Abstract
Context
Oligosaccharides are the third largest solid component in human milk. These diverse compounds are thought to have numerous beneficial functions in infants, including protection against infectious diseases. The structures of more than 100 oligosaccharides in human milk have been elucidated so far.
Objective
The aim of this review was to identify the main factors that affect the concentrations of oligosaccharides in human milk and to determine whether it is possible to calculate representative and reliable mean concentrations.
Data Sources
A comprehensive literature search on oligosaccharide concentrations in human milk was performed in 6 electronic databases: BIOSIS, Current Contents Search, Embase, Lancet Titles, MEDLINE and PubMed.
Study Selection
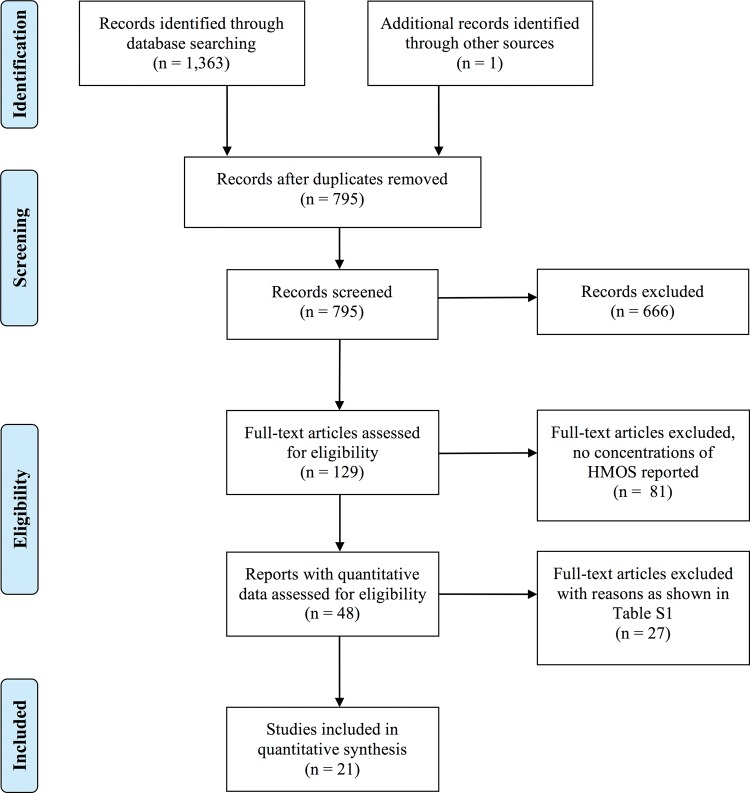
The initial search resulted in 1363 hits. After the elimination of duplicates, the literature was screened. The application of strict inclusion criteria resulted in 21 articles selected.
Data Extraction
Oligosaccharide concentrations, both mean values and single values, reported in the literature were sorted by gestational age, secretor status of mothers, and defined lactation periods.
Results
Mean concentrations, including confidence limits, of 33 neutral and acidic oligosaccharides reported could be calculated. Concentrations of oligosaccharides in human milk show variations that are dependent on both the secretor type of the mother and the lactation period as examined by analyses of variance. In addition, large interlaboratory variations in the data were observed.
Conclusions
Worldwide interlaboratory quantitative analyses of identical milk samples would be required to identify the most reliable methods of determining concentrations of oligosaccharides in human milk. The data presented here contribute to the current knowledge about the composition and quantities of oligosaccharides in human milk and may foster greater understanding of the biological functions of these compounds.
