Abstract
Regulatory frameworks for protein content claims in Canada and the United States are underpinned by the protein efficiency ratio and protein digestibility-corrected amino acid score (PDCAAS), respectively, which are used to assess the protein quality of a given food. The digestible indispensable amino acid score (DIAAS) is a novel approach to measuring the protein quality of foods and is supported by the Food and Agriculture Organization of the United Nations. Methodological concerns about the PDCAAS are addressed by the DIAAS through introduction of the use of ileal amino acid digestibility coefficients and untruncated protein scores. However, before the DIAAS is widely adopted within regulatory frameworks, a comprehensive assessment is required. Accordingly, this review addresses the potential impact of the DIAAS on regulation, communication, and public health, as well as knowledge gaps, analytical challenges, and cost of implementation. A pragmatic approach to addressing protein quality is advocated by suggesting the use of conservative coefficients of digestibility that are derived from in vitro methods. Before adopting the DIAAS as a framework for supporting protein content claims, updated food-related regulations and policies should also be evaluated through a lens that anticipates the impact on consumer-facing nutrition communication, the adoption of dietary patterns that are nutritionally adequate, and a food value chain that fosters a spirit of food and nutritional innovation.
Keywords: DIAAS, PDCAAS, PER, protein, protein quality, public health, health claims
INTRODUCTION
Although the quantity of protein within a given food source is primarily determined as a function of the true nitrogen content, this crude protein content is not considered a reliable indicator of the ability of a dietary protein to meet the metabolic needs of the host. The quality of dietary proteins is typically defined by the extent to which the constituent amino acids match the amino-acid needs of the consumer. This is coupled with some assessment of the efficiency with which the host extracts the amino acids from the dietary matrix and uses them for growth and/or maintenance purposes. Over the last 100 years, various methods for assessing the quality of dietary proteins have been developed. In some cases, these methods have been included in regulatory frameworks, specifically in the United States and Canada, as criteria for identifying and communicating that a food is a “source” of protein. In Canada, the protein rating system, which uses the protein efficiency ratio (PER) approach to protein quality assessment, is the approved system for protein content claims.1 In the United States, the protein digestibility-corrected amino acid score (PDCAAS) is used to characterize the protein quality of a given food.2 Since the endorsement of the PDCAAS by the Codex Alimentarius Commission’s Committee on Vegetable Proteins3 and the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Consultation on Protein Quality Evaluation,4 the PDCAAS has been widely adopted as the standard method for determining the quality of dietary protein and it remains so in the United States.
FAO/WHO committees have reconvened on numerous occasions to discuss measures of protein quality.5 Each time, the PDCAAS has remained the chosen method for quantifying and characterizing the quality of protein in food because sufficient data on alternative methodologies were not available to justify a change. For most dietary proteins, true fecal nitrogen digestibility values have been determined, which permits straightforward calculation of the PDCAAS by healthcare practitioners and industry stakeholders. However, following a 2012 meeting of an FAO Expert Consultation on Protein Evaluation in Human Nutrition, a report was published that advocates for a new method, called the digestible indispensable amino acid score (DIAAS), to be used as the standard for determining protein quality.6,7
Using as context the regulatory frameworks in Canada and the United States, where protein content claims are underpinned by metrics of protein quality, the purpose of this review is to discuss protein quality with a primary emphasis on the PDCAAS and DIAAS methodologies. Accordingly, public perception of food quality and downstream implications of adopting the DIAAS without due diligence are ongoing themes, and issues related to regulation, communication, and public health are given particular attention. This review introduces a pragmatic approach to addressing protein quality by suggesting a method that relies on conservative coefficients of digestibility that are derived from in vitro systems.
Protein quality assessment: the North American context
The protein regulatory framework in the United States: an overview of the protein digestibility-corrected amino acid score
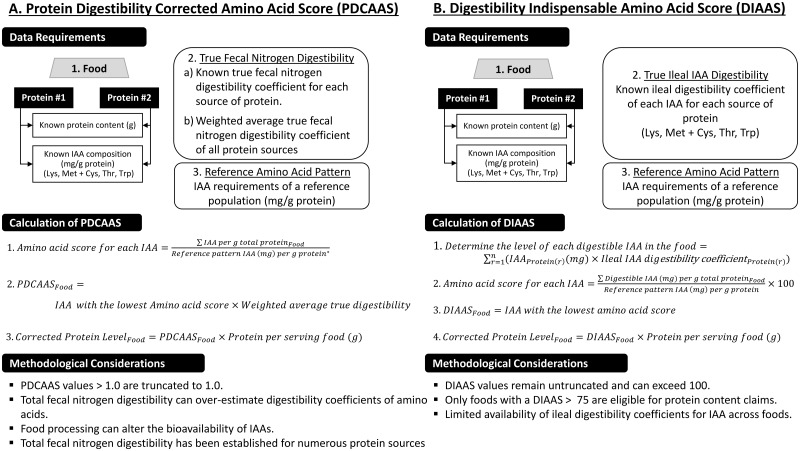
The PDCAAS methodology is summarized in Figure 1.4 Briefly, the PDCAAS of a food is determined as the product of (1) the amino-acid score of a dietary protein and (2) its true fecal nitrogen digestibility. True fecal nitrogen digestibility is the level of nitrogen excreted in feces relative to the level ingested (corrected for endogenous losses). The amino-acid score is determined as the lowest ratio value when the individual amino-acid contents of foods are divided by a reference amino-acid requirement pattern.8 Foods with lower levels of indispensable amino acids and/or lower coefficients of true fecal nitrogen digestibility will generate lower PDCAAS values and, thus, be characterized as a lower-quality protein. If both the true fecal nitrogen digestibility coefficient and the amino-acid profile of the food or ingredient are readily available, the PDCAAS can be calculated. The former has been determined for many animal and plant-based sources of protein currently consumed as stand-alone foods or as ingredients in processed food products.
