation is important to diagnose potential cause of hemorrhage, manage coagulopathy, and guide treatment with blood products in patients undergoing cardiac surgery with CPB. Approximately 20% of patients bleed significantly after cardiac surgery. However, a surgical cause is found only in 50% of reexplorations.[1] CPB is associated with complex hemostatic disturbances mainly contributing to nonsurgical bleeding.[2,3,4,5] The possible triggers of a coagulopathy leading to excessive bleeding are extensive surgical trauma, prolonged blood contact with the artificial surface of the CPB circuit, high doses of heparin, hypothermia, and platelet activation and dysfunction caused mainly by heparin and hypothermia.[1] It suggests a need to test these patients to determine hemostatic disorder and to differentiate it from a surgical cause of abnormal bleeding.
Blood products are often administered empirically in these patients, due to long turnaround times of laboratory based coagulation tests. Empiric transfusion of blood products is often inappropriate and may result in increased morbidity and mortality and hospital costs in these patients.[6,7,8]
There are few studies that have reported a better predictive value of TEG as compared to routine coagulation tests (RCT) for blood loss and consequently better use of blood products in patients undergoing cardiac surgery with CPB.[9,10,11] However, other studies have reported no predictive value of TEG for blood loss in such patients.[12,13,14,15]
This study was done to evaluate whether RCT correlate with TEG parameters and to evaluate the usefulness of these (RCT and TEG) to predict postoperative bleeding in patients undergoing cardiac surgery with CPB. This may be helpful in judicious use of blood products in these patients.
Materials and Methods
After obtaining approval of the Ethics Committee of our institute and written informed consent, fifty adult patients undergoing cardiac surgery with CPB were enrolled in this prospective study from January 2014 to April 2015. Adult patients with normal baseline coagulation profile and in whom anticoagulants and antiplatelet agents were discontinued at least 7 days before surgery were included in this study.
Blood samples were collected at two-time points. (1) Preoperatively (before induction of anesthesia and heparinization). (2) Postoperatively at admission to intensive care unit (ICU) once the patient was settled. Preoperative sample was collected immediately after a venous line was established. Three samples were collected: (1) K3 ethylenediaminetetraacetic acid (EDTA) vacutainers (2) 0.105–0.109 M tri-sodium citrate vacutainers (Becton Dickinson) were used to collect for routine hematological tests and RCT. (3) 1 ml nonanticoagulated blood sample was transferred immediately to Kaolin containing vial and Kaolin-activated TEG was done in OT using TEG® 5000 Thrombelastograph® Hemostasis Analyzer System (Haemonetics Corporation US).
In OT, all the patients were anesthetized, heparinized, put on CPB support, and surgery was done as existent standard protocol. The adequacy of heparin anticoagulation during CPB was monitored by activated clotting time as point-of-care test. Once the patient was weaned from CPB heparin reversal was done by injection protamine sulfate in the ratio of 1:1 for total heparin used. The adequacy of heparin reversal was again monitored by ACT, and it was ensured that it reached preheparinization value.
Postoperative sample collection: heparin-contaminated venous lines were avoided during postoperative sample collection, and in unavoidable circumstances, venous line was flushed with crystalloid and first few milliliters of blood were discarded, and whole blood sample was collected and transferred to (1) EDTA vial, (2) Citrate vial, and (3) 1 ml nonanticoagulated blood sample was transferred to heparinase-containing Kaolin vials available with TEG kit.
Following TEG parameters were studied: R = Reaction time: It is a measure of clotting factors, K = Kinetic time: It is a measure of the speed taken to reach a specific level of clot strength. Together with the alpha angle, it is a measure of clot kinetics. Alpha Angle: Measures the speed of fibrin build-up and cross-linking (clot strengthening), MA = maximum amplitude: it represents the ultimate strength of the clot and is a measure of platelet function, LY 30: It indicates breakdown of the clot, and therefore, gives an idea of clot stability.
