Abstract
Background
This study aims to subdivide BI-RADS-MRI (Breast Imaging Reporting and Data System Magnetic Resonance Imaging) Category 4 lesions and to evaluate the role of Fischer’s scoring system, apparent diffusion coefficient (ADC), and Fischer’s + ADC in differential diagnosis of breast lesions.
Material/Methods
This study retrospectively analyzed the data of 143 patients (150 breast lesions), who were diagnosed by biopsy, and received dynamic contrast enhancement and diffusion-weighted imaging. The diagnostic efficacies of ADC, Fischer’s scoring system, and the Fischer’s + ADC were analyzed by the receiver operating characteristics curve. The area under the curve (AUC) was calculated. Fischer’s scoring system and the Fischer’s + ADC were used to subdivide BI-RADS Category 4 breast lesions.
Results
ADC value was negatively correlated with the tumor grade. The AUC of Fischer’s + ADC (0.949) was significantly higher than that of ADC (0.855) and Fischer’s (0.912) (P=0.0008 and 0.001, respectively). Scored by Fischer’s scoring system, Category 4 and 5 indicated a likely malignant threshold with sensitivity and specificity of 98.70% and 65.75%, respectively. Scored by the Fischer’s + ADC method, Category 4B and 4C indicated a likely malignant threshold with sensitivity of 97.40% and specificity of 82.19%. Kappa values were 0.63 (ADC), 0.65 (Fischer’s), and 0.80 (Fischer’s + ADC), respectively. The positive predictive value of BI-RADS 4A, 4B, and 4C were 7.69%, 52.38% and 89.29%, respectively.
Conclusions
Fischer’s scoring system combined with ADC could reasonably subdivide Category 4 breast lesions with high specificity and sensitivity.
MeSH Keywords: Breast, Diffusion Magnetic Resonance Imaging, Magnetic Resonance Imaging
Background
In the last decade, magnetic resonance imaging (MRI) has increasingly been used to detect, localize, and characterize breast lesions. Many researchers focus on how to assess morphological characteristics for improving the diagnostic value of MRI in breast lesions [1–3]. In 1999, Fischer et al. [3] developed a scoring system to help the radiologist identify benign and malignant breast lesions. This scoring system emphasized the morphological characteristics (including shape, margin, and internal enhancement characteristics) and hemodynamic characteristics (including initial enhancement ratio and time-to-intensity curve (TIC) types) of breast lesions, and scored these characteristics. This scoring system can help radiologists to analyze imaging and identify benign and malignant breast lesions.
Breast Imaging Reporting and Data System (BI-RADS) was developed by the American College of Radiology (ACR) to standardize the description for mammography findings and reporting, and to overcome the difficulty of communicating results between radiologists and referring clinicians [4,5]. Then, ACR published a new part of BI-RADS: BI-RADS-MRI lexicon in 2003 [6] and updated it in 2013 [7,8]. BI-RADS-MRI defined 7 assessment categories. In Category 4 lesions, there was a great range of overlap between benign and malignant lesions (>2% but <95% likelihood of malignancy). In 2013, the ACR and BI-RADS identified the diagnostic positive predictive value (PPV) of cutoff points as 4A/4B/4C: Category 4A indicated low malignancy rate (2–10% likelihood of malignancy), Category 4B indicated moderate malignancy rate (10–50% likelihood of malignancy), and Category 4C indicated high malignancy rate (50–95% likelihood of malignancy) [7,8].
Diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) are 2 important methods used to distinguish benign and malignant breast lesions and to improve the diagnostic specificity [9–13]. In this study, we applied Fischer’s scores combined with ADC values for the subdivision of BI-RADS Category 4 lesions. Our results may further improve the diagnostic efficacy of breast MRI.