Figure 1.
Summary and comparison of the protein digestibility-corrected amino acid score (PDCAAS) (A) and the digestible indispensable amino acid score (DIAAS) (B). Asterisks represent protein content claims. The US regulations specify that the amino-acid requirements of preschool children aged 2–5 years, as outlined in the 1991 FAO report, “Protein Quality Evaluation: Report of Joint FAO/WHO Expert Consultation,”4 be used as the reference pattern.2Abbreviations: Cys, cysteine; DIAAS, digestible indispensable amino acid score; IAA, indispensable amino acid; Lys, lysine; Met, methionine; PDCAAS, protein digestibility-corrected amino acid score; Thr, threonine; Trp, tryptophan. Data for PDCAAS from the Food and Agriculture Organization of the United Nations/World Health Organization4; data for DIAAS from the Food and Agriculture Organization of the United Nations.6,7
The PDCAAS has been adopted in the United States as the method for determining the eligibility of foods targeted at children (≥4 years) through to adults for protein content claims (Figure 22). Furthermore, US regulations specify that the amino-acid requirements of preschool children aged 2–5 years—as outlined in the 1991 FAO report “Protein Quality Evaluation: Report of Joint FAO/WHO Expert Consultation”4—be used as the reference pattern to determine the PDCAAS of foods.2 After determining the corrected level of protein within a regulated serving of food or reference amount customarily consumed (RACC), the percent daily value (DV) is determined using 50 g as the daily reference value for protein. If a food contributes 10%–19.9% or ≥20% of the DV for protein, the food qualifies for a claim that the food is a “good source” and an “excellent source” of protein, respectively.2
Figure 2.
Summary of the regulatory framework for protein content claims using the protein digestibility-corrected amino acid score in the United States. Abbreviations: DV, daily value; PDCAAS, protein digestibility-corrected amino acid score; RAAC, reference amount customarily consumed. Data from the US Food and Drug Administration.2
Canada’s protein regulatory framework: the protein efficiency ratio and protein rating
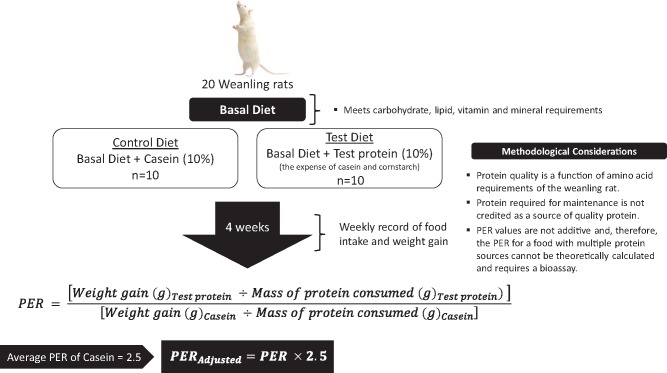
In Canada, the PER of foods is required to determine a protein rating for a given food. The PER method is summarized in Figure 3.9 The PER method is a bioassay that measures the body weight gain of rats over 28 days as a function of their protein intake, with the test article reflecting the sole source of dietary protein. To account for intralaboratory variability, the PER of casein (considered a high-quality protein) is measured concurrently, and the PER value of the test article is expressed relative to that of casein, with the latter being adjusted to a standardized value of 2.5.9 In Canada, foods qualify for “good source” and “excellent source” protein claims if their protein ratings are ≥20 and ≥40, respectively (Figure 41,12).1 Alongside the PER, the protein rating is determined by the amount of protein found in a reasonable daily intake of a given food, which is provided by Schedule K in the Canadian Food and Drug Regulations.10 If a reasonable daily intake is not available, the reference amount (Schedule M in the Canadian Food and Drug Regulations)11 for the food can be used.12
Figure 3.
Summary of the determination of the protein efficiency ratio (PER) of a food. Data from the Government of Canada.9
Figure 4.
