Abstract
Arboviruses are maintained and transmitted through an alternating biological cycle in arthropods and vertebrates, with largely incidental disease in humans and animals. As such, they provide excellent examples of One Health, as their health impact is inextricably linked to their vertebrate hosts, their arthropod vectors and the environment. Prevention and control requires a comprehensive understanding of these interactions, and how they may be effectively and safely modified. This review concentrates on human disease due to Ross River and Murray Valley encephalitis viruses, the two major arboviral pathogens in Australia. It describes how their pattern of infection and disease is influenced by natural climatic and weather patterns, and by anthropogenic activities. The latter includes human-mediated environmental manipulations, such as water impoundment infrastructures, human movements and migration, and community and social changes, such as urban spread into mosquito larval habitats. Effective interventions need to be directed at the environmental precursors of risk. This can best be achieved using One Health approaches to improve collaboration and coordination between different disciplines and cross-sectoral jurisdictions in order to develop more holistic mitigation and control procedures, and to address poorly understood ecological issues through multidisciplinary research.
Keywords: Ross River virus, Murray Valley encephalitis virus, Mosquito vectors, Vertebrate hosts, Climate, One Health
Background
Arboviruses, and particularly mosquito-borne arboviruses, provide excellent examples of One Health (OH) in action. OH recognizes that the health of humans, animals and their ecosystems are interconnected, and that to better understand and respond to zoonotic diseases requires coordinated, collaborative, multidisciplinary and cross-sectoral approaches.1 Arboviruses are perpetuated in complex ecological networks involving the viruses, their vertebrate hosts and the arthropod vectors, and each is governed by its own ecological and environmental parameters. The factors affecting these interactions may be biotic (such as viral strain, vector genetics, vector competence, vectorial capacity, host susceptibility and immune status) or abiotic.2,3 The latter includes the effects of climate and weather (moisture, temperature, humidity, wind velocity, atmospheric pressure), as well as anthropogenic factors such as urbanization, land use changes, dam building, population growth and globalization.2–6 These particularly impinge on the emergence and spread of viruses in existing and new geographic habitats, both directly and indirectly by influencing urban planning and community behaviour.5,7,8
Arboviruses exist in nature in transmission cycles between arthropod vectors and vertebrate hosts, replicating in each; and many also cause zoonotic infections of humans.3,5,9 These are frequently subclinical, but when they cause disease, it can vary from mild febrile illness, sometimes with rash and polyarthralgia, to severe neurological disease, such as encephalitis or haemorrhagic fever.9 Humans are generally dead-end or inefficient hosts, but for some viruses, such as chikungunya, dengue (DENV), yellow fever and Zika viruses, are able to be maintained in a human–mosquito cycle.10,11
Thus, arboviruses readily fulfil the basic tenets of OH, that is, the need to adopt an integrative effort across multiple disciplines addressing peoples, animals and the environment. Indeed, studies of arboviral ecology were using OH approaches long before the term was first employed. In this review, the experience with two Australian arboviruses, Murray Valley encephalitis virus (MVEV) and Ross River virus (RRV), is used to demonstrate the importance of OH approaches in developing mitigation and control strategies.
Australian arboviruses
Over 70 arboviruses have been reported from Australia, but only ten are associated with human infections. They are the flaviviruses MVEV, West Nile Kunjin strain (WNVkun), Edge Hill, Stratford and Kokobera viruses; the alphaviruses RRV, Barmah Forest (BFV) and Sindbis viruses; and the bunyaviruses Trubanaman and Gan Gan viruses.12–14 MVEV and RRV are the most important human pathogens.
Dengue is frequently reported in Australia, with over 1000 notifications annually, nearly all of whom are travellers.15 North Queensland, where Aedes aegypti mosquitoes occur, is the only region susceptible to local transmission and intermittent epidemic activity.16,17 Japanese encephalitis virus enters Torres Strait islands relatively frequently, but has only once caused human infection on mainland Australia.18,19
RRV and BFV are the most frequently notified arboviral diseases in Australia,20 averaging 5400 and 1600 cases annually over the last decade, although mild or asymptomatic infections are under-diagnosed. They cause similar illnesses and appear to share vector species, but less is known about the vertebrate hosts of BFV.12,14,21 While their seasonality and transmission cycles overlap, independent outbreaks also occur.
