Abstract
Background
Visceral leishmaniasis (VL) continues to be a deadly parasitic disease in Brazil but the epidemiology has changed. The objective of this study was to assess the evolution of urban VL in the city of Natal, Brazil, over the past 25 y.
Methods
A retrospective study of human VL was performed, considering reported cases over the past 25 y in Natal. Analyses considered the spatial distribution of VL cases, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) co-infection, Leishmania infantum infection in dogs, density of the insect vector (Lutzomyia longipalpis) and socio-economic factors.
Results
Paralleling migration of the population, VL cases changed from mostly rural to predominantly urban regions. The incidence of human VL was highest during the initial years (1990–1994) of our study. Human VL was positively associated with a high density of L. longipalpis, a high prevalence of canine L. infantum infection and HIV/AIDS co-infection. The average age at diagnosis increased over prior years and males were more frequently affected. The overall fatality rate was 6%. Socio-economic variables indicative of poverty were associated with a greater incidence of VL and clusters of VL.
Conclusion
VL has become endemic in Natal. The disease is associated with poverty and male gender. Surprisingly, there has been an increase in the age at diagnosis.
Keywords: Brazil, Leishmania infantum, Spatial distribution, Urbanization, Visceral leishmaniasis
Introduction
American visceral leishmaniasis (VL) was first reported in northeastern Brazil by Penna.1 In the mid-1980s there was a dramatic transformation in the geographical distribution of VL. The disease, previously restricted to the rural areas of northeastern Brazil, appeared on the periphery of large urban centres. This expansion was first observed in the cities of Teresina and São Luís.2,3 VL outbreaks subsequently occurred in Natal, Santarém, Corumbá, Rio de Janeiro, Montes Claros, Belo Horizonte, Campo Grande, Fortaleza and other cities.3–6 Currently, all geographic regions of Brazil report autochthonous cases of human and canine visceral leishmaniasis.3
In Natal there was a major outbreak of VL in the early 1990s6 coinciding with rapid population growth and massive urban migration. Impoverished people moved to the periphery of Natal and other urban areas where housing and sanitary conditions were poor. Concurrently, the sandfly vector Lutzomyia longipalpis adapted to the peridomestic environment and accounted for transmission of Leishmania infantum to humans and dogs.3,7
The outcome of infection with L. infantum or Leishmania donovani, the major aetiological agents of VL in the world, varies from asymptomatic infection to severe, life-threatening disease. The ratio of L. infantum infection to symptomatic VL in Brazilian children was reported to be 6:1 in the 1980s,8 but this has changed over the years.3 Malnourished children have an increased risk of developing VL.9,10 People who are immunocompromised are also at increased risk of disease. The emergence of co-infection with human immunodeficiency virus (HIV) in areas endemic for L. infantum has been a growing problem in Brazil and is similar to the situation in endemic regions of southern Europe11 where VL became an acquired immune deficiency syndrome (AIDS)-defining disease. A potential role for asymptomatically infected humans as reservoirs of L. infantum has not previously been a major consideration in Brazil,12 but it could be of concern as the incidence of HIV/AIDS and L. infantum co-infection expands.13
We previously reported an urban outbreak of 316 cases of VL occurring in the vicinity of Natal.6 Forty-nine percent of those cases were in children <5 y of age and the fatality rate was 9%. Only one person had concurrent HIV/AIDS. There was household clustering of cases of VL and asymptomatic Leishmania infection.6 The objective of the current study was to assess the evolution of VL in the city of Natal from 1990 through 2014, focusing on the spatial distribution of cases, density of the sandfly vector, prevalence of L. infantum infection in the domestic dog reservoir, HIV/AIDS co-infection and socio-economic factors. Our hypothesis was that the distribution of human cases of VL did not occur homogeneously in the city of Natal but was related to poverty, sandfly density and geographic positioning in the urban space.
Methods
Study area
The study was conducted in Natal, Rio Grande do Norte, in northeastern Brazil. Natal has an estimated population of 869 954 inhabitants, with a density of 4805 inhabitants/km2.14 It is divided into four administrative districts (North, South, East and West) with a total of 36 neighbourhoods. Natal has had several focal outbreaks of VL since the early 1990s,6 when it then had a population of approximately 588 207 inhabitants.
Study design and population
A retrospective study of all cases of VL reported in Natal between 1990 and 2014 was performed. Each of the 36 Natal neighbourhoods was used as a unit of analysis. Information was obtained from the SINAN database (National Notifiable Diseases System of the Brazilian Ministry of Health) and the Department of Health of the State of Rio Grande do Norte.
For all neighbourhoods with human VL cases occurring between 2007 and 2014, corresponding records determined canine L. infantum infection and sandfly density in the same neighbourhoods as VL cases were collected from the Natal Municipal Health database and from the Center for Zoonosis Control. Canine infection rates were determined by serological surveys. Sandfly density data were collected using Centers for Disease Control (CDC) light traps to attract insects, followed by species identification. The data on canine infection rates and sandfly density were collected within a month after the report of human VL. Rainfall data were obtained from the Agricultural Research Agency of Rio Grande do Norte.