Material and Methods
Patients
We retrospectively reviewed 143 patients (150 lesions) who underwent breast MRI between July 2013 and December 2015. All the patients were female, and their age ranged from 20 to 69 years (median age of 46.3 years). There were 14 patients with multiple lesions (31 lesions), of which 10 patients had 1 lesion per breast (20 lesions), 1 patient had 3 lesions in 2 breasts (3 lesions), 1 patient had 4 lesions in 2 breasts (4 lesions) and 2 patients had 2 lesions in 1 breast (4 lesions). Of these 31 lesions, 10 lesions did not receive surgery or biopsy and thus were excluded. The other 129 patients had only 1 lesion in 1 breast (129 lesions). Thus, a total of 150 lesions were included in study. All of the patients were not treated before MRI examination and were subsequently treated by surgery or puncture biopsy at 2 weeks after MRI examination. The breast puncture biopsy or tissue pathology results after surgery were used as gold standards to diagnose breast lesion. The patients with age younger than 18 years old, pregnant, breastfeeding, or with previous treatment before MRI (such as biopsy, chemotherapy, etc) were excluded. All patients signed informed consent and the study was approved by our hospital.
MRI scanning
Imaging was performed using a 1.5 Tesla Magnetic Resonance Imaging system (GE, Germany) in combination with the vendor-supplied receive-only 4-channel Breast Array coil. Patients were examined in the prone position. One standardized imaging protocol was applied for all patients. This protocol included a bilateral axial T2WI-FSE sequence (TR 4,660 ms, TE 89.2 ms, matrix 320×256, NEX 2, section thickness/interslice gap=4 mm/1 mm, FOV=320 mm), an axial DWI sequence (using a single shot echo planar imaging, TR 8,400 ms, TE 93.8 ms, NEX 2, matrix 128×128, b value: 0 s/mm2 and 800 s/mm2, diffusion mode 2-ScanTrace, section thickness/interslice gap=4 mm/1 mm, FOV=320 mm) and a DCE MRI of bilateral breasts. A 3-dimensional fat-suppressed axial VIBRANT (volume imaging for breast assessment) sequence (TR5.6 ms, TE2.6 ms, matrices 320×256, FOV=360mm, section thickness=1.0 mm) was used, which was acquired before and after intravenous bolus injection (automatic injector followed by 20 mL saline solution) of 0.1 mmol/kg gadopentetate dimeglumine (Gd-DTPA) with a flow of 2.0 mL/s. Twelve sequences were obtained.
Image analysis
All images were post-processed with an ADW4.3 workstation and evaluated by 2 experienced radiologists (with more than 5 years of experience in breast MRI analysis). ADC maps were calculated from raw DWI images using the scanner software (Functool 4.3, GE). The region of interest (ROI) was selected from the most homogeneous area of the lesion. Three ROI were selected, and the average was calculated. Dynamic enhancement curve measurement was performed in the most obvious areas of enhancement, and measurements were repeated 3 times. The curve with the most difference was selected as the dynamic enhancement of the time-signal intensity curve. The early signal enhancement rate of the second sequence was calculated. The morphological characteristics of the lesions were determined according to the image of dynamic enhancement.
Category of BI-RADS
The results of DCE MRI were interpreted according to Fischer’s scoring system (Table 1) [3,14] and the updates of BI-RADS-MRI mammography lexicon [7,8]. Lesions scored between 0 and 1 were defined as BI-RADS Category 1, lesions scored as 2 were defined as Category 2, lesions scored as 3 were defined as Category 3, lesions scored as 4–5 were defined as Category 4, and lesions scored as 6–8 were defined as Category 5.
Table 1.
Fischer’s scoring system.
| Points | 0 | 1 | 2 |
|---|---|---|---|
| Shape | Round Oval |
Linear Irregular |
– |
| Margin | Well-defined | Ill-defined | – |
| KM patterns | Homogeneous | Inhomogeneous | Ring-like (centripetal enhancement) |
| Initial enhancementa | <50% | 50–100% | >100% |
| Postinitial enhancementb | Continuous increasec | Plateaud | Wash oute |
(Signalmax 1–3 min – Sprecontrast)/Sprecontrast ×100 (%);
(Signal8 min – Signalmax 1–3 min)/Signalmax 1–3 min ×100 (%);
more than +10%;
ranging from +10 to −10%;
less than −10%.
Receiver operating characteristics (ROC) analysis was used to analyze the diagnostic value of ADC. The cutoff point was calculated. Lesions presenting ADC values below the threshold were assigned a score of 0.5 (likely malignancy), and lesions presenting ADC values above the threshold received a score of −0.5 (likely benign). The BI-RADS Category 4 lesions were further subdivided by Fischer’s + ADC scoring (Table 2). If a lesion had less points (4 points) and a higher ADC value (> the cutoff point), it was defined as Category 4A. Lesions with 5 points and a lower ADC value (≤ the cutoff point) were defined as Category 4C. If a lesion had 4 points and a lower ADC value or 5 points and a higher ADC value, it was defined as Category 4B. Lesions of Category 4A were defined as benign lesions, and Category 4B/4C lesions were defined as malignant.