MVEV and WNVkun are enzootic in northern Australia12–14,22 and, while the infections are predominantly mild or asymptomatic, cause a small number of sporadic human cases of encephalitis annually. Prior to 2000, encephalitis was associated with infections in indigenous populations, but as the mining and tourism industries have expanded in northern Australia, the risks to non-Aboriginal workers and tourists have increased.23,24
WNVkun causes mild human disease, with only rare cases of non-fatal encephalitis and rare cases of disease in horses. WNVkun and MVEV share similar vectors and vertebrate hosts, but vary in geographic incidence, possibly due to different vector preferences. WNVkun isolates comprise WNV lineage 1b,25 and are distinct from Kunjin strains from Sarawak.25,26 An unprecendented epidemic of approximately 900 cases of WNVkun equine encephalitis occurred in 2011 in southeastern Australia.27
The ecology of Ross River virus
RRV is a highly adaptable alphavirus, with outbreaks of human disease across environments ranging from tropical to temperate regions, as well as occasional acute activity in arid areas. The Northern Territory (NT) and Queensland have the highest mean annual incidence rates, at 117 and 59 per 100 000 population, respectively, compared with 33 per 100 000 in WA.20 While the incidence rate of RRV disease is highest in rural and remote regions of Australia, the largest number of human cases occur in the densely populated semi-rural and urban environments of Queensland.20 Clinical illness occurs in <2–75% of those infected, and typically includes malaise, fatigue, and muscle and joint pains, with fever in 50% and a generalized rash in 50–60%. Marked fatigue, worsening joint pain and swelling develop in 80–90% of patients and commonly persist for months.28 Severe illness is rare, although glomerulonephritis has been described.29 The burden of illness is related mainly to the social and economic costs of the prolonged post-acute illness and long-term sequelae. The average burden has been estimated at approximately AU$1100/per patient for the medical and social costs of the first week of the acute illness,30 and a further AU$4400/patient for post-acute illness and long-term sequelae, largely associated with loss of income.31
RRV has been isolated from over 40 mosquito species, many of which are implicated in its transmission.32 Principal rural vectors include Ae. camptorhynchus, Ae. vigilax (in coastal regions) and Culex annulirostris (inland and coastal areas), while a container-inhabiting species, Ae. notoscriptus, is also implicated in urban areas. Several other species act as vectors in certain regions or are associated with particular environmental conditions.
Marsupials, especially macropods (kangaroos and wallabies), are regarded as important amplifying hosts of RRV, as may be possums and horses.33–35 Several other domestic and wild animal species have also been implicated, and circumstantial evidence supports a role for humans in moving the virus from endemic to epizootic regions, and possibly maintaining low-level inefficient transmission cycles.32,35,36
The adaptability of RRV to Australian environments was illustrated by evidence of virus persistence in the arid north-west of Western Australia (WA) in desiccation-resistant eggs of Aedes spp. mosquitoes,37 which was linked to rapid reappearance of the virus and associated human infections soon after drought-breaking rain.
Surveillance of mosquito populations, infection rates in mosquitoes and analysis of environmental conditions (particularly rainfall, tides and temperature) have proved to be effective in several Australian jurisdictions as an early warning of increased risk of human RRV infection. In south-west WA, this work has also demonstrated the significance of other complex inter-relationships between RRV, its mosquito vectors, vertebrate hosts and the environment (natural and human-mediated) in determining the risk to human health. For example, major outbreaks of RRV in south-west WA occur every 3–4 years, even though environmental conditions support more frequent substantial mosquito breeding. Evidence from serosurveys and the known feeding habits of the major recognized mosquito vector in the region (Ae. camptorhynchus) implicate the western grey kangaroos (Macropus fuliginosus) as the major vertebrate host.34,38 Therefore, their abundance and immune status to RRV may be as important as that of the vector mosquito population in determining virus activity.
The ecology of Murray Valley encephalitis virus
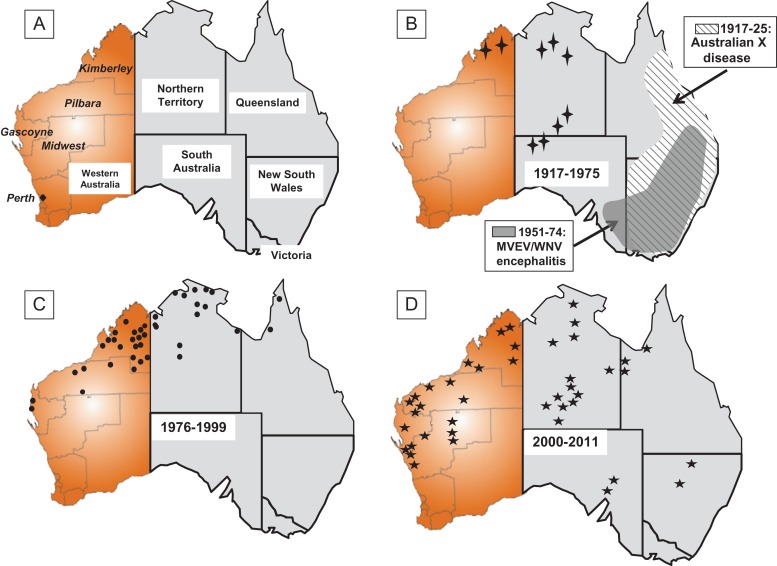
Virus detection and sentinel chicken data show that MVEV exists in enzootic foci in the Kimberley region of WA, the north of the NT and northern Queensland. The virus is epizootic in the Pilbara, Gascoyne and Mid-West regions of WA and southern parts of the NT.12,13,23,39 Very occasionally, MVEV spreads from enzootic areas to the south-eastern Australia, where it usually causes significant outbreaks of encephalitis, three of which occurred in 1950–51, 1974 and 2011,23,40 and four much earlier outbreaks of presumed MVEV encephalitis between 1917 and 1925 (Figure 1B).40,41 MVEV also occurs in Papua New Guinea, and possibly in the eastern Indonesia archipelago.26,40,42
Figure 1.