Of the 148 VL cases between 2007 and 2014, 143 cases were georeferenced. Socio-economic and environmental indicators for the neighbourhoods were obtained from the 2010 census of the Brazilian Institute of Geography and Statistics.14 Because secondary data on human VL was used, we cannot rule out bias due to a lack of VL reports. However, human VL has been a mandatory reportable disease in Brazil since early 1990s and treatment is only provided by the government, therefore it is reasonable to assume that the cases studied herein represent the majority of the cases that occurred during the period.
Statistical analysis
The incidence of VL in persons with or without HIV/AIDS, fatality rates, age and sex were extracted from the databases for the 1990–2014 time period. The covariate rainfall was represented as xt, indicating the percentage variation of the average rain during the rainy season (May–July) in year t relative to the average for the same 3-month time over the entire period studied. May–July is the usual time when rainfall indices are maximal for each year.
The association between human VL incidence and rainfall was evaluated through a linear structural model with autocorrelated errors following an autoregressive moving average (ARMA) (p, q) filter.15
The arima function of Stata version 10 was used (StataCorp, College Station, TX, USA). We assumed that a dynamic model system evolved annually over time, having as input a time series representing the rainfall component and as output another time series representing the incidence of human VL. Each series went through a process of identifying the most appropriate model using autocorrelation functions (ACFs) and a partial autocorrelation function (PACF). The best model fit was a structural linear model with error ARMA (1,0) or AR (1) with the following expression:
| (1) |
where {yt}, the dependent variable, is the natural logarithm of VL incidence plus 0.1. The independent variable is the covariate {xt}, in this case the rainfall component, and {at} is a random error process called white noise. The parameter β measures the association in question and the parameter φ measures the degree of autocorrelation of μt error.
Model (1) can be rewritten as
| (2) |
where the incidence in year t is a function of the incidence in the previous year plus a β multiple of the rainfall weighted difference in the biennium , plus the white noise error.
The human VL cases diagnosed in the city of Natal were overlaid on a more refined map at the level of their 910 census tracts, allowing calculation of incidence rates per 100 000 inhabitants for each tract using the population of the 2010 census, which is the middle year of the period 2007–2014. This procedure allowed determination of the city areas of greatest risk. The mean annual incidence per census tract I was calculated using the formula
Spatial regression models were used to analyse the association between the incidence of VL and socio-economic or structural housing factors.
The package spdep in R (R Project for Statistical Computing, Vienna, Austria) was used to adjust the spatial simultaneous autoregressive (SAR) lag model of the kind y=ρWy+Xβ+ε, where y was the vector of incidence in neighbourhoods. W was the spatial row standardized matrix, measuring the proximity between neighbourhoods based on the fraction of region i’s border that is shared with its neighbouring regions, so that the sum of the weights of each line was 1. X was the matrix with the covariate (factor) values and ε was the random vector. Gaussian error (ρ and β) were estimated parameters where ρ assessed the spatial autocorrelation involving each neighbourhood and its neighbours by using a lag term, ρWy, in the formula above. β was the vector of regression parameters that estimated the association between the covariates and the response.16
We also investigated the spatial dependence and existence of VL clusters by using Moran’s scatterplot,17 which consists of a plot of points (y, Wy), where y is the VL incidence in a neighbourhood and Wy is the weighted average of its surroundings calculated based on the adopted W proximity matrix (Vl spatially lagged). It is added to a regression line of Wy vs y, which has the known Moran’s I coefficient as its slope. Their points are distributed in four quadrants where the upper-right (high–high) and lower-left (low–low) quadrants represent a positive spatial association, in the sense that a neighbourhood is surrounded by neighbourhoods with similar values. Points (high–low) or (low–high) represent a negative association, that is, a (high–low) neighbourhood has a high incidence and is surrounded by neighbourhoods with a low incidence. The opposite occurs with (low–high) neighbourhoods.
The estimation of statistical models of spatial analysis was performed using the spdep package of R release 3.2.2 (https://www.r-project.org) and GIS tools using QGIS version 2.12.2 (http://www.qgis.org).