Table 2.
Category of BI-RADS lesions.
| Score | Category | Number of patients (n) | |
|---|---|---|---|
| Fischer’s Score | 0 | 1 | 3 |
| 1 | 1 | 6 | |
| 2 | 2 | 25 | |
| 3 | 3 | 15 | |
| 4 | 4 | 22 | |
| 5 | 4 | 40 | |
| 6 | 5 | 22 | |
| 7 | 5 | 17 | |
| Fischer’s + ADC | 3.5 | 4A | 13 |
| 4.5 | 4B | 21 | |
| 5.5 | 4C | 28 |
Statistical analysis
ROC curves of ADC values, Fischer’s and Fischer’s + ADC scoring were drawn. The cutoff value and area under the curve (AUC) were calculated and compared. The sensitivity, specificity and accuracy were calculated. Kappa analysis was used to analyze and compare the different methods. The Kappa value was interpreted as follows: poor agreement (Kappa ≤0.4), moderate agreement (4< Kappa <0.75), and good agreement (Kappa ≥0.75). Z Test was used. T Test was used to compare the ADC value of benign and malignant lesions. F Test was used to analyze the difference of ADC value among different tumor grade. Spearman correlation analysis was used to analyze the correlation between the ADC value and tumor grade. P<0.05 was considered statistically significant.
Results
MRI and histopathological findings
The imaging quality of the DWI and ADC scans was good. Among the 150 lesions, there were only 11 patients had equal signals on the DWI sequence. We could identify the lesions by referring to other sequences. Other lesions that could not be identified on DWI and ADC sequences were not included in the study. Out of the 150 lesions, 77 (22 non-mass and 55 mass) were malignant lesions and 73 (13 non-mass and 60 mass) were benign lesions. On DCE-MRI, 71.43% (55/77) malignant lesions were masses, and maximum diameters ranged from 7–61 mm (median 29 mm). Totally 82.19% (60/73) benign lesions were masses, and maximum diameters ranged from 7–41 mm (median 23 mm). There were 14 ductal carcinomas in situ, 28 invasive ductal carcinomas, 17 invasive lobular carcinomas, 9 invasive mixed carcinomas, 3 mucinous carcinomas, 3 papillary carcinomas, 1 medullary carcinoma, 1 metastatic tumor, and 1 neuroendocrine carcinoma. The benign breast lesions were: 24 cyclomastopathy, 9 fibroadenomatoid mastopathy, 38 fibroadenomas, 4 adenosis, 4 inflammation (2 with abscess), 1 benign papilloma, 1 cyst, and 1 benign phyllodes tumor. Among the 63 invasive breast carcinomas, there were 14 grade I lesions, 22 grade II lesions, and 27 grade III lesions.
On DWI, ADC value of benign lesions ranged from 0.41–1.89 (mean 1.32). The ADC value of malignant lesions ranged from 0.62–1.73 (mean 0.93), t=−8.997, P<0.01. The ADC value of grade I, II, and III lesions were 0.99±0.14, 0.87±0.12, 0.81±0.096, respectively. There was statistically significant difference in ADC value of three lesions (F=8.467, P<0.01). The Spearman correlation analysis found that the ADC value was negatively correlated with the tumor grade (r=−0.454, P<0.01).
ROC analysis of ADC, Fischer’s scoring system, and Fischer’s + ADC scoring
To identify the cutoff point for ADC value and compare the efficiency of the 3 methods, ROC curves were generated. The ROC showed that the cutoff point for ADC was 1.08×10−3 mm2/s and the AUC was 0.855 (Figure 1). The sensitivity, specificity, and accuracy of ADC were 83.12%, 79.45%, and 83.97%, respectively (Table 3). The AUC of Fischer’s and Fischer’s + ADC scoring systems were 0.912 and 0.949, respectively, and the difference between the areas was 0.037 (Z=3.27, P=0.001). For the Fischer’s + ADC scoring system, the sensitivity was 97.40%, the specificity was 82.19%, and the accuracy was 90.00% (Table 3). Category 4 as the likely malignant threshold had a sensitivity, specificity and accuracy of 98.70%, 65.75%, and 82.67%, respectively (Table 3). The Kappa value of Fischer’s + ADC scoring system method was 0.80, which was significantly higher than that of ADC (0.63) and the Fischer’s scoring system (0.65) (Table 3) (P=0.026 and 0.04, respectively). This result indicates that sensitivity, specificity, and accuracy of the Fischer’s + ADC method were higher than Fischer’s scoring system method alone.