Maps of mainland Australia showing human cases of encephalitis known or presumed to be due to Murray Valley encephalitis virus in Australia. Outbreaks 1917–1925 were clinical diagnoses only and labelled as Australian X disease. While the cases between 1951 and 1975 in south-eastern Australia could not be reliably attributed to MVEV or the Kunjin strain of West Nile virus, the great majority are believed to be MVEV infections. All other cases were confirmed MVEV infections: (A) map showing the states and territories, plus the regions in Western Australia where cases occurred; (B)  1969 and 1975; (C)
1969 and 1975; (C)  1976–1999; (D)
1976–1999; (D)  2000–2011.
2000–2011.
Most infections with MVEV are asymptomatic or cause a non-specific febrile illness usually accompanied by headache, myalgia and occasionally rash. Clinical encephalitis occurs in 1:150 to 1:1000 infections, with disease varying from fever, headache and altered mental state to coma and severe flaccid paralysis.43 The mortality rate is 15–30%, and long-term neurological sequelae occur in 30–50%,23,43 while depression or other psychiatric illnesses are common in survivors.44
The major vertebrate hosts are believed to be herons and egrets, particularly the Nankeen (or rufous) night heron (Nycticorax caledonicus). Members of other avian orders may also be adventitious hosts. Many domestic and wild animals have antibodies to MVEV, but only rabbits and possibly western grey kangaroos develop significant levels of viraemia regarded as sufficient to support local transmission cycles.40,45 MVEV also causes fatal encephalitis in horses,46 but levels of viraemia are probably insufficient to infect recipient mosquitoes.
The first mosquito isolates of MVEV were obtained from Cx. annulirostris mosquitoes, collected in north Queensland. This species is recognized as the principal vector, accounting for over 90% of all isolates, and is a competent laboratory vector of MVEV.12–14,40 MVEV has also been occasionally isolated from a number of other mosquito species, but it is not known whether they are competent to transmit the virus.12,14,40 MVEV is believed to survive over the dry season in the desiccation-resistant eggs of Aedes sp. and may lie dormant for several seasons in arid areas until sufficient rainfall occurs to cause flooding; support for this contention was obtained with the isolation of MVEV from infected male Ae. tremulus mosquitoes, but this does not preclude the possibility of a low level of localized transmission in some instances.47
Serological evidence indicates that MVEV has been present in the Kimberley region since at least 1959, although the first clinical case was not recorded until 1969, with a second case in 1974. This early activity in WA followed damming of the Ord River in the Kimberley region to provide water for irrigated agriculture, with a diversion dam that provided water to 14 000 ha of irrigated farmland completed in 1963 followed by a much larger dam, Lake Argyle, in 1972. The dams and irrigated agriculture attracted very large numbers of waterbirds of various species and increased mosquito populations.48 From 1975 onwards, cases of human encephalitis were recorded, initially in the Kimberley region, but later spreading further south into the Pilbara and Gascoyne regions, shown in Figure 1C and D, and comprised over 60% of all Australian cases. The population of northern WA is small, but rising rapidly due to increased mining, agriculture and tourism, which have driven immigration to support these industries, but this is not, of itself, sufficient to account for the increased incidence and spread of MVEV cases. It has been hypothesized that the dam and increased irrigated agriculture expanded the enzootic focus of MVEV activity in the region. That then acted as a source of virus spread to the south and east through the movement of viraemic waterbirds following monsoonal rainfall and flooding. This was accompanied by increased MVEV amplification between the birds and local mosquito populations, resulting in human infections. Virus may then persist across subsequent dry seasons by survival in desiccation-resistant mosquito eggs, and re-emerging from the infected eggs following periods of rainfall and flooding.