Blood samples were transported within 1 h at room temperature to laboratory where citrated sample was centrifuged at 2000 g for 10 min at 18°C–25°C to ensure platelet depletion. RCT and hematological tests were conducted within 4 h of sample collection. Routine coagulation studies PT, international normalized ratio (INR) (Neoplastin®CIPLUS, ISI value 1.30), APTT (C.K. PREST®), fibrinogen (Fibri-prest®), and D-dimer (DI TEST®) were performed on fully automated coagulation analyzer STA Compact (Diagnostica STAGO, France). Hematological parameters, i.e., hemoglobin, red blood cell (RBC) count, hematocrit, total leukocyte count, platelets were analyzed on Sysmex XE Alpha-N Automated Hematology Blood Analyzer.
Chest tube drain was recorded hourly till 24 h postoperatively as per existing ICU protocol in milliliters. Excessive bleeding was defined as >100 ml every hour in the first 3 h or 300 ml in any hour or >1000 ml in 24 h and such patients were classified as bleeders.
Statistical analysis
For statistical analysis, IBM SPSS, Version 20.0. (IBM Corp., Armonk, NY, USA) Data editor V20 was used. The data were expressed as the mean, standard deviation and range. The relationship between TEG-derived blood clotting variables and those from routine coagulation profile were obtained using correlation analysis. Mean values of CBC, coagulation tests, and TEG parameters in pre- and postoperative samples were compared by paired samples t-test, and significant (2-Tailed) values were derived. To assess whether standard coagulation tests correlate with TEG parameters, regression analysis and test of significance were performed between corresponding RCT and TEG parameters using Pearson's correlation coefficient. Pearson's correlation coefficient was also used to determine relation between blood loss at 24 h and routine coagulation parameters as well as TEG variables. Receivers operating characteristic (ROC) curves were derived to know how accurately different coagulation parameters detected bleeders. Regression analysis was done to study the relation between blood loss at 24 h and various coagulation parameters. Values of P < 0.05 were considered to be statistically significant.
Results
Fifty adult patients with a mean age of 32 ± 12.5 years (range 18–67) undergoing cardiac surgery with CPB were enrolled in this prospective study. During the procedure, aortic cross clamp time (ACC time), CPB time, drain output during surgery, and chest tube output at 24 h were noted. Mean ACC time was 50 ± 22.8 min; mean CPB time was 82 ± 33.5 min. Mean drain output after closure of surgery was 585 ± 192.7 ml, and chest tube output at 24 h was 509 ± 378.9 ml. The demographic characteristics and operative details of patients are shown in Table 1.
Table 1.
Patients demographics and surgical procedure details
| Patient demographics | Mean value (range) |
|---|---|
| Age (years) | 32±12.5 (18-67) |
| Sex (male/female) | 26/24 |
| Height (cm) | 156±7.6 (144-180) |
| Weight (kg) | 52±8.4 (29-71) |
| BSA (m2) | 1.49±0.14 (1.10-1.79) |
| Surgical procedures | |
| Mitral valve replacement | 31 |
| Double valve replacement | 9 |
| Aortic valve replacement | 6 |
| ASD closure | 1 |
| VSD closure | 1 |
| PS repair | 1 |
| ACC time (min) | 50±22.8 (19-104) |
| CPB time (min) | 82±33.5 (40-160) |
| Drain output during surgery (ml) | 585±192.7 (150-1000) |
| Chest tube output at 24 h (ml) | 509±378.9 (80-1960) |
CPB: Cardiopulmonary bypass, ACC: Aortic cross-clamp, BSA: Body surface area, ASD: Atrial Septal Defect, VSD: Ventral Septal Defect, PS: Pulmonary Stenosis
Hematological parameters
The mean values of baseline CBC parameters such as hemoglobin 12.1 ± 1.5 g/dl, hematocrit 36.1 ± 4.2%, RBC count 4.44 ± 0.59 × 1012/l, total leukocyte count: 7.5 ± 2.4 × 109/l, and platelet count 215 ± 76 × 109/l were within normal range. A significant decrease (in %) from baseline levels were observed in postoperative samples for hemoglobin (19.8%, P = 0.001), RBC count (22.72%, P = 0.001), hematocrit (19.9%, P = 0.001), platelet count (32.1%, P = 0.001), and plateletcrit (25%, P = 0.001) whereas Total Leucocyte count, (TLC) showed significant increase by 104%, P = 0.001 [Table 2].