Analysis of human VL and canine L. infantum infection
The incidence of human VL and the proportion of infected dogs with canine L. infantum (PCINF) were determined for 2007–2014, beginning when canine serological surveillance was first performed. In order to correct distortions in the proportion of dogs infected resulting from sample size variation, we applied a weighting on the proportion found in accordance with the number of dogs examined, generating the variable WPCINF as follows:
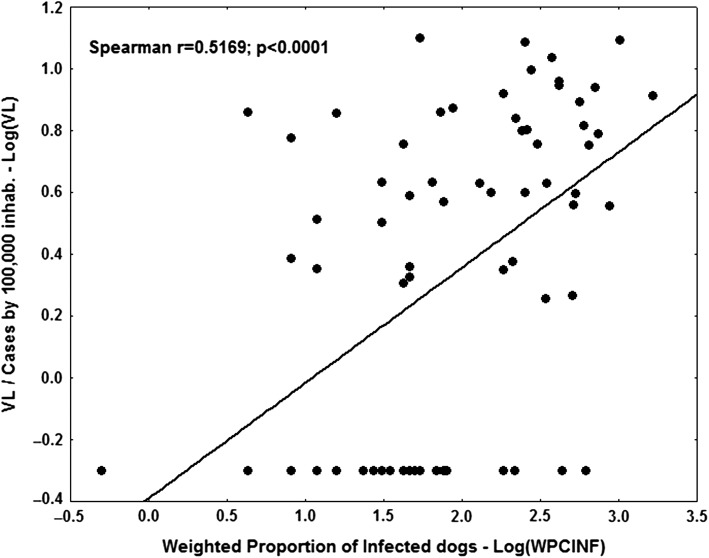
The level of association between human VL and canine Leishmania infection was evaluated by the Spearman correlation coefficient considering the variables log(VL+0.5) and log(WPCINF). It was also evaluated considering the unweighted index log (PCINF).
Human VL and sandfly density
Sandfly data were consolidated by neighbourhood and analysed, using vector infestation indices and relative abundance as indicators. Sandflies were captured using CDC light traps. The average of human VL, vector infestation and relative abundance of L. longipalpis in the 23 neighbourhoods where sandflies were collected from 2007 to 2014 were determined.
The infestation index (II) and the relative sandfly abundance (RA) were calculated using the following formulas (Ministry of Health 2006): II=(Total households positive for L. longipalpis in a neighbourhood/total number of households searched)×100 and RA=number of L. longipalpis collected in each household (outdoors)/total number of households surveyed (outdoors).
Ethical considerations
The protocol was reviewed and approved by the Federal University of Rio Grande do Norte Human Ethical Committee (CAAE: 12584513.1.000.5537).
Results
Characteristics of VL in Natal, Brazil
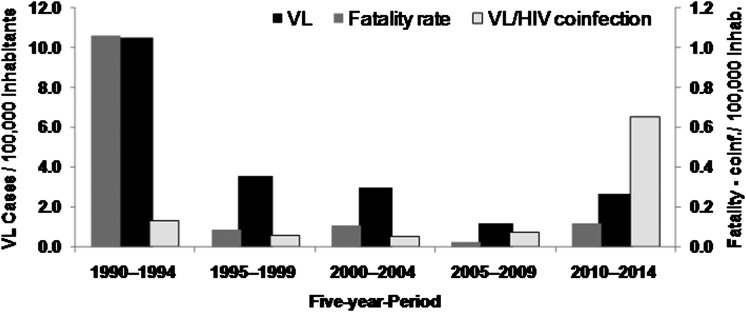
There were 718 human cases of VL in Natal between 1990 and 2014; 37.5% of these were diagnosed between 1990 and 1992. The annual incidence of VL peaked at 19.4/100 000 inhabitants in 1991. The mean incidence of VL was 4.2/100 000 over the study period with a standard deviation (SD) of 4.6. This is higher than the national average of VL for Brazil of 1.8 cases/100 000 people.18 The highest incidence of human VL was at the beginning of the outbreak (1990–1994). Cases of VL–HIV co-infection increased over time, reaching a peak in 2010–2014 (Figure 1). Of the 718 persons diagnosed with VL, 46 (6.4%) died. The fatality rate was higher at the start of the outbreak (12%) and decreased thereafter (Figure 1).
Figure 1.
Incidence of VL, fatality rate and VL/HIV co-infection per 100 000 inhabitants in Natal, Brazil between 1990 and 2014.
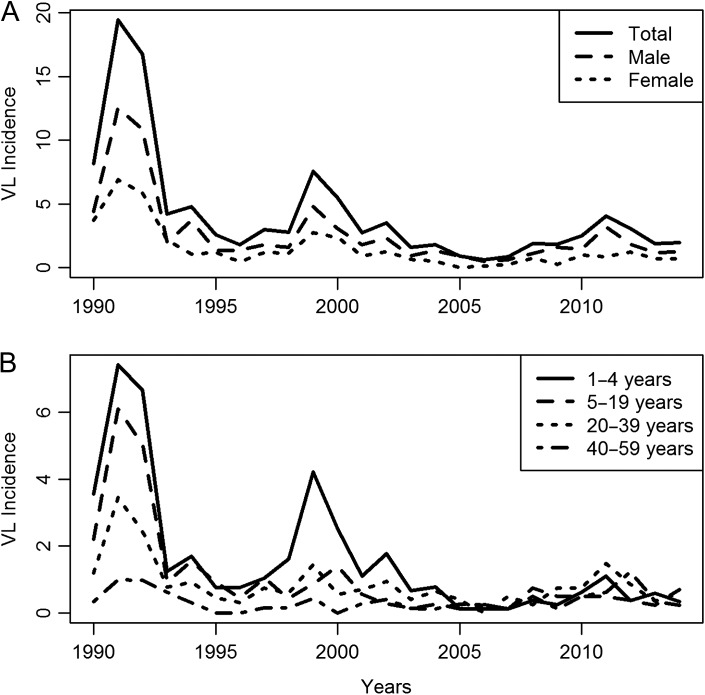
The incidences of VL by sex and age are shown in Figure 2A and B, respectively. In the period 1990–2014, the average male incidence was 5.80 (SD 1.26) and the female incidence was 2.89 (SD 0.63). The male incidence was always higher than that of females. In 1990–1992, 37% of all cases occurred in the age group 1–4 y. Overall, the percentage of VL in children <5 y of age was 26.8%; this age group made up <7% of the population of Natal. The average age at the time of diagnosis increased to 18.2 y in 2014. Males accounted for 64.2% of the VL cases. Overall there were 38 cases (4.9%) of VL with concurrent HIV/AIDS, however, 27 of these (71.1%) were diagnosed after 2010 (Figure 1).