An understanding of climate and weather patterns is critical to predicting risk, as studies have clearly demonstrated the importance of the annual monsoonal weather pattern in the Kimberley, and to a lesser extent the Pilbara, region in maintaining and amplifying enzootic MVEV activity.13,49 This generally occurs from the middle of the wet season and peaks towards the end of the wet season. Epizootic activity is more complex and multifaceted, but studies at Billiluna, an Aboriginal community in the arid south Kimberley, found that MVE activity required heavy rainfall and flooding, both locally and further upstream, in order to attract infected waterbirds from enzootic areas.50
How a One Health approach can be used to develop risk mitigation of future epidemic activity
Understanding the complex interplay between arboviruses, their vectors, animal reservoirs and the environment is crucial in developing risk assessment and mitigation strategies, and in determining research directions. However, these are only part of the OH equation: it is equally important to build strong cross-sectoral and cross-jurisdictional collaboration, and to integrate surveillance mechanisms to improve early warning of virus transmission. These are the OH approaches that together can provide strategic research into improved methodologies for the early detection and control of potential outbreaks.
For example, despite RRV being primarily a zoonotic infection, humans may have a role as secondary hosts, even though their viraemia is short-lived. Circumstantial evidence has implicated humans as sources of introduction and as amplifying hosts during a major epizootic of RRV in south-west Pacific Island nations in 1979–1982.32,36 Similarly, during an outbreak in metropolitan Perth in 2003–2004 the index cases in 42 suburbs had recently returned from epidemic areas in the rural south-west of the state, which had preceded small clusters of cases in those same suburbs in people who had no history of travel outside Perth during the incubation period (Lindsay MDA, unpublished observations). Therefore, humans appeared to have introduced the virus to urban mosquito vectors, and possibly through them to urban reservoir hosts such as possums or macropods. Management programmes for RRV in urban areas should therefore include consideration of urban mosquito larval habitats (vector), backyard and storm water infrastructure breeding sites (environment), and urban wildlife and domestic species as potential reservoir hosts.
The importance of the interactions of humans with urban wetlands and macropod populations was further implicated in a cluster of 111 human cases of RRV that occurred in 2011–2012. Cases were confined to suburbs with densely vegetated wetlands favouring mosquito breeding and a large western grey kangaroo population. That combination created the ideal situation for an epizootic of RRV, once the virus had been introduced into the locality, presumably via a human who returned there after being infected in the concurrent epidemic in south-west WA.
Similar findings of elevated disease risk in residents residing close to extensive natural saltmarsh mosquito habitats have been found for other parts of south-west Australia.51,52 These findings emphasize the importance of considering mosquito, wildlife (vertebrate host) and environmental factors in identifying appropriate uses and probable management requirements for future land-use development in these regions. This will become increasingly important as urban development encroaches on extensive natural mosquito and wildlife habitats, which will be retained and understandably protected from management practices that may impact adversely on the natural environment.
Increased risk of RRV disease resulting from human activities was seen in the tropical Kimberley region of WA following the Ord River irrigation development. That produced a proliferation of larval habitats for the major RRV vector species, which led to activity of RRV prior to the onset of the northern wet season.53 The influence of these permanent, artificial sources of water on population dynamics of vertebrate hosts of RRV in the region is undetermined but also likely to be important.
Current intervention strategies for RRV centre on mosquito surveillance, mosquito management and public awareness campaigns about mosquito avoidance in risk areas. Mosquito management can be effective for disease mitigation, but the necessary large-scale programmes are often beyond the resources of smaller local governments. Optimizing risk assessment and management practices in future require an OH vision that considers not only vector mosquito population and virus data, but also environmental health, wildlife ecology and human health.
Future research needs to include collaborative and multidisciplinary investigations to better understand the environmental conditions that favour amplifying vertebrate hosts, extreme and changing weather affecting transmission, and human activities, such as land-use and urban planning, adaptation to climate change and impact of water management practices on vector, host and human interactions. Such approaches will require coordination and data sharing between multiple state agencies and many local governments across Australia. Sharing resources and knowledge should lead to increased efficiencies, but may also be impacted by competing interests and priorities unless an OH approach is incorporated into policies and long-term strategic plans.
Not surprisingly, there has been discussion about the potential impacts of climate change on the incidence and distribution of RRV. Correlation between RRV activity and the Southern Oscillation Index has been well described, as has the influence of rainfall, tides and temperature in various regions, particularly the south-west of WA.54,55 The complex ecology means that changes in risk will be difficult to predict and will require detailed, locality-specific surveillance of vector, host, environmental and human influences on RRV ecology.
To assist with outbreak planning, various predictive models using meteorological and climatic conditions have been developed for MVEV epidemic activity. The Forbes model is based on above average rainfall in the river catchment basins of eastern Australia,56 while the Nicholls model is based on the Southern Oscillation Index.57 Unfortunately, neither of these two models is directly relevant to WA and, instead, a Bayesian Belief Network model has been proposed that combines various abiotic, biotic and anthropogenic factors that might affect the risk of MVEV into a predictive model, based on the ecology of the major mosquito vector and waterbird hosts of MVEV.58 So far its accuracy has not been prospectively tested, and it will require considerable coordination to gather the data required.