Table 2.
Baseline and postoperative complete blood count, coagulation, and thromboelastography parameters
| Tests | Mean±SD (range) | P | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| Hematological parameters | |||
| Hemoglobin (g/dl) | 12.1±1.5 (9.3-14.9) | 9.7±1.5 (7-14) | 0.001 |
| RBC count×1012/l | 4.44±0.59 (3.3-5.43) | 3.44±0.55 (2.39-4.84) | 0.001 |
| HCT (%) | 36.1±4.2 (26.4-44.8) | 28.9±4.1 (19.1-43) | 0.001 |
| TLC×109/l | 7.5±2.4 (3.48-9.38) | 15.3±4.8 (6.68-31) | 0.001 |
| Platelet count×109/l | 215±76 (114-582) | 146±43 (54-235) | 0.001 |
| Mean platelet volume (fl) | 11.7±1.0 (9.8-14.4) | 11.5±1.1 (9.10-14.5) | 0.06 |
| Plateletcrit (%) | 0.24±0.07 (0.12 0.62) | 0.18±0.06 (0.06-0.5) | 0.001 |
| Platelet distribution width (fl) | 14±2.7 (11.3-23.8) | 13.9±3.4 (10.3-23.6) | 0.7 |
| Coagulation parameters | |||
| PT (N=11-14 s) | 15.5±1.8 (11.8-21.5) | 20±3.1 (14.5-29.70) | 0.001 |
| INR | 1.3±0.21 (0.96-1.90) | 1.73±0.33 (1.24-2.81) | |
| APTT (N=25-34 s) | 34.4±7.2 (19.2-56.5) | 40.6±8.9 (28.9-79.0) | 0.05 |
| Fibrinogen (N=150-400 mg/dl) | 240±84 (124-438) | 176±82 (50-413) | 0.001 |
| D-dimer N <0.5 μg/ml | 0.63±0.92 (0.09-5.75/0 | 1.3±0.89 (0.25-4.75) | 0.01 |
| TEG parameters | |||
| r-time (N 4-8 min) | 7.2±1.8 (3.8-12.9) | 6.5±2.1 (3.4-15.5) | 0.96 |
| k-time (N 0-4 min) | 2.7±1.0 (1.2-5.1) | 2.4±0.9 (1.3-5.6) | 0.50 |
| Alpha angle (N 47°–74°) | 54.7±10.1 (34.1-69.2) | 58.6±9.3 (37.1-71.3) | 0.28 |
| Maximum amplitude (N 54-72 mm) | 65.4±5.4 (53.7-76.2) | 61±6.3 (47.3-77.5) | 0.64 |
| LY 30 (N 0-8%) | 0.19±0.6 (0.0-2.3) | 0.47±2.1 (0.0-14.5) | 0.27 |
SD: Standard deviation, RBC: Red blood cell, TEG: Thromboelastography, APTT: Activated partial thromboplastin time, PT: Prothrombin time, HCT: Hematocrit, TLC: Total Leucocyte count, LY: Lysis Fibrinogen , INR: International normalized ratio
Laboratory coagulation parameters
Coagulation parameters in pre- and postoperative samples also showed variation. The mean values of PT in postoperative were increased by 29% (P = 0.001) and APTT by 18% (P = 0.05). In six patients, PT and APTT were found to be deranged in preoperative samples which could be due to persistent effect of heparin given 24 h before surgery. The fibrinogen levels were also found to be significantly reduced (26.6%, P = 0.001) in postoperative samples as compared to baseline levels. D-dimer estimation showed a significant increase of 106% (P < 0.01) in postoperative samples from baseline values indicating ongoing fibrinolysis in these samples [Table 2].