Figure 1.
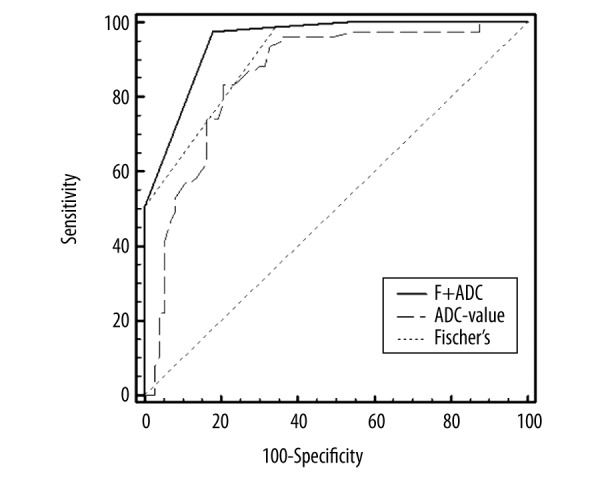
ROC curves analysis of ADC, Fischer’s scoring system, and Fischer’s + ADC for malignant breast lesions. The ROC curves showed higher diagnostic value (i.e., higher specificity, accuracy, and larger AUC) of Fischer’s + ADC method.
Table 3.
Diagnostic comparison of the three methods.
| Sensitivity (95% CI) | Specificity (95% CI) | Accuracy | PPV (95% CI) | NPV (95% CI) | Kappa (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|
| Fischer’s | 98.70% (93.0–100.0%) | 65.75% (53.7–76.5%) | 82.67% | 75.2% (65.6–83.3%) | 98.0% (89.1–99.9%) | 0.65 (0.534–0.766) | 0.912 (0.855–0.952) |
| Fischer’s + ADC | 97.40% (90.9–99.7%) | 82.19% (71.5–90.2%) | 90.00% | 85.2% (76.0–91.9%) | 96.8% (88.8–99.6%) | 0.799 (0.704–0.894) | 0.949 (0.901–0.978) |
| ADC | 83.12% (72.9–90.7%) | 79.45% (68.4–88.0%) | 83.97% | 81.0% (68.5–87.3%) | 81.7% (69.8–89.6%) | 0.626 (0.501–0.751) | 0.855 (0.789–0.907) |
PPV – positive predictive value; NPV – negative predictive value; AUC – area under the ROC curve.
Subdivision of BI-RADS-MRI category 4 lesions
Among the 150 lesions, there were 62 lesions that were classified into BI-RADS-MRI Category 4 by the Fischer’s scoring system (Table 2). The Fischer’s + ADC method was used to subdivide BI-RADS-MRI Category 4 lesions. As shown in Table 2, 13 lesions were classified as Category 4A, 21 lesions were classified as Category 4B, and 28 lesions were classified as Category 4C. Out of the Category 4A lesions, 1 was malignant and 12 were benign. There were 11 and 25 malignant lesions in Category 4B and Category 4C lesions, respectively. Figures 2–4 show the Category4A/4B/4C lesions, respectively. The PPV of BI-RADS Categories 4A, 4B, and 4C were 7.69%, 52.38%, and 89.29%, respectively (Table 4). This indicates that the Fischer’s + ADC method can subdivide Category 4 lesions reasonably.