Keywords: concentration, human milk, lactation period, oligosaccharide, secretor status
INTRODUCTION
Human milk oligosaccharides (HMOS) represent approximately 20% of the total carbohydrate content of human milk and are the third largest solid component, present at concentrations of up to 20 g/L or more in colostrum.1 A wide variety of oligosaccharides are synthesized in the mammary gland by the action of specific glycosyltransferases that sequentially add N-acetylglucosamine, galactose, fucose, and N-acetylneuraminic acid to the basic acceptor molecule, lactose.2 Currently, approximately 150 oligosaccharide structures in human milk have been elucidated,3–12 and many more are present, at least in small quantities.13 The presence of glycosyltransferases in the mammary gland is genetically determined. With the exception of fucosyltransferases, glycosyltransferases are common to all mothers. Fucosyltransferase 2 (corresponding to the secretor enzyme) transfers fucose residues as α1,2 linkages to acceptor molecules,14 whereas fucosyltransferase 3 (ie, the Lewis enzyme) transfers fucose residues predominantly as α1,4 linkages, leading to different patterns of fucosylated HMOS.2,15,16
Since the infant’s intestine cannot digest HMOS,17,18 the possible physiological role of HMOS was the subject of intensive research for more than 60 years. The prebiotic effect of HMOS was the first function discovered.19 There is increasing evidence that HMOS have other functions as well, as indicated by the direct interaction of HMOS with bacterial or protozoan lectins as well as with epithelial or immune cell receptors.20–23
Historically, the focus of HMOS research has been the development of methods to analyze the structure of HMOS. Since the biological functions of HMOS depend not only on the specific structure of an HMOS but also on the quantity of the HMOS present, different methods of quantitative measurements have also been developed24–30 to understand both the functional properties of HMOS and the role of HMOS in the development and well-being of the breastfed infant.
The wide variability in the pattern of HMOS present in different individuals is well known. There are several studies indicating that factors such as polymorphism of the Lewis and secretor genes2,31–33 as well as the period of lactation31,34,35 determine both the pattern and the quantities of HMOS present in milk. Since data on human milk are often used as the basis for establishing infant feeding regimens, this review aimed to determine whether standard concentrations of individual HMOS in human milk can be calculated and to identify which confounding factors must be considered. Such data would be important for calculating the physiological intakes of different HMOS, which have various biological functions. The findings presented here may serve as a basis for the application of oligosaccharides with structures identical to or different from those of HMOS.
METHODS
Literature search and literature selection
Comprehensive electronic literature searches were performed (August 2015) to find all relevant literature reporting quantitative data for HMOS. The databases BIOSIS, Current Contents Search, Embase, Lancet Titles, MEDLINE, and PubMed were screened by CM and GB. The following search strategy was applied: “(human OR breast) AND (milk) AND (oligosaccharide*) AND (quantification OR concentration* OR content*).” The only language limitation was that the titles and the abstracts had to be published in English.
The full texts of all articles that appeared to report quantitative data for HMOS were evaluated by applying the predetermined PICOS (Population, Intervention, Comparator, Outcome, and Study design) criteria described in Table 1.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Intervention | None | None |
| Comparator | None | None |
| Outcomes |
|
|
| Study design | Original articles from peer-reviewed journals | Abstracts, monographs, review articles Studies with data already reported |
Abbreviations: HMOS, human milk oligosaccharides.
Design of the systematic review
Concentrations of individual HMOS reported in the literature vary widely. Such variation may be attributable to the general biological variability as well as to the influence of gestational age, Lewis blood group, secretor status of the mother, lactation period, and analytical methods. Although the effect of gestational age on HMOS concentrations has not been clearly demonstrated in previous studies, an influence cannot be excluded. Gidrewicz and Fenton36 reported significantly higher amounts of oligosaccharides in preterm milk than in term milk during days 4 to 7 postpartum. Furthermore, lacto-N-tetraose (LNT), a nonfucosylated core HMOS, was more abundant in the milk of women who delivered preterm.37 Therefore, in the present review, documentation of the gestational age was an inclusion criterion, and data from term and preterm milks were analyzed and presented separately.