Thromboelastography parameters
The mean values of TEG parameters r-Time: 7.2 ± 1.8 min (3.8–12.9), k-Time: 2.7 ± 1.0 min (1.2–5.1), alpha angle: 54.7 ± 10.1 degrees (34.1–69.2), MA 65.4 ± 5.4 mm (53.7–76.2), and LY 30: 0.19 ± 0.6 (0.0–2.3%) were within normal range in baseline preoperative samples and showed no significant variation in postoperative samples. An increase in r-Time was seen in postoperative samples of 7 patients. Alpha angle values were found to be lower than normal range (<47 degrees) in 7 patients postoperatively. Lower value of MA <54 mm was seen in six patients postoperatively. However, this difference was not statistically significant for any of these parameters [Table 2].
Correlations of thromboelastography parameters with routine coagulation tests
To assess whether RCT correlate with TEG parameters, pearson's correlation coefficient was determined between PT and r-Time, INR and r-Time, APTT and r-time, platelet count and MA, fibrinogen and MA, fibrinogen and alpha angle, D-dimer and LY 30.
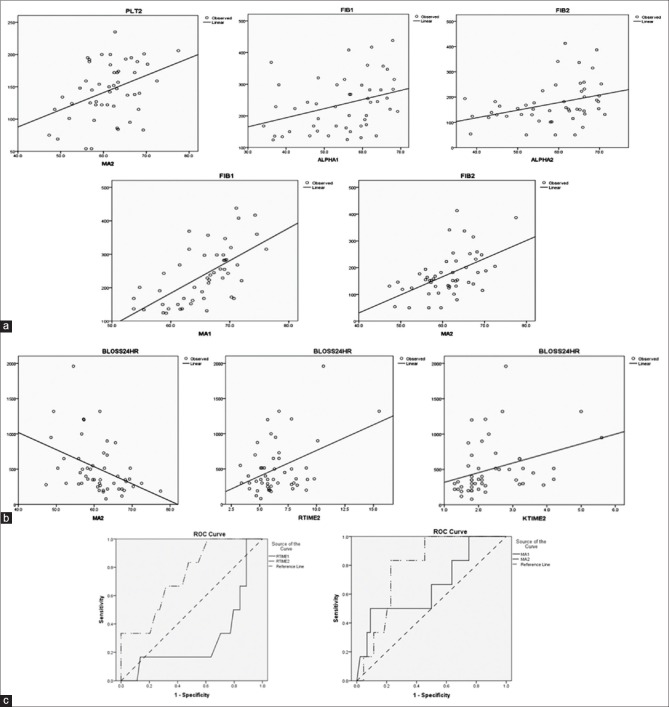
The RCT, i.e., PT, INR, APTT, and platelets showed no significant correlation with corresponding TEG parameters in preoperative samples. However, in postoperative samples, platelet count was significantly correlated (P = 0.004) with MA values [Figure 1a]. On the other hand, a significant correlation (P = 0.001) was seen between fibrinogen levels and alpha angles as well as with MA in both baseline preoperative and postoperative samples [Table 3 and Figure 1a].
Figure 1.
(a) Correlation between thromboelastography and routine coagulation tests parameters. (b) Correlation between thromboelastography parameters and blood loss. (c) Receivers operating characteristic curves for predicting bleeders
Table 3.