Figure 2.
Incidence of VL per 100 000 in Natal, Brazil between 1990 and 2014, plotted according to (A) sex and (B) age group.
Influence of rainfall in the incidence of VL
Rainfall significantly correlated with the incidence of VL. In total VL, for example, the estimated structural model was indicating an association between the incidence of total VL in the previous year (φ=0.8483, p<0.0001) and rainfall (% change from May to July, β=0.000336, p=0.044). In this case, the increment in the response from a one unit increase of xt would be β=0.000336. Therefore, with a relative 10% increase in the rainfall index in year t, one would expect an increase in the incidence of total VL of approximately exp[10(0.000336)]−0.1=0.9034, that is, nine cases per million. Similar associations occurred in males and in age groups <1 y, 1–4 y and 20–30 y (Table 1).
Table 1.
Structural model fits of incidence series VL versus rainfall, Natal, Brazil, 1990–2014
| Model: | yt=φyt−1+β(xt−φxt−1)+αt | |||
|---|---|---|---|---|
| VL incidence | Structural equation | Error ARMA (1, 0) | ||
| β | p-Value | φ | p-Value | |
| Total | 0.000336 | 0.044 | 0.848261 | <0.0001 |
| Male | 0.000533 | 0.003 | 0.784449 | <0.0001 |
| Female* | 0.00012 | 0.559 | 0.792703 | <0.0001 |
| Age group (years) | ||||
| <1 | 0.000799 | 0.021 | 0.533202 | 0.041 |
| 1–4 | 0.000559 | 0.01 | 0.936519 | <0.0001 |
| 5–19 | 0.00045 | 0.004 | 0.696984 | <0.0001 |
| 20–39 | 0.000442 | <0.0001 | 0.411926 | 0.053 |
| 40–59 | 0.000275 | 0.045 | 0.318921 | 0.125 |
Note: Bold values are significant at 5%.
Spatial distribution of human VL
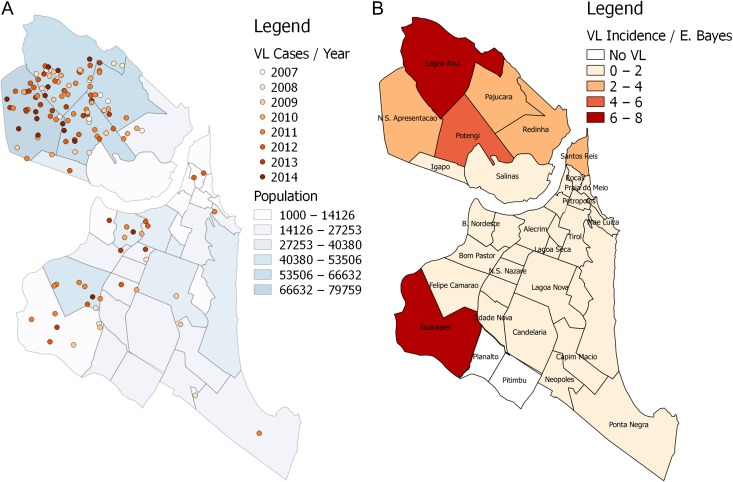
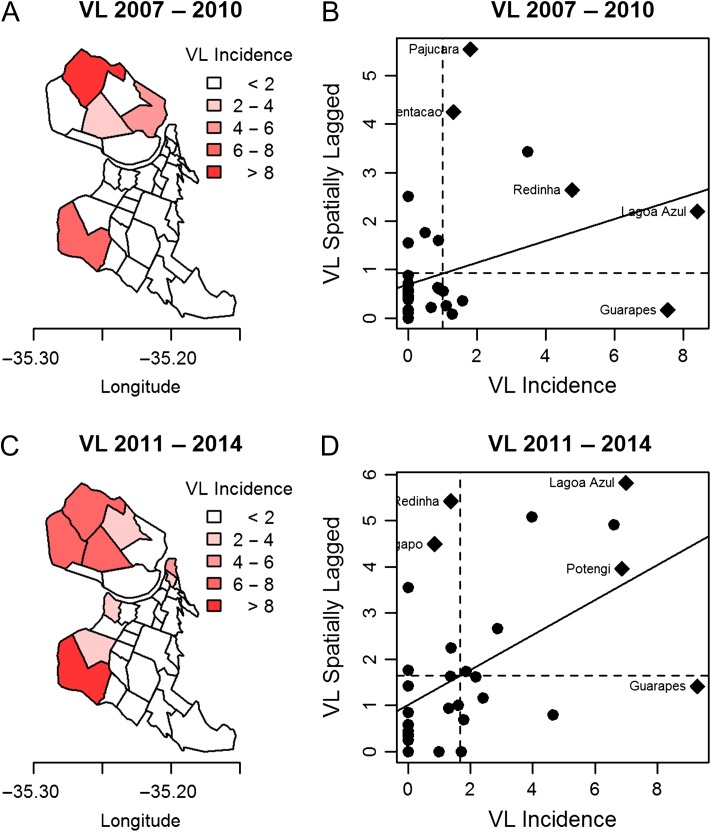
VL cases showed an uneven distribution among the neighbourhoods (Figure 3A), with 74.6% of the cases located in the northern neighbourhoods. Figure 3B shows the map of VL incidence in Natal during the study period 2007–2014. We used the empirical Bayesian estimator to allow for smoothing possible instabilities in the VL rate. In the 2007–2010 period, a focus with >8 cases per 100 000 inhabitants was observed for one of the neighbourhoods in the north zone (Lagoa Azul), however, the index of similarity with its neighbouring districts (N.S. Apresentação, Potengi and Pajuçara), represented by the mean of VL spatially lagged, was slightly >2. This can be considered an outlier, since two of its neighbours had practically no incidence of VL. The Guarapes neighbourhood, in the west, also had a high–low pattern, with high incidence but with low incidence in surrounding neighbourhoods. In this period the spatial association coefficient Moran’s I was 0.15 (p=0.056) (Figure 4A and B). In the 2011–2014 period, the spatial association was shown to be stronger, with Moran’s I=0.33 (p=0.0012). The Lagoa Azul neighbourhood continued