Abstract
Background
Cases of Zika virus were recently detected in Luanda, Angola, a major travel hub in Africa. The risk of Zika virus transmission throughout the continent from Angola is evaluated.
Methods
Travel volumes were assessed using monthly passenger-level flight data from Luanda to all locations throughout Africa. Travel data was superimposed onto seasonal environmental suitability maps that predict the potential for subsequent Zika virus transmission.
Results and Conclusions
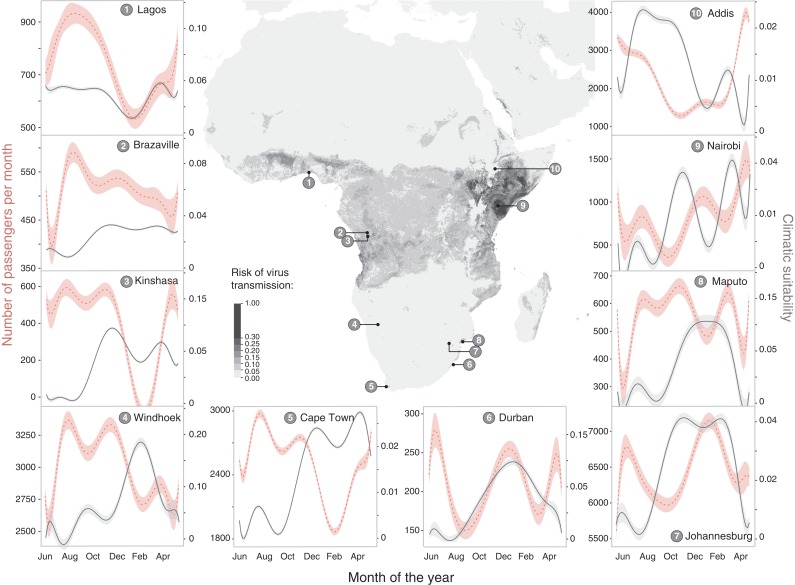
Windhoek, Maputo, Durban and Kinshasa have the greatest potential for Zika virus introduction and transmission during the southern hemisphere summer months, and Nairobi during the northern hemisphere summer months.
Keywords: Aedes, Africa, Air travel, Angola, Globalization, Mosquito, ZIKV
Introduction
Zika virus (ZIKV), originally endemic to parts of Africa and Asia, has now spread throughout most of Latin America and the Caribbean, and has infected millions of individuals. Infections with Zika virus are typically mild, although data emerging from the current epidemic confirmed that the infection is associated with significant birth defects and Guillain–Barré syndrome, and that it can be transmitted sexually. ZIKV has spread internationally due to the movement of infected individuals and autochthonous transmission is now occurring in several regions that have suitable conditions, namely the appropriate climate and Aedes mosquito vectors. Although the current Pan-American outbreak strain is caused by the Asian genotype, the virus has recently been detected in at least three African settings: Guinea-Bissau (African genotype), Angola (genotype yet to be determined)1 and Cape Verde (Asian genotype). The African ZIKV genotype has also been known to be circulating in West Africa at low levels for the last few decades, possibly supported by a sylvatic cycle,2 although there are suggestions that the ZIKV stain currently circulating in the Americas has significantly higher infectivity.3 The more recent cases in urban areas of Angola are of potential concern. Angola is a major gateway between Africa and South America with significant connectivity via air travel and is a plausible location for ZIKV re-introduction.4 Furthermore, Angola, its neighbours, and much of the continent is vulnerable to arboviral infections year-round due to conducive climate conditions, and many countries have sub-optimal surveillance systems in place for disease detection. Should Angola become a focal point for ZIKV introduction, directing limited public health resources to strengthen surveillance and control measures in that country, while also predicting where subsequent regional and continental transmission may occur could help mitigate ZIKV-related complications.
Methods
We analysed monthly passenger-level flight data from Luanda (where two ZIKV cases were recently detected)1 to all locations throughout Africa. We analysed all airport destinations and corresponding volumes of travellers for the period of October 2015 to September 2016 using worldwide ticket sales data from the International Air Transport Association. To estimate the potential for local virus transmission arising from ZIKV introduction, we then combined predictions of local monthly variation in ZIKV transmission suitability,4 and show seasonal variation in local transmission risk for the 10 most visited African cities from Luanda, Angola.