Correlation between thromboelastography parameters and corresponding routine coagulation tests
| Parameters | Preoperative | Postoperative | ||
|---|---|---|---|---|
| r | P | r | P | |
| r-time-PT | 0.12 | 0.39 | 0.10 | 0.48 |
| k-time-PT | 0.005 | 0.98 | 0.18 | 0.20 |
| r-time-APTT | 0.11 | 0.43 | 0.19 | 0.19 |
| k-time-APTT | 0.08 | 0.55 | 0.09 | 0.52 |
| Alpha angle-FIB | 0.34 | 0.001 | 0.34 | 0.001 |
| MA-B | 0.63 | 0.001 | 0.53 | 0.02 |
| MA-PLT count | 0.11 | 0.43 | 0.4 | 0.004 |
| LY 30-D dimer | 0.02 | 0.88 | 0.09 | 0.53 |
APTT: Activated partial thromboplastin time, PT: Prothrombin time, FIB: Fibrinogen, LY: Lysis, MA: Maximum amplitude
Laboratory parameters in Bleeder and nonbleeder patients
On the basis of criteria mentioned in material and methods, excessive bleeding was seen in 6 patients. These six bleeders belonged to MVR (n = 3) and DVR (n = 3) groups. None of the patients from other operative procedure group were identified as bleeders. Three of these patients were reexplored in OT, and clot evacuation was done followed by medical management and other three were managed medically with blood components and drugs without reexploration.
Regarding patient demographics of bleeders, no significant differences in age, sex, height, weight, and drain output during surgery data were noted when compared to nonbleeders. However, ACC time (24%), CPB time (20%), and chest tube output at 24 h (237%) were found to be increased in bleeders as compared to nonbleeders [Table 5]. The platelet count and coagulation parameters of bleeders and nonbleeders were compared. The mean baseline platelet count among bleeders was 26% lesser as compared to nonbleeders; however, it was not statistically significant (P = 0.08) though mean platelet values were within normal range in both the groups. On comparison of routine coagulation profile, no significant differences in mean PT and APTT values were seen in bleeders as compared to nonbleeders in both baseline preoperative as well as postoperative samples.
Table 5.
Comparison of pre- and post-operative routine coagulation tests and thromboelastography parameters among bleeders (n=6) and nonbleeders (n=44)
| Parameters | Preoperative | P | Postoperative | P | ||
|---|---|---|---|---|---|---|
| Nonbleeders | Bleeders | Nonbleeders | Bleeders | |||
| RCT | ||||||
| PT (s) | 15.5±1.8 | 15.4±2.3 | 0.12 | 20±2.8 | 20.2±4.9 | 0.08 |
| APTT (s) | 34.7±7.5 | 31.7±3.0 | 0.19 | 41.0±8.8 | 37.8±9.7 | 0.34 |
| FIB (mg/dl) | 244±84 | 202±54 | 0.06 | 182±85 | 132±43 | 0.04 |
| D-dimer | 0.54±0.6 | 1.2±2.2 | 0.06 | 1.2±0.8 | 1.8±1.2 | 0.08 |
| PLT | 222±76 | 164±63 | 0.08 | 149±41 | 125±52 | 0.08 |
| TEG | ||||||
| r-Time | 7.3±1.9 | 6.2±1.4 | 0.98 | 6.2±1.6 | 8.7±3.7 | 0.06 |
| k-Time | 2.7±1.0 | 2.6±1.0 | 0.43 | 2.3±0.9 | 2.8±1.1 | 0.52 |
| Alpha angle | 54.0±10.3 | 56.9±9.5 | 0.92 | 58.9±9 | 56.2±9.2 | 0.52 |
| MA | 65.8±9.4 | 62.5±6.5 | 0.54 | 61.7±6.3 | 56.0±4.0 | 0.34 |
| LY 30 | 0.13±0.3 | 0.6±1.5 | 0.10 | 0.4±2.1 | 0.8±2.0 | 0.08 |
RCT: Routine coagulation tests, TEG: Thromboelastography, APTT: Activated partial thromboplastin time, PT: Prothrombin time, PLT: Platelet, FIB: Fibrinogen, LY: Lysis, MA: Maximum amplitude
Although PT and APTT in both groups were not different, fibrinogen levels were found to be 17% lower in bleeders as compared to nonbleeders in baseline samples. This decrease in fibrinogen was even more marked (27%) and statistically significant (P = 0.04) in postoperative samples.