to be an outlier, but its index of similarity with its neighbourhood increased greatly, becoming close to 6, thus building a cluster of high VL incidence. Guarapes, in the west, increased the VL incidence to >8 per 100 000 inhabitants, and the similarity with its neighbours rose above 1 (Figure 4C and D). Overall, the data suggest that there has been a persistence of VL in the northern neighbourhoods across the years.
Figure 3.
Spatial distribution of human VL in Natal, Brazil. (A) Human VL cases according to the year of occurrence. (B) Empirical Bayesian estimator on the map of human VL cases per 100 000 inhabitants by neighbourhood.
Figure 4.
Maps and Moran diagram incidence of VL per 100 000 inhabitants of Natal, Brazil in 4-y periods: (A, B) 2007–2010 and (C, D) 2011–2014.
Each of the predictors of socio-economic and infrastructure variables associated with the incidence of VL are shown in Table 2. A strong spatial association was seen (ρ, p<0.05) for those variables. Variables associated with a higher standard of living in the neighbourhood had a negative β coefficient and were associated with a reduction in the incidence of VL. The variables that indicated a lack of infrastructure and poverty were associated with a higher incidence of VL. The variable with the greatest explanatory power was the percent of households with garbage collection (R2=0.68), followed by households without sanitation (R2=0.58), average number of residents per household (R2=0.58) and literacy rate in the neighbourhood (R2=0.54). All those variables reflect a lack of infrastructure and are a proxy of poverty. A multiple regression model with all parameters would explain more than 80% of the spatial value of VL.
Table 2.
Association* in the incidence of VL with socio-economic and structural variables, Natal, Brazil, 2007–2014
| Model | β | p-Value | ρ | p-Value | R2 | Predictor |
|---|---|---|---|---|---|---|
| 1 | −0.3147 | 0.1000 | 0.5403 | 0.0033 | 0.51 | % households with toilets |
| 2 | −0.0163 | 0.0339 | 0.4047 | 0.0403 | 0.51 | % households with city water |
| 3 | 0.0414 | 0.0007 | 0.2143 | 0.3268 | 0.52 | % households with septic tank |
| 4 | 1.4775 | 0.0020 | 0.5154 | 0.0036 | 0.58 | % without sanitation households |
| 5 | −0.5633 | <0.0001 | 0.4699 | 0.0044 | 0.68 | % households with garbage collection |
| 6 | 0.0484 | 0.0151 | 0.6118 | 0.0002 | 0.49 | % households with income <1 minimum wage |
| 7 | −0.0341 | 0.0136 | 0.3891 | 0.0476 | 0.52 | % households with income >3 minimum wage |
| 8 | 3.8266 | 0.0028 | 0.4166 | 0.0247 | 0.58 | % average residents per household |
| 9 | −0.1394 | 0.0082 | 0.4222 | 0.0254 | 0.54 | % of literacy rate in the neighbourhood |
*All nine variables showed R2=0.82 with significance in variables 5 (p<0.0001), 6 (p=0.0467) and 9 (p<0.0001).