Four different human milk types, characterized by the presence or absence of specific fucosylated oligosaccharides, have been described. The presence or absence of fucosylated oligosaccharides depends on the Lewis blood type of the lactating woman.2,16 Furthermore, the 4 milk types contain different amounts of HMOS; these amounts are not directly affected by either of the 2 fucosyltransferases, ie, the secretor enzyme and the Lewis enzyme.31–33 There are only a few HMOS studies that report the Lewis blood group of the donors, and even fewer compare HMOS concentrations of the different milk types. However, several authors report the secretor status of the mothers without indicating the exact milk group (secretors produce milk belonging to groups 1 and 3, whereas nonsecretors produce milk belonging to groups 2 and 4). For the purpose of this systematic review, HMOS data was included if the secretor status of the mothers was reported, even if the exact milk group was not.
Several studies have demonstrated the influence of the lactation period on HMOS concentrations.31,34,35,38,39 There is no international consensus about how to define lactation periods. The following 6 lactation periods were defined on the basis of the most commonly used definition in the articles selected for the present review: 0 to 4 days postpartum (colostrum), 5 to 10 days postpartum (transitional milk), 11 to 30 days postpartum, 31 to 60 days postpartum, 61 to 100 days postpartum, and more than 100 days postpartum. The effects of the lactation periods were examined in conjunction with the documented gestational age and with secretor status, respectively.
The above-mentioned variability in concentrations of different HMOS is probably also attributable to the various methods used to quantify free oligosaccharides in human milk. Although details about quantification methods were recorded for this review, the effect of these methods could not be tested because nearly every study used a unique methodology with regard to the preparation, derivatization, and separation of samples and the detection and quantification of HMOS.
Data extraction and data processing
From the studies selected, absolute concentrations of HMOS were extracted if the HMOS structures could be identified on the basis of several recent reports.6,8–10 The nomenclature according to Urashima et al.8 served as a guideline for the designation of oligosaccharide structures. The structures of 3 HMOS reported in 1 study were identified after the main author was contacted.34
Most reports selected indicated the secretor status of the mothers as determined either by serological tests or by interpretation of the HMOS patterns found in human milk. The secretor status of the mothers in 2 studies40,41 could be determined from the individual HMOS patterns reported. The secretor-positive status of the donors in 1 study42 could be identified by combining the HMOS patterns and the concentration ranges reported. Erney et al.35 did not mention the secretor status of the mothers. Nevertheless, the concentrations of α1,2 fucosylated HMOS were included because the authors indicated that zero values were excluded from the calculations of mean values. This was interpreted to mean that only secretor mothers were taken into account. Furthermore, data of other HMOS analyzed in milks from mothers from Latin America were also included in the present review because the HMOS patterns reported showed that these mothers were almost exclusively secretor donors.35
In most cases, concentrations of the HMOS were reported as grams per liter or milligrams per liter. Concentrations reported as millimoles per liter were converted to grams per liter by multiplying by the average molecular mass of the corresponding HMOS. Data presented in non-numerical form in graphs were measured manually with rulers, using multiple enlarged figures that were translated into numbers.43–46 Two mean values of the acidic HMOS LST a were reported as “not determined.”34 During data processing, these expressions were transduced to zero values.
Usually, authors reported mean values as well as standard deviations, number of samples, and number of mothers. When concentrations from single milk samples were reported, mean values were calculated, with data sorted according to gestational age, secretor group, and lactation period as defined in the present review.40,41,47,48 When authors reported several mean values within a certain lactation period at different days postpartum, the corresponding mean of the entire period was calculated. As a result, the different numbers of mothers or samples were not taken into account. Similarly, means reported for milk groups 1 and 3 as well as for milk groups 2 and 4 were averaged to yield the corresponding data for secretors and nonsecretors, respectively.33,41 In addition, means reported from different world regions at given lactation times were also averaged.35 As such, each study was represented at a given lactation period with 1 mean value.