Near normal mean value of D-dimer (0.54 μg/ml) was seen in nonbleeder group while it was higher (1.2 μg/ml) in bleeders in preoperative samples though it was short of statistical significance (P = 0.06). D-dimer was found to be increased in both nonbleeder (1.2 μg/ml) and bleeder (1.8 μg/ml) groups in postoperative samples; however, the magnitude of this increase was still lower by 50% in nonbleeders as compared to bleeders [Table 5].
TEG parameters of bleeders when compared to nonbleeders did not show statistically significant differences in either preoperative or postoperative samples in our study [Table 4].
Table 4.
Demographic data of bleeders and nonbleeders
| Patient demographics | Mean value (range) | |
|---|---|---|
| Nonbleeders | Bleeder | |
| Age (years) | 32±12.5 (18-67) | 29±9.7 (18-40) |
| Sex (male/female) | 23/21 | 3/3 |
| Height (cm) | 156±7.6 (144-180) | 153±5.4 (146-160) |
| Weight (kg) | 52±8.5 (29-69) | 50±8.6 (39-60) |
| BSA (m2) | 1.49±0.14 (1.1-1.8) | 1.50±0.12 (1.30-1.65) |
| ACC time (min) | 48±20.6 (19-104) | 62±29.4 (25-92) |
| CPB time (min) | 80±30.2 (40-156) | 99±40.8 (52-159) |
| Drain output during surgery (ml) | 580±180 (150-1000) | 616±231 (300-900) |
| Chest tube output at 24 h (ml) | 396±365 (80-950) | 1335±327 (1000-1960) |
CPB: Cardiopulmonary bypass, ACC: Aortic cross clamp, BSA: Body surface area
Correlation of coagulation and thromboelastography parameters with postoperative blood loss
In preoperative samples, none of the TEG parameters showed correlation with blood loss. However, in postoperative samples, among TEG parameters MA, r-time, and k-time showed a significant correlation with blood loss. Negative significant correlation was observed between blood loss and postoperative MA (r = −0.4 and P = 0.003) whereas positive significant correlation was seen between blood loss and postoperative r-Time (r = 0.4 and P = 0.003) and k-Time (r = 0.3 and P = 0.01) [Figure 1b].
ROC curves were derived to know how accurately different coagulation and TEG parameters detected bleeders. The accuracy of the test which means how well the test separates the bleeders from nonbleeders is measured by the area under the ROC curve. TEG parameters R time and MA [Figure 1c] in postoperative samples were the only parameters that predicted bleeders with fair accuracy (area under the curve was between 0.70 and 0.80). No RCT was found to be able to predict blood loss.
Discussion
Cardiac surgery with the use of CPB is a scenario that results in widespread, multifactorial activation of the hemostatic system. The various factors that disturb hemostasis during cardiac surgery are as follows: induced hypothermia, hemodilution, coagulation activation, endothelial injury, platelet activation, and dysfunction and fibrinolytic system activation.[1]
Bleeding management after CPB is commonly guided by plasma-based assays, i.e., PT, APTT, and fibrinogen level in addition to platelet count. However, these tests have long (30–60 min) turn-around times. TEG – a point-of-care test for monitoring coagulation during or after CPB.