Canine L. infantum infection, sandfly density and human VL
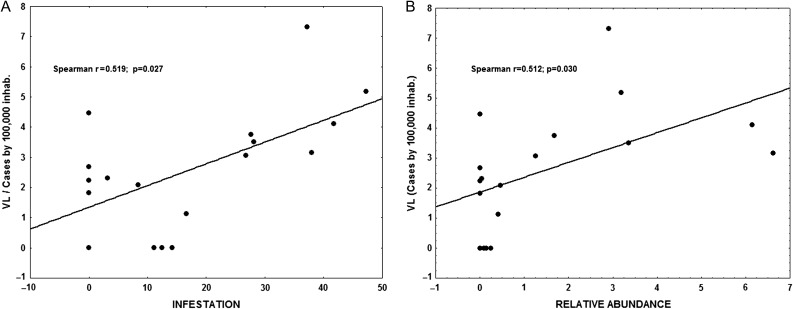
A total of 50 181 blood samples were collected from dogs in neighbourhoods of reported VL subjects between 2007 and 2014. Among those, 4256 (8.4%) were positive for L. infantum infection. The ratio of L. infantum infected:uninfected dogs varied in accordance with the districts, with highest in the north (9.8%), followed by the west (4.0%), east (2.8%) and south (2.1%). The average rates of L. longipalpis infestation and the highest relative sandfly abundance were 32% and 3.52, respectively. The highest rate was in the north district, with 38.4% L. longipalpis infestation and 4.2 relative abundance. Spearman’s correlation coefficient was calculated showing that there was a significant association between human VL and canine L. infantum infection (rs=0.517, p<0.001; Figure 5), using the WPCINF weighted index as the canine infection indicator. Using the unweighted PCINF version, the coefficient decreases (rs=0.328, p=0.004). A correlation between the incidence of human VL and vector infestation (rs=0.519, p=0.027) as well as relative abundance of L. longipalpis (rs=0.512, p=0.030) was found and is shown in Figure 6A and B.
Figure 5.
Log-log diagram of the correlation of the incidence of human VL and the weighted proportion of infected dogs, Natal, Brazil, 2007–2014.
Figure 6.
Human visceral leishmaniasis and vector infestation. (A) Correlation between the incidence of VL and entomological indicators of vector infestation. (B) Relative abundance of L. longipalpis in Natal.
Discussion
VL has become endemic in the city of Natal, northeastern Brazil, although the incidence of new cases and fatality rate have declined since the early 1990s.6 Despite the decrease in the incidence of VL, there has been continuous ongoing transmission as estimated by the high level of asymptomatic L. infantum infection,12 and there is the risk of new epidemics in the future.
Historically, children have been the most commonly affected hosts, but the average age at diagnosis has increased. This could be due to decreased exposure to the parasite and/or improved health, nutrition and medical care. As in the past, males account for the majority of cases. The higher frequency of VL in males is in agreement with other reports, including our own.6 In Sudan, the risk of developing VL is also higher in males.19 Given that the risk of exposure and infection occurs primarily in the household in Natal and that males and females, in all age groups, appear to be equally exposed to Leishmania infection,20 it seems likely that the male predominance is due to its greater risk of progression from asymptomatic infection to disease. Male predominance has also been observed in studies of Mycobacterium tuberculosis infection.21 Our prior studies in Natal revealed that the incidence of VL is comparable in males and females <10 y of age, but the male:female ratio increases thereafter.20 We hypothesize that immunologic effects of sex hormones are responsible for the increased risk of VL in males.22
Changes in the epidemiological pattern of VL in Brazil were noted in the 1980s when outbreaks of VL were reported in Natal and other large Brazilian cities in the setting of mass urban migration. A cyclic pattern of VL over time has also been noted in Brazil3 and in endemic regions of India.23 This is likely due to global environmental factors that affect sandfly density such as El Niño.24
Of note, 4.9% of persons with VL in the current study were co-infected with HIV. More cases of concurrent VL/AIDS in the Americas have been reported from Brazil than any other country. In some areas of Africa, the number of co-infected individuals has increased dramatically due to mass migration and war. In northwestern Ethiopia, for example, 30% of patients with VL have AIDS.25 VL re-emerged in Europe during the HIV epidemic because of needle sharing.11,25 Approximately 10% of cases of VL in Brazil are now estimated to be associated with HIV/AIDS. We anticipate that co-infection may become greater in the future.