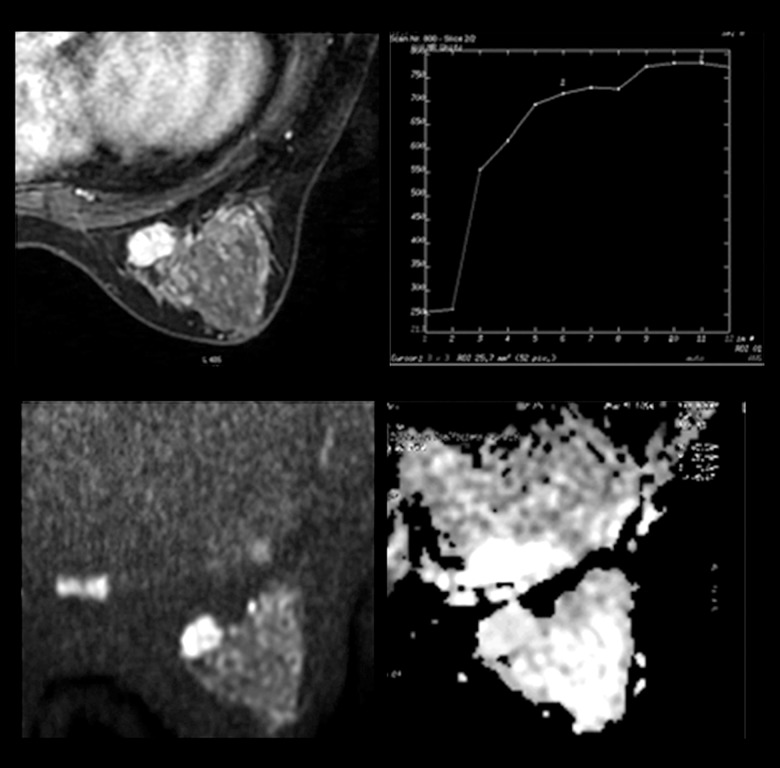
Figure 2.
MRI of a 40-year-old patient showing a lesion in the left breast. Dynamic contrast-enhanced images revealed an oval mass enhancement with in-heterogeneous internal enhancement, and a continuous increasing curve type. Initial enhancement was 120% and the ADC value was 1.77×10−3 mm2/s. The lesion was rated as BI-RADS Category 4A. The pathology was fibroadenomas.
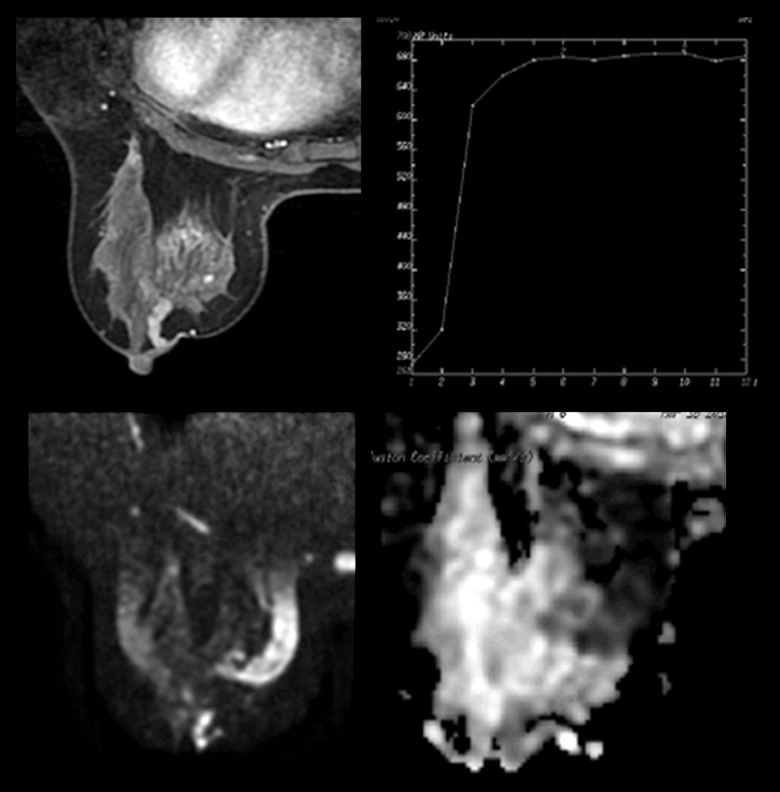
Figure 3.
MRI finding of a 36-year-old patient with a lesion in the left breast. Dynamic contrast-enhanced images showed a linear non-mass enhancement with heterogeneous internal enhancement, circumscribed margin and a plateau curve type. Initial enhancement was 105% and the ADC value was 1.04×10−3 mm2/s. The lesion was rated as BI-RADS Category 4B. The pathology was ductal carcinoma in situ.