In WA, sentinel chicken serosurveillance and climate surveillance have been the cornerstones in predicting MVEV epidemic activity.13 Across most of the risk areas, the presence of high rainfall and seroconversion of sentinel chickens was predictive of human risk for MVEV infection.59 The exceptions to this were sentinel flocks at permanent dam sites built in the 1980s to provide water to mine sites in the arid Pilbara region, where chicken seroconversions were often restricted to that site and were not necessarily predictive of a human risk in that specific area. This suggested that these reservoirs support focal flavivirus activity between mosquitoes and resident waterbirds, as had been seen in the more northerly Ord River development.48 This was further supported by multiple detections of WNVkun in mosquitoes trapped at one of these dams in 2016 in the absence of activity in other traps in the region. Of concern is that these foci could act as sources for more extensive outbreaks when local flooding occurs.
A similar effect from an anthropogenic change to a water body was seen in a larval habitat near Alice Springs in Central Australia, where effluent discharges from an adjacent sewage farm increased MVEV and WNVkun vector species breeding.60 Following re-emergence of MVEV encephalitis cases in 2000 and 2001, drainage was undertaken, and resulted in a decline in mosquito numbers and sentinel chicken seroconversions.
Getting rapid and reliable information about arbovirus activity in mosquitoes in these remote areas is challenging. Sentinel chicken flocks are difficult to maintain and seroconversions are delayed indicators of virus activity, and they are not useful for viruses, such as RRV, whose vector species do not feed on them. Future surveillance may use a passive sentinel mosquito arbovirus capture kit (SMACK) that detects arboviruses by PCR on honey-soaked nucleic acid preservation cards (Flinders Technology Associates; FTA®) deployed in purpose-built CO2-baited traps.61 These are simple and effective tools that may be used instead of traditional overnight mosquito traps or as a practical substitute for sentinel animal programmes.
Other changes in human activities and human behaviour have dramatically modified the patterns of human infections due to MVEV and RRV. A review of cases of MVE following a national outbreak in 2011 demonstrated that cases had shifted from occurring predominantly among Aboriginal children to occurring mainly in non-Aboriginal adults.23 Increasing movement of non-indigenous adults from the south of the state for work or tourism had preceded this change. Furthermore, most cases had been undertaking specific actives, such as outdoor sports or camping, that led to heavy mosquito exposure. Similarly, most cases of RRV also link to outdoor activities in areas where natural transmission cycles occur close to human habitation, and demonstrate the importance of urban planning to reduce this contact and of mosquito avoidance to reduce personal exposure. These examples serve to underline the necessity for cross-sectoral and cross-jurisdictional coordination of data sharing to provide advice to the public and urban planners.
Conclusions
This review has demonstrated that the two most important arboviruses in Western Australia causing human disease, RRV and MVEV, are perpetuated in complex ecological networks. The viruses, their vertebrate hosts and their arthropod vectors are each subject to various ecological and environmental factors that determine the risk of human infection. These interconnections between humans, animals and their ecosystems have been recognized in the development of the OH paradigm, which asserts that to better understand and respond to zoonotic diseases, it is necessary to have coordinated, collaborative, multidisciplinary and cross-sectoral approaches. Employing OH strategies has great potential to help prevent or ameliorate human diseases caused by RRV and MVEV, and is relevant to the control of other arboviruses, such as Japanese encephalitis virus.62
Much of our knowledge of the ecology of these viruses has been derived from de facto OH studies that long preceded the introduction of the OH concept, but the OH concept has greatly assisted the recognition of the complexity and in developing a productive approach to elucidating some of the more intractable ecological problems through collaboration and multidisciplinary approaches. These include the determination of the role of different wildlife species as arbovirus hosts, the relevance and involvement of human-to-human transmission in RRV outbreaks, and improved predictive models of MVEV and RRV to provide more accurate early warning of possible outbreaks and identify interventions.
However, OH is also about bringing together the various health sectors to amplify the benefits to all of those sectors. Breaking down disciplinary and jurisdictional silos is an essential requirement for improving future mitigation and control of outbreaks of these two viruses in Australia, and indeed arboviruses elsewhere around the globe. They are prime examples of the benefits of an OH approach in improving coordination and collaboration across multiple disciplines, cross-sectors and cross-jurisdictions essential to planning and initiating interventions to mitigate risk and for providing research directions likely to improve our prevention and control measures.
Acknowledgments
Authors’ disclaimers: All the authors have no conflicts of interest or other disclaimers.
Authors’ contributions: JSM was the principal contributor, but all authors were involved in all aspects of the manuscript, including this revised version.