Young age has commonly been identified as a risk factor for morbidity and mortality with VL due to L. infantum.3 In the current study, 37% of cases occurred among children 1–4 y of age. This group accounts for <7% of the population of Natal. This finding corroborates studies conducted in other Brazilian states such as São Paulo, Alagoas and Minas Gerais.26
With respect to environmental factors, the incidence of VL in Natal correlates with rainfall, similar to other areas of Brazil. The association of VL with rainfall is likely due to the abundance of the vector when environmental conditions allow sandflies to thrive.7,27 However, the incubation period between Leishmania infection and the development of VL varies, precluding precise determination of the time of infection. The length of time between Leishmania seroconversion and the development of human VL varied from a few weeks to 14 months in a study conducted in a rural area of Brazil in late 1980s.4
The heterogeneous distribution of VL within regions of Natal is likely due at least in part to variations in local factors that determine sandfly breeding and abundance. The highest incidence of VL was in the north of the city of Natal and was associated with low income and neighbourhoods that lacked proper infrastructure. The expansion of residential areas, mostly settled by ‘land squatting’, has been associated with disturbances in wild ecosystems for sandfly breeding.28 VL affects mainly individuals with low income whose living conditions result in greater exposure to sandflies capable of transmitting Leishmania. A meta-analysis also showed that factors linked to poverty and low education levels were associated with not only VL, but also HIV/AIDS.3,29
Finally, there was an association between the prevalence of L. infantum–infected dogs and the incidence of human VL, similar to that reported in other areas of the state.12 This would make it appear that a reduction in infection of the canine reservoir might result in a reduction of human VL. However, to date, dog culling has not proven to be effective in controlling VL in Brazil.30 This may be due to the high replacement rate of dogs, some of which are infected. There is usually a lag between implementation of control measures and the identification of L. infantum transmission in a neighbourhood and there is also the lack of clarity about the nuances of the dynamics of Leishmania transmission in dogs and the uncertain role of humans as a potential reservoir of infection. A more effective approach may be to improve infrastructure, sanitation and education at the fringes of cities, which might not only decrease the incidence of VL but also improve the quality of life in those areas.
In summary, VL has become endemic in the city of Natal, Brazil, over the past 25 y and remains an important cause of fatality. The highest incidence of VL is among males and the average age at diagnosis has increased. Co-infection with HIV/AIDS has become increasingly prevalent. The environment, social context and factors such as land use and occupation, lack of infrastructure, vector density and canine infection appear to be responsible for the aggregation of cases. A better understanding of the complexity of L. infantum infection in humans and dogs, factors that enhance transmission by the sandfly vector and socio-economic determinants should allow for improved control measures in the future.
Acknowledgments
Authors’ contributions: ALML, IDL, JWQ and SMBJ conceived the study. ALML, JWQ and SMBJ designed the study protocol. ALML, IDL, JFVC, UPSTS and MAGR collected the data. JWQ performed data analysis and interpretation. ALML, JWQ, RDP, MEW and SMBJ drafted the manuscript. All authors critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. SMBJ is the guarantor of the paper.
Acknowledgments: We thank the Zoonosis Control Center staff, from the Natal Health Secretariat, for sharing data on canine infection and sandflies for the city of Natal.
Funding: The project was funded by the National Institutes of Health (AI-030639).
Competing interests: None declared.
Ethical approval: This study was approved by the Universidade Federal do Rio Grande do Norte Ethics Committee.
References
- 1. Penna HA. Leishmaniose visceral no Brasil. J Bras Med 1934;48:949–50. [Google Scholar]
- 2. Costa CH, Pereira HF, Araujo MV. Visceral leishmaniasis epidemic in the State of Piaui, Brazil, 1980–1986. Rev Saude Publica 1990;24(5):361–72. [DOI] [PubMed] [Google Scholar]
- 3. Maia-Elkhoury AN, Alves WA, Sousa-Gomes ML et al. Visceral leishmaniasis in Brazil: trends and challenges. Cad Saude Publica 2008;24(12):2941–7. [DOI] [PubMed] [Google Scholar]
- 4. Evans TG, Teixeira MJ, McAuliffe IT, et al. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis 1992;166(5):1124–32. [DOI] [PubMed] [Google Scholar]
- 5. Marzochi MC, Marzochi KB, Carvalho RW. Visceral leishmaniasis in Rio de Janeiro. Parasitol Today 1994;10(1):37–40. [DOI] [PubMed] [Google Scholar]
- 6. Jeronimo SM, Oliveira RM, Mackay S, et al. An urban outbreak of visceral leishmaniasis in Natal, Brazil. Trans R Soc Trop Med Hyg 1994;88(4):386–8. [DOI] [PubMed] [Google Scholar]
- 7. Brazil RP. The dispersion of Lutzomyia longipalpis in urban areas. Rev Soc Bras Med Trop 2013;46(3):263–4. [DOI] [PubMed] [Google Scholar]
- 8. Badaro R, Jones TC, Lorenco R, et al. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis 1986;154(4):639–49. [DOI] [PubMed] [Google Scholar]
- 9. Harrison LH, Naidu TG, Drew JS, et al. Reciprocal relationships between undernutrition and the parasitic disease visceral leishmaniasis. Rev Infect Dis 1986;8(3):447–53. [DOI] [PubMed] [Google Scholar]
- 10. Maciel BL, Lacerda HG, Queiroz JW, et al. Association of nutritional status with the response to infection with Leishmania chagasi. Am J Trop Med Hyg 2008;79(4):591–8. [PubMed] [Google Scholar]
- 11. Alvar J, Gutierrez-Solar B, Molina R, et al. Prevalence of Leishmania infection among AIDS patients. Lancet 1992;339(8806):1427. [DOI] [PubMed] [Google Scholar]
- 12. Lima ID, Queiroz JW, Lacerda HG, et al. Leishmania infantum chagasi in northeastern Brazil: asymptomatic infection at the urban perimeter. Am J Trop Med Hyg 2012;86(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gidwani K, Picado A, Ostyn B, et al. Persistence of Leishmania donovani antibodies in past visceral leishmaniasis cases in India. Clin Vaccine Immunol 2011;18(2):346–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Instituto Brasileiro de Geografia e Estatistica Cidades [Rio Grande do Norte] Natal. Instituto Brasileiro de Geografia e Estatistica, 2016. https://www.ibge.gov.br [accessed 9 March 2016].