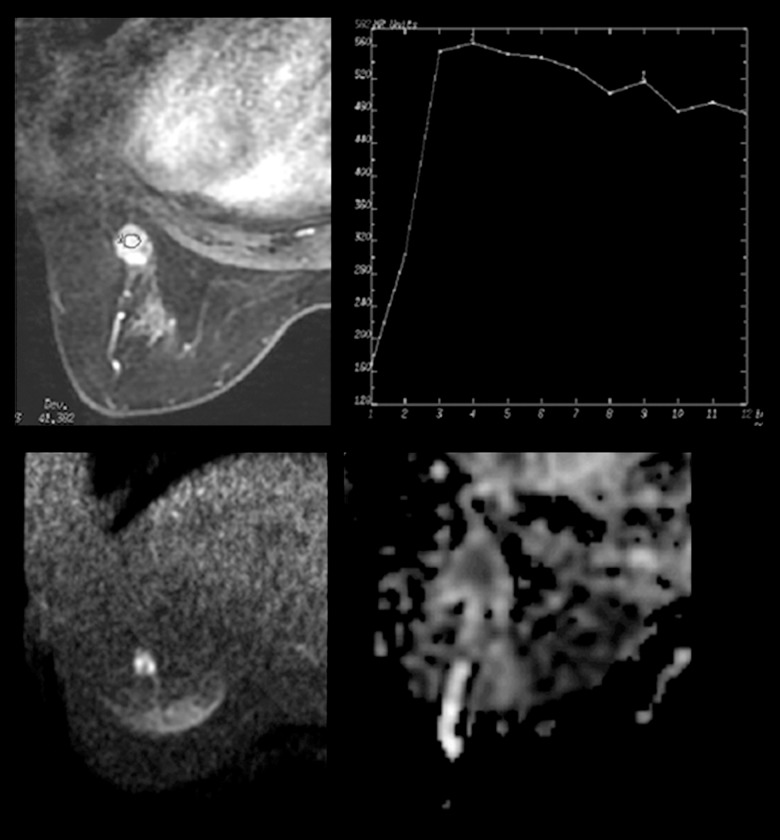
Figure 4.
MRI finding of a 58-year-old patient with a lesion in the left breast. Dynamic contrast enhanced images showed a round mass enhancement with in-heterogeneous internal enhancement, a regular circumscribed margin and a washed-out curve type. Initial enhancement was 228% and the ADC value was 0.96×10−3 mm2/s. The lesion was rated BI-RADS Category 4C. The pathology was invasive lobular carcinomas.
Table 4.
The PPV of Category 4A, 4B and 4C.
| Category | Benign | Malignant | PPV (95%CI) |
|---|---|---|---|
| 4A | 12 | 1 | 7.7% |
| 4B | 10 | 11 | 52.4% (29.8–74.3%) |
| 4C | 3 | 25 | 89.3% (71.3–97.8%) |
Discussion
As we know, the sensitivity of contrast enhanced MRI diagnosis of breast lesions has improved significantly, but the specificity is low [15,16]. In our study, we found the sensitivity was 98.70%, but the specificity was 65.75%. However, high sensitivity leads to the problem of over-treatment, resulting in a high rate of puncture biopsies [17–19]. Many studies have tested the effectiveness of DWI in benign-malignant differentiation of breast lesions and find that ADC values can increase the specificity of breast MRI [20,21]. For example, Li et al. [22] found that ADC values were significantly lower in malignant than in benign lesions. Consistently, we found that the malignant lesions had lower ADC value and benign lesions had higher ADC value. And, the ADC value was negatively correlated to the tumor grade. ADC values can also significantly reduce the false positive rate (FPR) and avoid unnecessary puncture biopsies [19,21,23]. For example, Pasian et al. [19] found that DCE MRI combined with ADC could reduce the FPR and the rate of puncture biopsies. However, the role of DWI and DCE MRI in subdividing BI-RADS-MRI Category 4 is unclear. Therefore, how to combine ADC and DCE effectively is crucial.