Summary of the regulatory framework for protein content claims in Canada using the protein efficiency ratio (PER) and protein rating system. Data from the Government of Canada1 and the Canadian Food Inspection Agency.12
The PER/protein rating method, as used in Canada, has the strength of providing evidence of the ability of a given protein to support growth in a rapidly-growing-animal model. For this reason, PER is also used in the United States as the official method to assess the protein quality of foods intended as sole-source foods for infants.2 However, the use of a growing rat model means that protein used for maintenance is not credited as a source of quality protein.4 Also, rats have higher requirements for sulfur-containing amino-acid than humans.13 Therefore, PER values generated from the required rat bioassay underestimate the protein quality of a food targeted for human consumption. Another major limitation with the PER method relates to the fact that PER values of protein mixtures cannot be derived in any meaningful way by determining the average of PER values of constituent protein sources. The challenges associated with PER are recognized by the international community and are reflected in the limited use of PER within regulatory frameworks around the world. Consequently, relatively few PER values are available for stand-alone foods or food products comprised of ≥2 protein sources, and they are nonexistent for innovative food products. Given the above-mentioned challenges, Canada remains the only developed nation that uses PER to validate protein content claims on noninfant foods.
Making the case for transitioning to the digestible indispensable amino acid score
As a result of prevalent criticisms of the PDCAAS (Box 16), a 2013 FAO report introduced the DIAAS as a method for evaluating protein quality.6Figure 1 provides a summary of the DIAAS and associated methodological considerations. The DIAAS confronts concerns over the use of true fecal digestibility as a proxy for amino-acid digestibility and associated challenges within the PDCAAS methodology. Briefly, similar to the PDCAAS, determination of the DIAAS requires the absolute protein content and levels of indispensable amino acids for a given food.6 However, in contrast with the PDCAAS, which uses the true fecal digestibility of the entire protein, the DIAAS uses the ileal digestibility coefficients of each amino acid as a constituent of food to determine the true ileal digestibility of the indispensable amino acids present within the food mixture. When multiple protein-containing ingredients are present, the sum of digestible amino acids for each ingredient is calculated. As a final step, the proportion of each digestible amino acid, relative to a reference amino-acid pattern (milligram/gram), is determined. Depending on the application, different amino-acid reference patterns for specific age groups are available. The lowest value across amino acids is multiplied by 100 to convert the ratio to a percentage, and this represents the DIAAS of the food.6
Box 1Summary of the principal challenges of using the protein digestibility-corrected amino acid score (PDCAAS) for determining the protein quality of food. Data from Food and Agriculture Organization of the United Nations.6
Given that the PDCAAS relies on true fecal nitrogen digestibility, the PDCAAS does not account for the bioavailability of individual indispensable amino acids.
PDCAAS values are overestimated because of limited bioavailability of specific forms of amino acids. Bacterial assimilation of amino acids can falsely enhance values of true protein digestibility.
PDCAASs truncate at 1, and, therefore, proteins of higher quality are not identified or highlighted.
True fecal protein digestibility values are determined using rats. Rats have different pattern of requirement for amino acids for both growth and maintenance than humans.
In contrast with the PDCAAS, the DIAAS for individual ingredients remains untruncated and can exceed 100. Given that a DIAAS of >100 can be used to inflate the protein content of a diet, DIAASs for mixed meals (i.e. multiple foods or ingredients) and sole-sourced foods (i.e. infant formulas or enteric formulations) remain truncated at 100. 6 Interestingly, regulatory considerations to prohibit protein content claims on foods if the DIAAS is <75 have been proposed.6 Justification and rationale for a DIAAS of ≥75 as the threshold for protein content claims have not been provided.6
It is acknowledged that the PDCAAS methodology has weaknesses. However, even though reports from the FAO provide a compelling case for replacing the PDCAAS with the DIAAS,6,7 the implications of this transition should be fully considered before any widespread adoption of DIAAS as a benchmark for protein quality.
Delineating impact of the digestible indispensable amino acid score on food systems
Regulatory impact and nutrition communication
Determination of the protein quality of food can have broad implications for jurisdictional regulatory frameworks as well as public health. From the perspective of the food industry, in some regions such as Canada and the United States, the ability to communicate that a food is a source of protein is dependent on estimates of both protein quantity and quality. Therefore, a manufacturer that desires to have protein content underpin the nutritional messaging of a food must consider the types of protein that are used during the initial stages of the food innovation process. With the development of the DIAAS, there could be pressure from certain industries to replace the PDCAAS and the PER with the DIAAS as the preferred measure of protein quality for informing food policy and for protein content claims within US and Canadian regulations, as well as in international standards.