- 15. Box GEP, Jenkins GM, Reinsel GC. Time series analysis: forecasting and control, 3rd ed Englewood Cliffs, NJ: Prentice-Hall, 1994. [Google Scholar]
- 16. Waller LA, Gotway CA. Applied spatial statistics for public health data Hoboken, NJ: John Wiley & Sons, 2004. [Google Scholar]
- 17. Anselin L. The Moran scatterplot as an ESDA tool to assess local instability in spatial association In: Fischer M, Scholten HJ, Unwin D, editors. Spatial analytical perspectives on GIS London: Taylor & Francis, 1996; 111–25. [Google Scholar]
- 18. Minesterio Saude Situação Epidemiológica—Dados. Portal da Saúde. Ministerio da Saude, 2016. http://portalsaude.saude.gov.br [accessed 24 February 2016].
- 19. Nackers F, Mueller YK, Salih N, et al. Determinants of visceral leishmaniasis: a case-control study in Gedaref State, Sudan. PLoS Negl Trop Dis 2015;9(11):e0004187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeronimo SM, Duggal P, Braz RF, et al. An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral leishmaniasis in northeast Brazil. Scand J Infect Dis 2004;36(6–7):443–9. [DOI] [PubMed] [Google Scholar]
- 21. Mukhopadhyay D, Mukherjee S, Ghosh S, et al. A male preponderance in patients with Indian post kala-azar dermal leishmaniasis is associated with increased circulating levels of testosterone. Int J Dermatol 2016;55:e250–5. [DOI] [PubMed] [Google Scholar]
- 22. Arcay L. [Effect of sex hormones on experimental infections induced by a strain of Leishmania mexicana amazonensis from Venezuela]. Rev Latinoam Microbiol 1985;27(3):195–207. [PubMed] [Google Scholar]
- 23. Singh OP, Hasker E, Boelaert M, et al. Elimination of visceral leishmaniasis on the Indian subcontinent. Lancet Infect Dis 2016;16(12):e304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franke CR, Ziller M, Staubach C, et al. Impact of the El Niño/Southern Oscillation on visceral leishmaniasis, Brazil. Emerg Infect Dis 2002;8(9):914–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alvar J, Aparicio P, Aseffa A, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 2008;21(2):334–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menezes JA, Luz TC, Sousa FF, et al. Peridomiciliary risk factors and knowledge concerning visceral leishmaniasis in the population of Formiga, Minas Gerais, Brazil. Rev Bras Epidemiol 2016;19(2):362–74. [DOI] [PubMed] [Google Scholar]
- 27. Macedo-Silva VP, Martins DR, De Queiroz PV, et al. Feeding preferences of Lutzomyia longipalpis (Diptera: Psychodidae), the sand fly vector, for Leishmania infantum (Kinetoplastida: Trypanosomatidae). J Med Entomol 2014;51(1):237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ximenes MF, Castellon EG, de Souza MF, et al. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in the state of Rio Grande do Norte, Brazil. J Med Entomol 2000;37(1):162–9. [DOI] [PubMed] [Google Scholar]
- 29. Belo VS, Struchiner CJ, Barbosa DS, et al. Risk factors for adverse prognosis and death in American visceral leishmaniasis: a meta-analysis. PLoS Negl Trop Dis 2014;8(7):e2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werneck GL, Costa CHN, de Carvalho FAA, et al. Effectiveness of insecticide spraying and culling of dogs on the incidence of Leishmania infantum infection in humans: a cluster randomized trial in Teresina, Brazil. PLoS Negl Trop Dis 2014;8(10):e3172. [DOI] [PMC free article] [PubMed] [Google Scholar]