The malignant possibility of BI-RADS-MRI Category 4 is 2–95% [7,8], and the ACR suggests that a biopsy should be considered [7,8]. Benign lesions account for a large part of the Category 4 lesions and thus many benign lesions have been treated with unnecessary puncture biopsies [19,21]. It is necessary to subdivide BI-RADS Category 4 lesions to avoid unnecessary biopsies. Siegmann et al. [24] carried out a study on 100 lesions by scoring, with pathology as the gold standard, to calculate the PPV scores, and then they were translated into BI-RADS categories. They found that there was a good correspondence between BI-RADS categories and scores, and they defined 4 points as BI-RADS-MRI Category 4A, for which the PPV was 18.2%. In this study, we combined ADC values and DCE imaging to subdivide Category 4 lesions. Fischer’s scoring system was performed to analyze the scores of DCE imaging, and lesions with scores of 4 and 5 points were considered BI-RIDS Category 4. If a lesion had less points (4 points) and an ADC value >1.08×10−3 mm2/s, it tended to be benign, and it was defined as Category 4A. Lesions with 5 points and an ADC value ≤1.08×10−3 mm2/s tended to be malignant, and they were defined as Category 4C. If a lesion had 4 points and a lower ADC value or 5 points and a higher ADC value, it was defined as Category 4B. We found the PPV of the Categories 4A, 4B, and 4C were 7.69%, 52.38% and 89.29%, respectively. The results of 4A and 4C were in accordance with the criteria for the subdivision of BI-RADS Category 4 lesions [7]. But, the PPV of Category 4B was little higher than the established reporting guidelines (10–50% likelihood of malignancy). We speculate that this may be due to the small sample size. Therefore, the Fischer’s + ADC method can be used to subdivide BI-RADS Category 4 lesions.
In our study, we assigned BI-RADS-MRI Category 4A and the subsequent lesions as benign lesions, and BI-RADS-MRI Category 4B and more were assigned as malignant lesions. The results showed that the combined method was better than Fischer’s scoring method, with excellent sensitivity (97.40%), increased diagnostic specificity (82.19% vs. 65.75%) and improved diagnostic consistency significantly. We suppose that the increased specificity is due to the reasonable subdivision of BI-RADS-MRI Category 4 lesions. In our study, among the 13 lesions of Category 4A, 12 lesions were pathologically diagnosed as benign lesions and 1 as malignant lesion, with decreased FPR and improved diagnosis specificity. As a functional MRI method, DWI can improve the diagnostic accuracy of breast MRI [9–13,21]. Our study showed that the accuracy of combined methods (90.00%) was higher than the Fischer’s (82.67%) and ADC (83.97%) method. Thus, the diagnostic value of breast MRI can be substantially improved by combining functional imaging with morphologic and hemodynamic characteristics of the lesion, avoiding missed diagnoses and unnecessary treatments.
Our study had some limitations. First, there were few non-mass lesions (only 35 lesions) in our study. Therefore, mass and non-mass lesions were not analyzed separately. Second, the sample size was relatively small, which may cause some bias. In our study, the sensitivity of all the methods was high and therefore large sample studies are needed for confirmation. Third, this study only assessed the value of MRI in the diagnosis of breast lesions. Recently, researchers have found that contrast-enhanced spectral mammography is also a good method for detecting malignant breast lesions [25]. So, the effective combination of MRI, x-ray, and ultrasound still needs further study.