Acknowledgments: None.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
Guarantor: DWS.
References
- 1. Mackenzie JS, McKinnon M, Jeggo M. One Health: from concept to operationalization In: Yamada A, Kahn LH, Kaplan B, Monath TP, Woodall J, Conti LA (eds) Confronting emerging zoonoses: the One Health paradigm. Tokyo: Springer, 2014;163–89. [Google Scholar]
- 2. Kramer LD. Complexity of virus-vector interactions. Curr Opin Virol 2016;21:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall RA, Prow N, Peters J et al. . Ecological and epidemiological factors influencing arbovirus diversity and evolution In: Vasilakis N and Gubler DJ (eds) Arboviruses: molecular biology, evolution and control. Norwich: Caister Academic Press; 2016, pp. 135–66. [Google Scholar]
- 4. Mackenzie JS, Lindsay MD, Daniels PW. The effect of climate on the incidence of vector borne viral diseases: the potential value of seasonal forecasting In: Hammer G, Nicholls N, Mitchel VI (eds) Applications of seasonal climate forecasting in agriculture and natural ecosystems—the Australian experience. Dordrecht: Kluwer Academic Publishers; 2000, pp.429–52. [Google Scholar]
- 5. Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect 2015;4(3):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev 2004;17:136–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smolinski MS, Hamburg MA, Lederberg J. (eds) Microbial threats to health. Emergence, detection, and response. Washington DC: The National Academies Press, 2003; 1–366. [PubMed] [Google Scholar]
- 8. Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004;10(12 Suppl.):S98–109. [DOI] [PubMed] [Google Scholar]
- 9. Smith DW, Hall RA, Johansen CA et al. . Arbovirus infections In: Cook GC and Zumla AI (eds) Manson's tropical diseases, 22nd edn Philadelphia: Saunders Elsevier; 2009, pp. 715–51. [Google Scholar]
- 10. Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop 2017;166:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martina BE, Barzon L, Pijlman GP et al. . Human to human transmission of arthropod-borne pathogens. Curr Opin Virol 2016;22:13–21. [DOI] [PubMed] [Google Scholar]
- 12. Mackenzie JS, Lindsay MD, Coelen RJ et al. . Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol 1994;136:447–67. [DOI] [PubMed] [Google Scholar]
- 13. Spencer JD, Azoulas J, Broom AK et al. . Murray Valley encephalitis virus surveillance and control initiatives in Australia. National Arbovirus Advisory Committee of the Communicable Diseases Network Australia. Commun Dis Intell Q Rep 2001;25(2):33–47. [PubMed] [Google Scholar]
- 14. Russell RC. Mosquito-borne arboviruses in Australia: the current scene and implications of climate change for human health. Int J Parasitol 1998;28:955–69. [DOI] [PubMed] [Google Scholar]
- 15. Department of Health , Summary information about overseas- acquired vectorborne disease notifications in Australia. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-vectorborne-overseas-acquired.htm (accessed 25 August 2017).
- 16. Warrilow D, Northill JA, Pyke AT. Sources of dengue viruses imported into Queensland, Australia, 2002–2010. Emerg Infect Dis 2012;18:1850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viennet E, Ritchie S, Faddy H, Williams C, Harley D. Epidemiology of dengue in a high-income country: a case study in Queensland, Australia. Parasit Vectors 2014;7:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mackenzie JS, Johansen CA, Ritchie SA et al. . Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia. Curr Topics Microbiol Immunol 2002;267:49–73. [DOI] [PubMed] [Google Scholar]
- 19. van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographic expansion of Japanese encephalitis virus. Ann Rev Entomol 2009;54:17–35. [DOI] [PubMed] [Google Scholar]
- 20. Australian Department of Health , National Notifiable Diseases Surveillance System. http://www9.health.gov.au/cda/source/rpt_4.cfm (accessed 25 August 2017).