Very little, if any, work has been done to determine the impact of the DIAAS on industrialized food systems. Using a rat model, a recent study compared the DIAAS and the PDCAAS for a variety of protein sources. Although it was concluded that PDCAAS values were inflated compared with DIAAS values, differences among plant-based foods that would provide substantial levels of protein to the diet were marginal, ranging from 1.8% to 9%.14 For wheat bran and rolled oats, protein scores were, respectively, 11.4% and 12.8% lower when the DIASS was used instead of the PDCAAS. However, the contribution of these foods to daily protein intake is relatively small. Conversely, animal sources of protein, such as milk protein concentrate (MPC) and whey protein isolate (WPI), stood to benefit the most if the PDCAAS (MPC: 1.0; WPI: 1.0) were to be replaced by the DIAAS (MPC: 1.18; WPI: 1.09).14 However, given that MPC and WPI are added to foods as ingredients, from a regulatory point of view, it is unknown whether these variances would impact the potential for a finished food to meet the quality threshold for a protein content claim; as this is dependent on the inclusion levels of all ingredients. Further, for foods that already qualify for a protein source claim based on the PDCAAS in the United States or the PER in Canada, would the application of the DIAAS make them ineligible for the claim? In Table 1,1,2,6,9,11,12 several foods for which established PER values are available are described and the potential for these foods to carry protein content claims are noted based on regulations existing within Canada and the United States as well as the approach proposed approach by the FAO. In general, the existing (PER and PDCAAS) and proposed (DIAAS) systems position animal-based foods as good to excellent sources of protein with general consistency. Single-source cereal-based foods tend to not reach the level required for protein content claims regardless of the system used. For meals with multiple foods and ingredients, limited data for PER values make an exhaustive comparison of systems impossible. In general, the lack of accessible PER values for modern, processed foods limits the usefulness of the protein rating system. It is important to consider that the purpose of any nutrient content claim is to steer the public toward foods that contribute to daily nutrient requirements. This purpose is lost when one considers that a seemingly arbitrary DIAAS of 75 has been identified as the cutoff for making a protein content claim.6 As depicted in Table 2,2,6 foods considered as “meat alternatives” or “protein foods” tend to be disadvantaged by the DIAAS system because of failure to meet the DIAAS claim threshold of 75. As recognized in the FAO report, this cutoff value must be evaluated in the context of national dietary guidelines.
Table 1.
Comparison of permitted protein content claim statements on select foods based on established and proposed regulations
| Food (NDB)a | Canadian regulatory systemb |
US regulatory systemc |
FAO proposed systemd |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reasonable daily intake or reference amounte | Adjusted PER | Protein rating | Claim permittedf | RACCg | PDCAAS (untruncated)h | Corrected protein content (g)i | Claim permittedj | DIAASk | Proposed claim permittedl | |
| Animal-derived foods | ||||||||||
| Milk, whole (01077) | 852 mL | 2.5 | 72.1 | Excellent source | 240 mL | 1.00 (1.10) | 7.69 | Good source | 114 | Good source |
| Eggs, hard-boiled (01129) | 100 g | 3.1 | 39.0 | Good source | 50 g | 1.00 (1.05) | 6.29 | Good source | 113 | Good source |
| Chicken breast (05064) | 100 g | 2.7 | 83.8 | Excellent source | 100 g | 1.00 (1.01) | 31.02 | Excellent source | 108 | Excellent source |
| Cereal-based foods | ||||||||||
| Oatmeal (08121) | 250 mL | 1.8 | 10.7 | No claim | 250 mL | 0.82 | 4.87 | No claim | 84 | Good source |
| Wheat bread, white (20083) | 150 g | 1.0 | 18.0 | No claim | 50 g | 0.28 | 1.68 | No claim | 29 | No claim |
| Rice, white (20045) | 140 g | 1.5 | 5.7 | No claim | 140 g | 0.56 | 2.11 | No claim | 57 | No claim |
| Mixed dishesm | ||||||||||
| Macaroni and cheese (36009) | 250 mL | 2.1 | 15.2 | No claim | 240 mL | 0.89 | 6.44 | Good source | 90 | Good source |
| Beef stew (22905) | 250 mL | 1.8 | 15.6 | No claim | 240 mL | 0.35 | 3.02 | No claim | 45 | No claim |
| Legume/pulse foods | ||||||||||
| Baked beans (16006) | 250 g | 1.51 | 17.9 | No claim | 130 g | 0.60 | 3.71 | No claim | 56 | No claim |
| Chickpeas, boiled (16057) | 250 mL | 2.32 | 33.7 | Good source | 90 g | 0.74 | 5.90 | Good source | 83 | Good source |
| Soy-based tofu (16426) | 85 g | 2.3 | 33.8 | Good source | 85 g | 0.56 | 8.22 | Good source | 52 | No claim |
Abbreviations: DIAAS, digestible indispensable amino acid score; NDB, USDA nutrient database; PDCAAS, protein digestibility-corrected amino acid score; PER, protein efficiency ratio; RACC, reference amount customarily consumed.
aNutrient Database Number from the USDA Nutrient Database USDA National Nutrient Database for Standard Reference: Release 28. http://www.ars.usda.gov/Services/docs.htm?docid=8964. Accessed August 12, 2016.
bBased on protein rating, approved method FO-1.9
cBased on FDA: 21CFR-101.9.2
dFood and Agriculture Organization of the United Nations Food and Nutrition paper 92, chapter 4.1: The digestible indispensable amino acid score (DIAAS).6
eReasonable daily intake as per schedule K, Canadian Food and Drug Regulations10; when a reasonable daily intake is not available, the reference amount (schedule M) for a food is used.11,12
fProtein rating of 20–39.9 = good source; ≥40 = excellent source.1
gRACC from FDA: 21CFR101.12.2
hValues in parenthesis equal to nontruncated PDCAAS values.
iCorrected protein content = crude protein content in RACC × PDCAAS.2
j5–9.9 g = good source; ≥10 g = excellent source.2
kDIAAS calculated using available digestibility coefficients (ileal or fecal) or using estimates of 0.85.
lClaim based on both quantity (crude protein 5–9.9 g qualifies for good source if the DIAAS is >75; ≥10 g qualifies for excellent source only if the DIAAS is ≥100.6
mMixed dish selection limited to those for which PER values were available.12
Table 2.