In the present study, we evaluated relationship of TEG parameters with corresponding routine laboratory coagulation tests. In our study, the TEG parameters, i.e., alpha angle and MA had a significant correlation with fibrinogen and strong correlation between MA and platelet count in postoperative samples. These findings are similar to the previous studies by Welsby et al.,[16] Welsh et al.,[17] and Ozolina et al.,[11] who had also reported strong correlation between postoperative MA and postoperative platelet counts whereas Welsby et al.[16] also reported moderate correlation between postoperative MA and postoperative fibrinogen level. Moderate correlation was shown between the postoperative r-Time and PT by Welsh et al.,[17] however, no such correlation was seen in our as well as by Welsby et al.[16] Ozolina et al.[11] has shown correlation between preoperative r-Time and preoperative APTT; however, our study and studies by Welsby et al.[16] Welsh et al.[17] showed no such correlation. Contrary to these and our study, Dorman et al.[12] have shown that there is no correlation between preoperative and postoperative TEG and RCT parameters. Narani[18] has reported that it is not possible to correlate TEG parameters with conventional coagulation profile as both techniques are different. Contrary to which our study has shown that moderate significant correlation exists between fibrinogen and alpha angle as well as MA in both pre- and postoperative samples. However, such significant correlation between MA and platelet count was seen in only postoperative samples.
We also evaluated the utility of TEG and routine coagulation parameters to predict postoperative blood loss in patients undergoing cardiac surgery on CPB. Based on criteria of postoperative blood loss mentioned in material and methods, we identified six bleeders. The incidence of bleeders in our study (12%) was lower as compared to other studies in which it ranged from 17% to 36%.[11,13,17]
Spiess et al.[9] in their study concerning TEG and blood loss in cardiac surgery patients had shown that TEG was a significantly better predictor (87% accuracy) of postoperative hemorrhage than was the activated clotting time (30%) or coagulation profile (51%). Ereth et al.[10] have reported that TEG was more predictive of blood loss than PACT, ACT, and clotting studies.
In our study, postoperative r-Time, k-Time, and MA were found to be significantly better predictor of postoperative bleeding than other TEG parameters. None of RCT showed any correlation studies with postoperative blood loss. ROC curves were derived to know how accurately different coagulation parameters detect bleeders. Among these parameters, postoperative r-Time and MA have shown fair accuracy in predicting bleeders. Studies by Welsby et al.,[16] Ozolina et al.,[11] and Welsh et al.[17] have shown that TEG is a better predictor of postoperative bleeding than RCT similar to our study.
There are some studies, for example, Wang et al.[14] which found no correlation between the amount of postoperative chest tube drainage and TEG variables. Dorman et al.[12] also reported that TEG failed to predict intraoperative blood loss. Nuttall et al.[13] found no correlation between TEG done after CPB and 24-h blood loss. Sharma et al.[15] in their retrospective study found that adding TEG angle and MA to clinical parameters did not improve chest tube output predictability.
Routine coagulation parameters were not found to be associated with postoperative blood loss in our study similar to study by Ti et al.[19] though Ozolina et al.[11] has reported that preoperative APTT can predict blood loss.
In our study, we could not evaluate the utility of TEG parameters in guiding blood component therapy as the number of bleeders in our study was relatively small and only five out of six patients of this group were provided blood component support by packed RBC, platelet, fresh frozen plasma, or cryoprecipitate.
Conclusion
Although RCT measures the various components of the hemostasis in isolation, while TEG measures the various components of hemostasis as they interact with one another in vivo, a few RCT, for example, platelet count and fibrinogen correlated with corresponding TEG parameters, i.e., MA and alpha angle. TEG parameters (R time and MA in postoperative samples) were able to predict blood loss better than RCT.
Financial support and sponsorship
Nil.
Conflicts of interest
It has been partially presented as:
“Correlation of TEG parameters with routine coagulation tests in patients undergoing cardiac bypass surgery” in ISLH 2016, and has been abstracted in International Journal of Laboratory Hematology, Volume 38, Issue S2, May 2016, Page 96.