- 21. Jacups SP, Whelan PI, Currie BJ. Ross River virus and Barmah Forest virus infections: a review of history, ecology, and predictive models, with implications for tropical northern Australia. Vector Borne Zoonotic Dis 2008;8:283–97. doi:10.1089/vbz.2007.0152. [DOI] [PubMed] [Google Scholar]
- 22. Hall RA, Scherret JH, Mackenzie JS. Kunjin virus: an Australian variant of West Nile? Ann NY Acad Sci. 2001;951:153–60. [PubMed] [Google Scholar]
- 23. Selvey LA, Dailey L, Lindsay M et al. . The changing epidemiology of Murray Valley encephalitis in Australia: the 2011 outbreak and a review of the literature. PLoS Negl Trop Dis 2014;8(1):e2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith DW, Speers DJ, Mackenzie JS. The viruses of Australia and the risk to tourists. Travel Med Infect Dis 2011;9:113–25. [DOI] [PubMed] [Google Scholar]
- 25. Scherret JH, Poidinger M, Mackenzie JS et al. . The relationships between West Nile and Kunjin viruses. Emerg Infect Dis 2001;7:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health 2009;56:338–56. [DOI] [PubMed] [Google Scholar]
- 27. Roche SE, Wicks R, Garner MG et al. . Descriptive overview of the 2011 epidemic of arboviral disease in horses in Australia. Aust Vet J 2013;91:5–13. [DOI] [PubMed] [Google Scholar]
- 28. Smith DW, Mackenzie JS, Frolov I, Weaver SC. The Alphaviruses In: Richman DD, Whitley RJ, Hayden FG (eds) Clinical Virology (4th edn). Washington DC: ASM Press;2017, pp. 1347–79. [Google Scholar]
- 29. Fraser JR, Cunningham AL, Muller HK et al. . Glomerulonephritis in the acute phase of Ross River virus disease (epidemic polyarthritis). Clin Nephrol 1988;29:149–52. [PubMed] [Google Scholar]
- 30. Woodruff R, Bambrick H Climate change impacts on the burden of Ross River virus disease. Garnaut Climate Change Review. http://garnautreview.org.au/CA25734E0016A131/WebObj/03-BRossRivervirus/$File/03-B%20Ross%20River%20virus.pdf (accessed 25 August 2017).
- 31. Prow NA. Epidemiology of Ross River virus in the south-west of Western Australia and assessment of serotype involvement in Ross River virus pathogenesis. PhD Thesis, The University of Western Australia; 2006.
- 32. Claflin SB, Webb CE. Ross River Virus: many vectors and unusual hosts make for an unpredictable pathogen. PLoS Pathog 2015;11(9):e1005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyd AM, Hall RA, Gemmell RT, Kay BH. Experimental infection of Australian brushtail possums, Trichosurus vulpecula (Phalangeridae: Marsupialia), with Ross River and Barmah Forest viruses by use of a natural mosquito vector system. Am J Trop Med Hyg 2001;65:777–82. [DOI] [PubMed] [Google Scholar]
- 34. Potter A, Johansen CA, Fenwick S et al. . The seroprevalence and factors associated with Ross River virus infection in western grey kangaroos (Macropus fuliginosus) in Western Australia. Vector Borne Zoonotic Dis 2014;14:740–5. [DOI] [PubMed] [Google Scholar]
- 35. Koolhof IS, Carver S. Epidemic host community contribution to mosquito-borne disease transmission: Ross River virus. Epidemiol Infect 2017;145:656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harley D, Sleigh A, Ritchie S. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev 2001;14:909–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindsay MD, Broom AK, Wright AE et al. . Ross River virus isolations from mosquitoes in arid regions of Western Australia: implication of vertical transmission as a means of persistence of the virus. Am J Trop Med Hyg 1993;49:686–96. [DOI] [PubMed] [Google Scholar]
- 38. Johansen CA, Power SL, Broom AK. Determination of mosquito (Diptera: Culicidae) bloodmeal sources in Western Australia: implications for arbovirus transmission. J Med Entomol 2009;46:1167–75. [DOI] [PubMed] [Google Scholar]
- 39. Cordova SP, Smith DW, Broom AK et al. . Murray Valley encephalitis in Western Australia in 2000, with evidence of southerly spread. Commun Dis Intell 2000;24:368–72. [PubMed] [Google Scholar]
- 40. Marshall ID. Murray Valley and Kunjin encephalitis In: Monath TP. (ed.) Arboviruses: Epidemiology and Ecology, Volume 3 Boca Raton, FL: CRC Press; 1988, 151–90. [Google Scholar]
- 41. Mackenzie JS, Broom AK. Australian X disease, Murray Valley encephalitis, and the French Connection. Vet Microbiol 1995; 46:79–90. [DOI] [PubMed] [Google Scholar]
- 42. Johansen CA, van den Hurk AF, Ritchie SA et al. . Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea. Am J Trop Med Hyg 2000;62:631–38. [DOI] [PubMed] [Google Scholar]
- 43. Knox J, Cowan RU, Doyle JS et al. . Murray Valley encephalitis: a review of clinical features, diagnosis and treatment. Med J Aust 2012;196: 322–26. [DOI] [PubMed] [Google Scholar]