Prior to the analyses of variance, outlier diagnostic tests were conducted using a cutoff value of 3.0. The rare studies that did not meet this criterion were excluded from the data processing. Furthermore, the prerequisites for the analysis of variance—homoscedasticity (Brown-Forsythe test) and homogeneity of variance (Shapiro–Wilk test)—were checked. These requirements were fulfilled in all cases. Data processing was performed with the software SAS, version 9.3, applying the procedures GLM (general linear models), MIXED (mixed linear models), and ROBUSTREG (robust regression) (using option outlier diagnostics).49
A one-way analysis of variance followed by a Tukey test of the means was applied to the factor “lactation period” as defined in this section. Tukey tests were carried out on a level of significance of α = 5%. In addition, 95% confidence limits were calculated for all mean values. All analyses were performed separately for term and preterm milks from secretor mothers. A two-factor analysis of variance with the factors “lactation period” and “secretor status” was performed when authors provided data for defined lactation periods from secretor as well as from nonsecretor mothers. Because these two-factor analyses were based on unequal numbers of observations, adjusted means (so-called least squares means) had to be calculated. Out of 24 HMOS tested, only 2 (3′-FL in term milks and DF-LNH II in preterm milks) showed significant interactions between the factors “lactation period” and “secretor status” detected. Hence, both factors were largely independent.
RESULTS
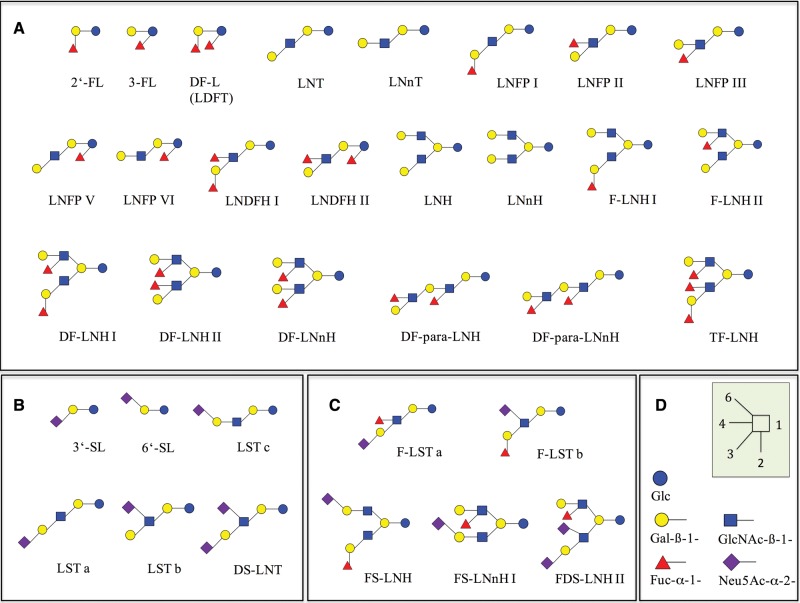
The application of a relatively simple search strategy using the keywords “human milk oligosaccharides” in combination with “quantification” in the most common medical and life science databases yielded 1363 hits (Figure 1). After duplicates were removed, the titles and abstracts of the articles were screened. Reports with irrelevant topics as well as monographs, meeting abstracts, and review articles were excluded, resulting in 129 articles. The corresponding full-text articles were examined intensively. These included all relevant articles already known to the authors, with 1 exception.39 Forty-eight research studies reported quantitative data on single HMOS compounds. Of these, 27 had to be excluded because 1 or several inclusion criteria had not been fulfilled24,25,27–29,37,38,50–69 (see Table S1 in the Supporting Information online). Several articles could not be included because the secretor status of the mothers could not be determined.24,29,38,58,61,67 Finally, 21 studies meeting the predefined inclusion criteria were selected.31–35,39–48,70–75Table 231–35,39,40–48,70–75 lists the main characteristics of these studies, the countries of the mothers, the secretor status, the gestational stage, and the quantification methods applied. The secretor status was either reported or could be deduced from 15 of the 21 studies selected, as shown in Table 2. The remaining 6 studies, which investigated acidic HMOS, lacked information on the secretor status of the mothers but were included since the effect of secretor status on the concentrations of acidic compounds is of minor importance, as noted in the next section. From all these reports, absolute concentrations of 33 HMOS (Figure 2) were extracted. The 33 oligosaccharides were classified according to their degree of fucosylation and sialylation: 22 were neutral, nonsialylated HMOS and 11 were acidic HMOS, half of which also contained fucose residues.