“Utility of thromboelastography (TEG) and routine coagulation tests to predict postoperative bleeding in cardiac surgery with cardiopulmonary bypass” in ILSH 2017 has been abstracted in International Journal Of Laboratory Hematology, Volume 39, Issue S2, May 2017, Page 106.
References
- 1.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med. 2004;30:1873–81. doi: 10.1007/s00134-004-2388-0. [DOI] [PubMed] [Google Scholar]
- 2.Espinosa A, Stenseth R, Videm V, Pleym H. Comparison of three point-of-care testing devices to detect hemostatic changes in adult elective cardiac surgery: A prospective observational study. BMC Anesthesiol. 2014;14:80. doi: 10.1186/1471-2253-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curry AN, Pierce JT. Conventional and near-patient tests of coagulation. Contin Educ Anaesth Crit Care Pain. 2007;7:45–50. [Google Scholar]
- 4.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 5.Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680–97. [PubMed] [Google Scholar]
- 6.Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–33. doi: 10.1213/ANE.0b013e3182354b7e. [DOI] [PubMed] [Google Scholar]
- 7.Chandler WL. Effects of hemodilution, blood loss, and consumption on hemostatic factor levels during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2005;19:459–67. doi: 10.1053/j.jvca.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 9.Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD, et al. Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit. 1987;3:25–30. doi: 10.1007/BF00770880. [DOI] [PubMed] [Google Scholar]
- 10.Ereth MH, Nuttall GA, Klindworth JT, MacVeigh I, Santrach PJ, Orszulak TA, et al. Does the platelet-activated clotting test (HemoSTATUS) predict blood loss and platelet dysfunction associated with cardiopulmonary bypass? Anesth Analg. 1997;85:259–64. doi: 10.1097/00000539-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ozolina A, Strike E, Vangas I. The predictive value of thromboelastography and routine coagulation tests for postoperative blood loss in open heart surgery. Acta Chir Latviensis. 2010;10:34–8. [Google Scholar]
- 12.Dorman BH, Spinale FG, Bailey MK, Kratz JM, Roy RC. Identification of patients at risk for excessive blood loss during coronary artery bypass surgery: Thromboelastography versus coagulation screen. Anesth Analg. 1993;76:694–700. doi: 10.1213/00000539-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:815–23. doi: 10.1016/s1053-0770(97)90112-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang JS, Lin CY, Hung WT, O’Connor MF, Thisted RA, Lee BK, et al. Thromboelastogram fails to predict postoperative hemorrhage in cardiac patients. Ann Thorac Surg. 1992;53:435–9. doi: 10.1016/0003-4975(92)90264-5. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AD, Al-Achi A, Seccombe JF, Hummel R, Preston M, Behrend D, et al. Does incorporation of thromboelastography improve bleeding prediction following adult cardiac surgery? Blood Coagul Fibrinolysis. 2014;25:561–70. doi: 10.1097/MBC.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsby IJ, Jiao K, Ortel TL, Brudney CS, Roche AM, Bennett-Guerrero E, et al. The kaolin-activated thrombelastograph predicts bleeding after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:531–5. doi: 10.1053/j.jvca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Welsh KJ, Padilla A, Dasgupta A, Nguyen AN, Wahed A. Thromboelastography is a suboptimal test for determination of the underlying cause of bleeding associated with cardiopulmonary bypass and may not predict a hypercoagulable state. Am J Clin Pathol. 2014;142:492–7. doi: 10.1309/AJCPVB73TMIDFNCB. [DOI] [PubMed] [Google Scholar]
- 18.Narani KK. Thrombelastography in the perioperative period. Indian J Anaesth. 2005;49:89–95. [Google Scholar]
- 19.Ti LK, Cheong KF, Chen FG. Prediction of excessive bleeding after coronary artery bypass graft surgery: The influence of timing and heparinase on thromboelastography. J Cardiothorac Vasc Anesth. 2002;16:545–50. doi: 10.1053/jcan.2002.126945. [DOI] [PubMed] [Google Scholar]