Impact of using either the protein digestibility corrected amino acid score or digestible indispensable amino acid score for determining protein content claims for nonanimal foods identified as protein foods or meat alternatives within US national dietary standards
| Protein food categories (NDB)a | RACC (g)b | Application of PDCAAS method |
Application of DIAAS method |
||||
|---|---|---|---|---|---|---|---|
| PDCAAS | Corrected protein content in RACC (g)c (%DRV)d | Permitted protein claime | DIAASf | Crude protein content in RACC (g)g (%DRV)d | Permitted protein claimh | ||
| Nuts and seeds | |||||||
| Almonds (12 061) | 30 g | 39 | 2.5 (5.0) | No claim | 40 | 6.3 (12.7) | No claim |
| Sunflower seeds (12 036) | 30 g | 66 | 4.1 (8.2) | No claim | 67 | 6.2 (12.5) | No claim |
| Peanut butter (16 167) | 32 g | 45 | 3.2 (6.3) | No claim | 46 | 7.0 (14.0) | No claim |
| Legumes/pulsesi | |||||||
| Navy beans | 35 g dry | 67 | 5.7 (11.5) | Good source | 51 | 8.6 (17.2) | No claim |
| Whole green lentils | 35 g dry | 63 | 5.8 (11.6) | Good source | 65 | 9.2 (18.4) | No claim |
| Split red lentils | 35 g dry | 54 | 5.6 (11.2) | Good source | 50 | 10.3 (20.7) | No claim |
| Split yellow peas | 35 g dry | 64 | 5.7 (11.4) | Good source | 73 | 8.8 (17.7) | No claim |
| Chickpeas (16 057) | 35 g dry | 74 | 5.9 (11.8) | Good source | 83 | 7.7 (15.3) | Good source |
| Soy products | |||||||
| Tofu (16 426) | 85 g | 56 | 8.22 (16.4) | Good source | 52 | 14.7 (29.4) | No claim |
Abbreviations: DIAAS, digestible indispensable amino acid score; DRV, daily reference value; NDB, USDA nutrient database; PDCAAS, protein digestibility-corrected amino acid score; RACC, reference amount customarily consumed.
aNDB is the Nutrient Database Number from the USDA Nutrient Database USDA National Nutrient Database for Standard Reference: Release 28. http://www.ars.usda.gov/Services/docs.htm?docid=8964. Accessed August 12, 2016.
bRACC from FDA: 21CFR101.12.2
cCorrected protein content = crude protein content in RACC × PDCAAS.
dValues in parentheses reflect % DRV, where the DRV for protein = 50 g2
e5–9.9 g = good source; ≥10 g = excellent source.2
fDIAAS calculated using available digestibility coefficients (ileal or fecal) or using estimates of 0.85.
gCrude protein content per RACC, based on proposed approach in Food and Agriculture Organization of the United Nations 2013 report.6
hClaim based on both quantity (if crude protein, 5–9.9 g = good source if the DIAAS is >75; ≥10 g = excellent source only if the DIAAS is ≥100.6
iData from pulses, unless noted, are derived from the author’s (J.D.H.) laboratory (unpublished data).
In developed jurisdictions such as the United States and Canada where protein intake is sufficient15–18 and widespread evidence of protein deficiencies are not evident, a comprehensive review of the benefits of implementing the DIAAS for assessing protein quality is required. Although it is likely that the DIAAS provides a more accurate assessment of protein quality, if the adoption of the DIAAS does not impact the potential for foods to qualify for protein content claims and affect consumer’s food choices, the advantage of transitioning to the DIAAS is questionable. Any analysis of protein regulations should also consider that use of the DIAAS may mitigate the potential for healthy foods, such as legumes, to qualify for protein claims that also direct consumers toward dietary patterns that align with national dietary guidance.19,20
Interestingly, in other developed regions, protein claims are not contingent on protein quality. Instead, protein claims are solely based on the overall protein content of food. In Europe, claims that foods are a “source” or a “high source” of protein are permitted if protein contributes 12% and 20% of energy from protein, respectively.21 In Australia, 5 g of protein per serving qualifies a food for a general claim, and 10 g of protein per serving is the threshold for claiming that a food is a “good source” of protein.22 Survey data demonstrate that protein consumption in regions of the European Union and Australia are adequate.23,24 Epidemiologic data from the same work also show that food groups that encompass “high-quality protein,” such as meat, poultry, eggs, and milk, comprise a significant portion of total energy intake—19%, 24%, 29%, and 27% in Australia, the United States, Denmark, and the Netherlands, respectively.24 This suggests that implementation of the DIAAS would marginally shift the protein quality ratings of diets because these foods already contribute significant levels of protein to diets and the DIAASs of these foods already have higher protein scores compared to plant-based sources of protein.