Results
Passengers arriving to African cities conducive to local arbovirus transmission were highest in neighbouring Namibia (Windhoek) followed by Addis Ababa (Ethiopia), which experienced a significant increase in passengers in the months of November to January (Figure 1). Johannesburg and Cape Town (South Africa) both had the highest number of passengers arriving from Luanda, but did not have Aedes aegypti suitability for subsequent ZIKV transmission. Areas that have high risk of ZIKV introduction and potential for local transmission are Windhoek, Maputo, Durban and Kinshasa (all during the southern hemisphere summer months, between November and February, inclusive). Transmission potential is expected to shift to the northern hemisphere during their spring and summer months. In this analysis, Nairobi had both the highest potential of ZIKV introduction reflected by the number of arriving passengers coupled with suitable environmental potential for local transmission (Figure 1).
Figure 1.
Number of flights per month from Luanda to other African destinations with monthly climatic suitability for Zika virus transmission in passenger-receiving cities. Axes labels show climatic suitability and flight passenger numbers tailored to each location independently to illustrate seasonality and inter-annual variation. Addis Ababa, Cape Town, Johannesburg, Nairobi, and Windhoek have considerably higher numbers of travellers arriving from Angola.
Discussion
It is currently unclear if the cases of ZIKV detected in Angola are of the African or Asian lineage. Genetic data could help with answering this question and elucidate whether the detection of these cases is indicative of local transmission. If so, there are presumably more unreported cases. This is of concern for pregnant women or women considering pregnancy, given the association between ZIKV infection and foetal complications.5 Prior studies evaluating the potential for ZIKV introduction or re-introduction to African and Asia-Pacific settings highlighted Angola as being of increased risk of importation during the months of November to April given the confluence of travel patterns from epidemic locations (from Brazil in particular) and congruent seasonal environmental conditions suitable for ZIKV transmission in the arrival locations of these passengers.4 Our results reveal the potential for distant spread of ZIKV from Angola throughout the African continent via air travel, highlighting that Namibia and Mozambique are at highest risk for importation and subsequent transmission. Beyond continental spread via air travel, spread of the virus via land travel is possible, as recently observed with yellow fever cases spreading from Angola to the Democratic Republic of Congo.6 Unfortunately, the limited diagnostic, surveillance and public health infrastructure in many at-risk African settings poses several challenges, including providing quality maternal care and mosquito control efforts. Recent advances in rapid and even portable genomic sequencing of arboviruses could improve surveillance, but currently there a dearth of data evaluating the burden of illness in African settings.7
It is unclear what the underlying extent of protective immunity to ZIKV exists in African settings. It is also unclear whether the African genotype provides some or any cross-protection against the epidemic Asian strain. These two unknown issues have the potential to significantly influence transmission dynamics of ZIKV in Africa. Regardless of the genotype circulating in Angola, these recently discovered cases have been suggested to be the result of local transmission. This further highlights the potential of arboviruses to spread locally and regionally in African settings, requiring a coordinated public health response.
Supplementary data
Supplementary data are available at Transactions online (http://trstmh.oxfordjournals.org/).
Supplementary Material
Acknowledgments
Authors’ contributions: MUGK, OB, KK and IB contributed to the idea conception, data analysis, data interpretation, and writing of the manuscript. AW, MG, and SIH contriubted to data interpretation and the writing of the manuscript.
Funding: This work was supported, in part, by the Canadian Institutes of Health Research. SIH is funded by grants from the Bill & Melinda Gates Foundation (OPP1106023, OPP1093011, OPP1132415, OPP1159934 and OPP1176062) and the Wellcome Trust (#209142) and the Fleming Fund. MUGK is supported by The Branco Weiss Fellowship - Society in Science, administered by the ETH Zurich and acknowledges funding from a Training Grant from the National Institute of Child Health and Human Development (T32HD040128) and the National Library of Medicine of the National Institutes of Health (R01LM010812 and R01LM011965). OJB was funded by a Sir Henry Wellcome Fellowship funded by the Wellcome Trust (grant number 206471/Z/17/Z). The funders did not influence the content of this manuscript nor the decision to submit it for publication.
Ethical approval: Not required.
References
- 1.ProMed-mail. Archive Number: 20170117.4772206. Published Date: January 17, 2017. www.promedmail.org [accessed 22 March 2017].
- 2. Herrera BB, Chang CA, Hamel DJ et al. Continued transmission of Zika virus in humans in West Africa, 1992–2016. J Infect Dis 2017;215:1546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Liu J, Du S et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017;545:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogoch II, Brady OJ, Kraemer MU et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia–Pacific region: a modelling study. Lancet Infect Dis 2016;16:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brasil P, Pereira JP Jr, Moreira ME et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraemer MUG, Faria NR, Reiner RC Jr et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect Dis 2017;7:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faria NR, Quick J, Claro IM et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017;546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.