- 44. Selvey LA, Speers DJ, Smith DW. Long term outcomes of Murray Valley encephalitis cases in Western Australia—what have we learnt? Intern Med J 2016;46:193–201. [DOI] [PubMed] [Google Scholar]
- 45. Carver S, Bestall A, Jardine A, Ostfeld RS. Influence of hosts on the ecology of arboviral transmission: potential mechanisms influencing dengue, Murray Valley encephalitis, and Ross River virus in Australia. Vector-borne Zoo Dis 2009;9:51–64. [DOI] [PubMed] [Google Scholar]
- 46. Mann RA, Fegan M, O'Riley K et al. . Molecular characterization and phylogenetic analysis of Murray Valley encephalitis virus and West Nile virus (Kunjin subtype) from an arbovirus disease outbreak in horses in Victoria, Australia, in 2011. J Vet Diagn Invest 2013;25:35–44. [DOI] [PubMed] [Google Scholar]
- 47. Broom AK, Lindsay MD, Johansen CA et al. . Two possible mechanisms for survival and initiation of Murray Valley encephalitis virus activity in the Kimberley region of Western Australia. Am J Trop Med Hyg 1995;53:95–9. [PubMed] [Google Scholar]
- 48. Mackenzie JS, Broom AK. Ord River irrigation area: the effect of dam construction and irrigation on the incidence of Murray Valley encephalitis In: Kay BH. (editor). Water Resources – Health Environment and Development. London: E & FN Spon; 1999, pp. 108–22. [Google Scholar]
- 49. Mackenzie JS, Lindsay MDL, Broom AK. Climate changes and vector-borne diseases: potential consequences for human health In: Ewan CE, Bryant EA, Calvert GD, Garrick JA (eds) Health in the Greenhouse. The Medical and Environmental Health Effects of Global Climate Change. Canberra: Australian Government Publishing Service; 1993, 229–34. [Google Scholar]
- 50. Broom AK, Lindsay MD, Wright AE et al. . Epizootic activity of Murray Valley encephalitis and Kunjin viruses in an aboriginal community in the southeast Kimberley region of Western Australia: results of mosquito fauna and virus isolation studies. Am J Trop Med Hyg 2003;69:277–83. [PubMed] [Google Scholar]
- 51. Jardine A, Neville PJ, Lindsay MDL. Proximity to Mosquito Breeding Habitat and Ross River Virus Risk in the Peel Region of Western Australia. Vector Borne Zoo Dis 2015; 15:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vally H, Peel M, Dowse GK et al. . Geographic Information Systems used to describe the link between the risk of Ross River virus infection and proximity to the Leschenault estuary, WA. Aust NZ J Public Health 2012;36:229–35. [DOI] [PubMed] [Google Scholar]
- 53. Jardine A, Lindsay M, Heyworth J, Weinstein P. Dry-season mosquito breeding associated with irrigation in the northeast Kimberley Region of Western Australia: potential impact on mosquito-borne disease transmission. Eco Health 2004;1:387–98. [Google Scholar]
- 54. Lindsay MD, Mackenzie JS, Condon RJ (1993). Ross River outbreaks in Western Australia: epidemiological aspects and the role of environmental factors In: Ewan CE, Bryant EA, Calvert GD, Garrick JA (eds) Health in the Greenhouse. The Medical and Environmental Health Effects of Global Climate Change. Canberra: Australian Government Publishing Service; 1993, 85–100. [Google Scholar]
- 55. Woodruff RE, Guest CS, Garner MG et al. . Early warning of Ross River virus epidemics: combining surveillance data on climate and mosquitoes. Epidemiology 2006;17:569–75. [DOI] [PubMed] [Google Scholar]
- 56. Forbes JA. Murray Valley encephalitis 1974, also, the epidemic variance since 1914 and predisposing rainfall patterns. Glebe: Australasian Medical publishing Co; 1978. [Google Scholar]
- 57. Nicholls N. A method for predicting Murray Valley encephalitis in southeast Australia using the Southern Oscillation. Aust J Exp Biol Med Sci 1986;64:587–94. [DOI] [PubMed] [Google Scholar]
- 58. Ho SH, Speldewinde P, Cook A. A Bayesian Belief Network for Murray Valley encephalitis virus risk assessment in Western Australia. Int J Health Geogr 2016;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Selvey LA, Johansen CA, Broom AK et al. . Rainfall and sentinel chicken seroconversions predict human cases of Murray Valley encephalitis in the north of Western Australia. BMC Infect Dis 2014, 14:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jacups S, Kurucz N, Whitters R, Whelan P. Habitat modification for mosquito control in the Ilparpa Swamp, Northern Territory, Australia. J Vector Ecol 2011;36:292–9. [DOI] [PubMed] [Google Scholar]
- 61. Johnson BJ, Kerlin T, Hall-Mendelin S et al. . Development and field evaluation of the sentinel mosquito arbovirus capture kit (SMACK). Parasit Vectors 2015;8:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Impoinvil DE, Baylis M, Solomon T. Japanese encephalitis: On the One Health agenda. Curr Top Microbiol Immunol 2013;365:205–47. [DOI] [PubMed] [Google Scholar]