Implications for public health
Generally, across metrics, plant-based protein sources generate lower values for protein quality because they have reduced levels of 1 or more indispensable amino acids. Thus, these protein sources are often characterized as “incomplete.” However, plant-based sources of protein can be complementary to each other, which reduces independent shortfalls in indispensable amino acids. For example, when legumes and cereals are compared, legumes typically have lower levels of sulfur-containing amino acids and higher levels of lysine. The opposite is true for cereals. When legumes and cereal grains are consumed together, or throughout a given day, indispensable amino-acid requirements can be met.25
Around the world, individuals and entire cultures thrive on vegetarian diets, which have been shown to decrease the risk of chronic disease.26,27 When vegetarian diets are critically evaluated for their nutrient density, there is little concern about the diet meeting protein needs, let alone protein quality.28 For growing children, however, the ability of vegetarian diets to supply appropriate levels of vitamin A, vitamin B12, riboflavin, calcium, iron, and zinc has been identified as an area of nutritional concern.29 A review of the nutritional adequacy of vegetarian diets has identified that, when malnutrition is considered, the primary concern is adequate protein intake rather than protein quality.30 While the importance of protein quality should not be marginalized, it is important to emphasize the need for a balanced approach to public health communications in developed and developing countries.
The use of vegetarian, particularly vegan, diets in developed regions allows for the discussion of possible nutrition-related implications of consuming protein sources that are of lower protein quality. Indeed, even outside the scope of veganism, healthy omnivorous dietary paradigms that include foods that rank lower on the scale of protein quality can help individuals meet amino-acid requirements. For example, the Mediterranean dietary pattern, which has been shown to reduce the risk of cardiovascular disease,31,32 includes animal food sources as well as plant-based foods that provide significant levels of nutrients, including protein. The merits of all foods, plant- and animal-based, should be acknowledged as part of healthful dietary patterns.
Rethinking the need for complex assessments of protein quality within regulatory frameworks of the developed world
Figure 1 demonstrates that generating ileal amino-acid digestibility coefficients for all ingredients used in foods requires more sophisticated methods than generating the true fecal nitrogen digestibility.
In Canada and the United States, where protein quality is part of the framework that allows for protein content claims, harmonization of the regulatory approaches seems warranted. In Canada, the protein rating system has reached a point where it can no longer serve the needs of the broader food system, for both industry and consumer stakeholders alike. Conceptually, both the PDCAAS and DIAAS values for mixed foods can be derived from the available data from single ingredients, and the DIAAS method may be better positioned to lead to more accurate estimates. But at what cost? The use of ileal-cannulated pigs, as recommended in the FAO report, to derive digestibility coefficients for hundreds, if not thousands, of food items within today’s modern food system is not practical. Considering that each food undergoes 6 different analyses to determine coefficients of ileal digestibility for indispensable amino acids, the possibility of compounding errors with each subsequent analytical step should be acknowledged as discussions around the implementation of the DIAAS move forward. The knowledge that multiple sources of variation exist to influence the quality of dietary proteins (eg, analytical, plant and animal genetics, processing steps) necessitates a re-envisioning of a more practical approach to assessing protein quality. The following items are offered for consideration.
First, protein remains the only nutrient that requires an animal-based bioassay for routine regulatory and labeling purposes. Ethical considerations around the use of animals for the purpose of substantiating the application of basic nutrition regulations should be reflected in initiatives that support regulatory modernization.
Second, the use of fixed, conservative estimates of ileal digestibility coefficients (ie, 0.80) could be considered; these could then be routinely applied to available amino-acid data in national nutrient databases for the calculation of DIAAS values. The DIAAS calculation approach is defensible on the basis of available evidence derived from the livestock feeding arena. This approach to using a fixed coefficient was adopted by the Institute of Medicine during the establishment of the dietary folate equivalent, where dietary folate was assumed to be 50% bioavailable.
Third, as an additional alternative to bioassay-derived digestibility coefficients, efforts to robustly assess in vitro methods for measuring the DIAAS and the PDCAAS are warranted. The use of in vitro methods to measure digestibility coefficients would provide a rapid and inexpensive approach to bioassays and potentially afford sufficient sensitivity for regulatory needs. This would need to be determined in a risk-assessment exercise directly comparing in vivo versus in vitro PDCAAS/DIAAS values relative to their potential impact on protein nutrition guidelines.
Although debate persists on the future use of the DIAAS, sufficient evidence is available to support an interim move by Canada to harmonize its protein quality regulations to those of the United States. The joint usage of a PDCAAS-based system would enhance innovation in the development of protein-containing foods by removing the need to use an antiquated system based on the PER. Efforts are needed to assess the potential impact of moving to the use of the PDCAAS, and to the use of in vitro assessments of digestibility, on nutrition messaging and policies in Canada.