Conclusions
In summary, Fischer’s method combined with ADC can subdivide BI-RADS Category 4 lesions easily, reasonably, and practically. Lesions categorized as 4A and below are determined to be benign, which can significantly improve the diagnostic specificity and accuracy of diagnosis, and maintain high sensitivity. The method is a clinically applicable way of improving the diagnostic accuracy of breast MRI in the clinics.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Gilles R, Guinebretiere JM, Toussaint C. Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology. 1994;191:633–38. doi: 10.1148/radiology.191.3.8184039. [DOI] [PubMed] [Google Scholar]
- 2.Heywang-Kobrunner SH. Contrast-enhanced magnetic resonance imaging of the breast. Invest Radiol. 1994;29:94–104. doi: 10.1097/00004424-199401000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881–88. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 4.American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS) Reston, VA: American College of Radiology; 1995. [Google Scholar]
- 5.American College of Radiology. Illustrated breast Imaging Reporting and Data System (BI-RADSTM) Reston, VA: American College of Radiology; 1998. [Google Scholar]
- 6.D’Orsi CJ, Mendelson EB, Ikeda DM. Breastimaging reporting and data system: ACR BI-RADS breast imaging atlas. Reston (VA): American College of Radiology; 2003. [Google Scholar]
- 7.D’Orsi CJ, Sickles EA, Mendelson EB. ACR BI-RADS atlas, breast imaging reporting and data system. 5th ed. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 8.Mercado CL. BI-RADS Update. Radiol Clin N Am. 2014;52:481–87. doi: 10.1016/j.rcl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Baltzer A, Dietzel M, Kaiser CG, Baltzer PA. Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol. 2016;26:884–89. doi: 10.1007/s00330-015-3886-x. [DOI] [PubMed] [Google Scholar]
- 10.Marini C, Iacconi C, Giannelli M, et al. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007;17:2646–55. doi: 10.1007/s00330-007-0621-2. [DOI] [PubMed] [Google Scholar]
- 11.Çabuk G, Nass Duce M, Özgür A, et al. The diagnostic value of diffusion-weighted imaging and the apparent diffusion coefficient values in the differentiation of benign and malignant breast lesions. J Med Imaging Radiat Oncol. 2015;59:141–48. doi: 10.1111/1754-9485.12273. [DOI] [PubMed] [Google Scholar]
- 12.Kul S, Eyuboglu I, Cansu A, Alhan E. Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J Magn Reson Imaging. 2014;40:1158–64. doi: 10.1002/jmri.24491. [DOI] [PubMed] [Google Scholar]
- 13.Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: A systematic review and meta-analysis. Eur Radiol. 2014;24:2835–47. doi: 10.1007/s00330-014-3338-z. [DOI] [PubMed] [Google Scholar]
- 14.Baum F, Fischer U, Vosshenrich R, Grabbe E. Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol. 2002;12:1087–92. doi: 10.1007/s00330-001-1213-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Guan H, Li M, et al. Significance of the ADC ratio in the differential diagnosis of breast lesions. Acta Radiol. 2016;57:422–29. doi: 10.1177/0284185115590286. [DOI] [PubMed] [Google Scholar]
- 16.Aribal E, Asadov R, Ramazan A, et al. Multiparametric breast MRI with 3T: Effectivity of combination of contrast enhanced MRI, DWI and 1H single voxel spectroscopy indifferentiation of breast tumors. Eur Radiol. 2016;85:979–86. doi: 10.1016/j.ejrad.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–78. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 18.Partridge SC, DeMartini WB, Kurland BF, et al. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. Am J Roentgenol. 2009;193:1716–22. doi: 10.2214/AJR.08.2139. [DOI] [PubMed] [Google Scholar]
- 19.Parsian S, Rahbar H, Allison KH, et al. Nonmalignant breast lesions: ADCs of benign and high risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265:696–706. doi: 10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Çabuk G, Nass Duce M, Özgür A, et al. The diagnostic value of diffusion-weighted imaging and the apparent diffusion coefficient values in the differentiation of benign and malignant breast lesions. J Med Imaging Radiat Oncol. 2015;59:141–48. doi: 10.1111/1754-9485.12273. [DOI] [PubMed] [Google Scholar]
- 21.Rubesova E, Grell AS, De Maertelaer V, et al. Quantitative diffusion imaging in breast cancer: A clinical prospective study. J Magn Reson Imaging. 2006;24:319–24. doi: 10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Wang K, Sun XL, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–82. doi: 10.12659/MSM.892534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: Clinical applications. Am J Roentgenol. 1992;159:591–99. doi: 10.2214/ajr.159.3.1503032. [DOI] [PubMed] [Google Scholar]
- 24.Siegmann KC, Moron HU, Baur A, et al. Diagnostic value of a breast MRI score for the prediction of malignancy of breast lesion detected solely with MRI. Rofo. 2009;181:556–63. doi: 10.1055/s-0028-1109156. [DOI] [PubMed] [Google Scholar]
- 25.Łuczyńska E, Paluchowska SH, Hendrick E, et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit. 2015;21:1358–67. doi: 10.12659/MSM.893018. [DOI] [PMC free article] [PubMed] [Google Scholar]