Conclusion
The primary areas of study required to determine the impact of the DIAAS as a standard of protein quality within regulatory frameworks have been discussed throughout this review. These include the potential impact on perceptions of healthy foods in the context of nutrition communication and public health.
Communication and perception are ongoing themes throughout this review and it is important to have a comprehensive understanding of the impact that the DIAAS could have in these areas if implemented as a standard for evaluating protein quality. For example, how great is the risk that the positive attributes, with respect to protein content, of healthy foods could be downplayed if they have a lower DIAAS relative to corresponding animal-based products? In addition, if Canada and the United States continue to use protein quality to support protein content claims for food, a harmonized system would benefit both countries to mitigate consumer confusion. Whether or not adoption of the PDCAAS in Canada is realized, there is validity to challenging the need for such complex methods, inclusive of the PDCAAS and the DIAAS, for determining the protein quality of diets in developed and, perhaps, developing regions. Based on the marginal differences in protein digestibility between foods, a simplified determination of protein quality that is based on essential amino-acid scores, coupled with general digestibility coefficients that are established by in vitro methodologies, is proposed.
It is recognized that the overarching philosophies supporting the use of the DIAAS are based on a positive intent. Proponents of the DIAAS are striving for a more accurate depiction of protein quality within the food supply, and the DIAAS method is supportive of this mandate. A recent review by Wolfe et al.33 discusses the DIAAS and provides the rationale for its development and use. In the spirit of advancing the nutritional sciences, there is certainly value in pursuing ongoing research to delineate the impact of protein quality on nutritional and health outcomes, as well as to identify foods, especially in vegetarian diets, that optimize the intake of indispensable amino acids. Also, given its improved accuracy over the PDCAAS, there are situations in which the DIAAS could be beneficial as a protein quality standard, including for the development of specialized or targeted diets for disease states, such as renal disease or malnutrition, as well as diets formulated for elite athletes. This is captured in a recent report from the Codex Alimentarius Commission, in which participating jurisdictions, such as Brazil and Canada, acknowledge that ready-to-use therapeutic foods should be subject to the most up-to-date methodologies, such as DIAAS, to assess their protein quality.34 However, participating jurisdictions also acknowledged that, at the present time, insufficient data are available to permit implementation of the DIAAS into regulatory standards for therapeutic foods. This further supports the position that, before considering the widespread use of DIAAS within regulatory and policy frameworks, a thorough and pragmatic assessment that considers both health and cost outcomes is required. The alternative approach to protein quality suggested herein—ie, the use of a fixed digestibility coefficient and/or the removal of in vivo analyses—considers these principles and could serve as a comprehensive model for promoting healthful plant-based and omnivorous diets.
Modernization of regulatory frameworks involves the integration of advances made in the field of nutrition science, including assessments of protein quality. Before adopting the DIAAS as a framework for supporting protein content claims, it is prudent for updated food-related regulations and policies to also be evaluated through a lens that anticipates the likely return on investment. Factors to consider include the impact on consumer-facing nutrition communication, the adoption of dietary patterns that are nutritionally adequate, optimized health and well-being, and a food value chain that fosters a spirit of food and nutritional innovation.
Acknowledgments
Author contributions. C.P.F.M. and J.D.H. cowrote the manuscript.
Funding. J.D.H. has received funding from Agriculture and Agri-food Canada to determine factors that influence protein quality of plant-based proteins. No agency provided funding for the preparation, research, or publication of this manuscript.
Declaration of interest. C.P.F.M. is an employee of Pulse Canada and a former employee of Kellogg Canada. J.D.H. has conducted research funded by Pulse Canada.
References
- 1. Government of Canada. Food and Drug Regulations: Nutrient Content Claims, B.01.500. Canada: Ottawa; 2016. [Google Scholar]
- 2. US Food and Drug Administration. Electronic code of federal regulations: title 21: food and drugs. Part 101—food labeling. 2016. http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=bad23c28ebd662323b3ace1e3f5ee94f&mc=true&n=pt21.2.101&r=PART&ty=HTML#se21.2.101_154. Accessed May 17, 2016. [Google Scholar]
- 3. Codex Alimentarius Commission. Report of the Fifth Session of the Codex Committee on vegetable proteins, Ottawa, Canada February 6–10, 1989; Rome, Italy. [Google Scholar]
- 4. Food and Agriculture Organization of the United Nations/World Health Organization. Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, FAO Food and Nutrition: Paper 51. Rome, Italy: Food and Agriculture Organization of the United Nations; 1991. [Google Scholar]
- 5. Gilani GS. Background on international activities on protein quality assessment of foods. Br J Nutr. 2012;108(suppl 2):S168–S182. [DOI] [PubMed] [Google Scholar]
- 6. Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition: Paper 92. Rome, Italy: Food and Agriculture Organization of the United Nations; 2013. [Google Scholar]
- 7. Food and Agriculture Organization of the United Nations. FAO Expert Working Group: Research Approaches and Methods for Evaluating Protein Quality of Human Foods. Rome, Italy: Food and Agriculture Organization of the United Nations; 2014. [Google Scholar]
- 8. Schaafsma G. The protein digestibility-corrected amino acid score. J Nutr. 2000;130(Suppl):1865S–1867S. [DOI] [PubMed] [Google Scholar]
- 9. Government of Canada. Method FO-1: Determination of Protein Rating. Ottawa, Canada: Health Protection Branch; 1981. [Google Scholar]
- 10. Government of Canada. Food and drug regulations: schedule K—reasonable daily intake for various foods. 2016. http://laws-lois.justice.gc.ca/eng/regulations/c.r.c.,_c._870/page-159.html#h-351. Accessed December 15, 2016. [Google Scholar]
- 11. Government of Canada. Food and drug regulations: schedule M—reference amounts. 2016. http://laws-lois.justice.gc.ca/eng/regulations/c.r.c.,_c._870/page-163.html#h-353. Accessed December 15, 2016. [Google Scholar]
- 12. The Canadian Food Inspection Agency. Food labeling for industry: elements within the nutrition facts table—protein. 2016. http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrition-labelling/elements-within-the-nutrition-facts-table/eng/1389206763218/1389206811747?chap=7. Accessed July 26, 2016. [Google Scholar]
- 13. Sarwar G, Peace RW, Botting HG. Corrected relative net protein ratio (CRNPR) method based on differences in rat and human requirements for sulfur amino acids. J Assoc Off Anal Chem. 1985;68:689–693. [PubMed] [Google Scholar]
- 14. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145:372–379. [DOI] [PubMed] [Google Scholar]
- 15. Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87(Suppl):1554S–1557S. [DOI] [PubMed] [Google Scholar]
- 16. Health Canada. Do Canadian adults meet their nutrient requirements through food intake alone? 2012. http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-adult-eng.php. Accessed January 3, 2017. [Google Scholar]
- 17. Health Canada. Do Canadian children meet their nutrient requirements through food intake alone? 2012. http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-child-enf-eng.php. Accessed January 3, 2017. [Google Scholar]
- 18. Health Canada. Do Canadian adolescents meet their nutrient requirements through food intake alone? 2012. http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-adol-eng.php. Accessed January 3, 2017. [Google Scholar]
- 19. US Department of Health and Human Services, US Department of Agriculture. 2015–2020 dietary guidelines for Americans, 8th ed.2015. http://health.gov/dietaryguidelines/2015/guidelines/. Accessed Febrary 4, 2016. [Google Scholar]
- 20. Health Canada. Eating well with Canada’s food guide—a resource for educators and communicators. 2011. http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/pubs/res-educat-eng.pdf. Accessed July 28, 2016. [Google Scholar]
- 21. European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Official Journal of the European Union; 2006. [Google Scholar]
- 22. Food Standards Australia New Zealand. Food standards code: 1.2.7 nutrition, health and related claims. Standard 1.2.7. Federal Register of Legislative Instruments. 2015. [Google Scholar]
- 23. Waern RV, Cumming RG, Blyth F et al. . Adequacy of nutritional intake among older men living in Sydney, Australia: findings from the Concord Health and Ageing in Men Project (CHAMP). Br J Nutr. 2015;114:812–821. [DOI] [PubMed] [Google Scholar]
- 24. Auestad N, Hurley JS, Fulgoni VL 3rd, Schweitzer CM. Contribution of food groups to energy and nutrient intakes in five developed countries. Nutrients. 2015;7:4593–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marsh KA, Munn EA, Baines SK. Protein and vegetarian diets. Med J Aust. 2013;199(4 suppl):S7–S10. [DOI] [PubMed] [Google Scholar]
- 26. Chiu YF, Hsu CC, Chiu TH et al. . Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: a matched cohort study. Br J Nutr. 2015;114:1313–1320. [DOI] [PubMed] [Google Scholar]
- 27. Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health. 2014;35:83–103. [DOI] [PubMed] [Google Scholar]
- 28. Sanders TA. The nutritional adequacy of plant-based diets. Proc Nutr Soc. 1999;58:265–269. [DOI] [PubMed] [Google Scholar]
- 29. Murphy SP, Allen LH. Nutritional importance of animal source foods. J Nutr. 2003;133(11 suppl 2):3932S–3935S. [DOI] [PubMed] [Google Scholar]
- 30. Muller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ. 2005;173:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez-Gonzalez MA, Salas-Salvado J, Estruch R et al. . Benefits of the Mediterranean diet: insights From the PREDIMED Study. Prog Cardiovasc Dis. 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
- 32. Ros E, Martinez-Gonzalez MA, Estruch R et al. . Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5:330S–336S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolfe RR, Rutherfurd SM, Kim IY, Moughan PJ. Protein quality as determined by the digestible indispensable amino acid score: evaluation of factors underlying the calculation. Nutr Rev. 2016;74:584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme Codex Committee on Nutrition and Foods for Special Dietary Uses: Thirty-eighth Session. Proposed Draft Guideline for Ready-to-Use Therapeutic Foods: CX/NFSDU 16/38/9-Add.1. Rome: Italy: Food and Agriculture Organization of the United Nations, World Health Organization; 2016. [Google Scholar]