Figure 1.
Flow diagram of the literature search process.
Table 2.
Studies included in the systematic review
| Reference | Mothers |
Quantification methods | Type of HMOS quantified | |||
|---|---|---|---|---|---|---|
| Site | Gestation | Secretor status | N | |||
| Asakuma et al. (2007)70 | Japan | Term | Unknown | 20 | HPLC-UV | Acidic |
| Asakuma et al. (2008)42 | Japan | Term | + | 12 | HPLC-UV | Neutral |
| Bao et al. (2007)47 | USA | Term | Unknown | 8 | CE-UV | Acidic |
| Bao et al. (2013)48 | USA | Term | + | 4 | LC-MS | Neutral |
| Chaturvedi et al. (2001)43 | Mexico | Term | + | 11 | HPLC-UV | Neutral |
| Coppa et al. (1999)34 | Italy | Term | + | 18 | HPAEC-PAD | Neutral, acidic |
| Coppa et al. (2011)32 | Italy | Term | +/− | 16/23 | HPAEC-PAD | Neutral |
| Erney et al. (2000)35 | Various world regions | Term | + | 197 | HPAEC-PAD | Neutral |
| Gabrielli et al. (2011)33 | Italy | 27.9 wk | +/− | 42/21 | HPAEC-PAD | Neutral, acidic |
| Goehring et al. (2014)71 | USA | Term | +/− | 13/4 | LC-MS | Neutral, acidic |
| Hong et al. (2014)72 | USA | Term | +/− | 10/10 | LC-MS | Neutral, acidic |
| Kunz et al. (1999)40 | Germany | Term | +/− | 2/2 | HPAEC-PAD | Neutral, acidic |
| Leo et al. (2010)73 | Samoa | Term | Unknown | 16 | HPLC-UV | Neutral, acidic |
| Martin-Sosa et al. (2003)44 | Spain | Term | Unknown | 12 | HPLC-UV | Acidic |
| Nakhla et al. (1999)41 | USA | 29.5 wk | + | 12 | HPAEC-PAD | Acidic |
| Olivares et al. (2015)74 | The Netherlands | Term | +/− | 7/5 | CE-FL | Neutral |
| Smilowitz et al. (2013)45 | USA | Term | +/− | 40/12 | NMR | Neutral |
| Spevacek et al. (2015)75 | USA | Term/preterm | Unknown | 15/13 | NMR | Neutral, acidic |
| Sumiyoshi et al. (2003)39 | Japan | Term | Unknown | 20 | HPLC-UV | Acidic |
| Thurl et al. (2010)31 | Germany | Term | + | 21 | HPAEC-PAD | Neutral, acidic |
| Van Niekerk et al. (2014)46 | South Africa | 500–1250 ga | +/− | 20/21 | HPLC-UV | Neutral, acidic |
Abbreviations: CE-FL, capillary electrophoresis with fluorescence detection; CE-UV, capillary electrophoresis with UV detection; HPAEC-PAD, high-pH anion-exchange chromatography with pulsed amperometric detection; HPLC-UV, high-performance liquid chromatography with UV detection; LC-MS, liquid chromatography with mass spectrometric detection; NMR, nuclear magnetic resonance spectrometry; +, positive; −, negative.
aBirth weight of the premature infants.
Figure 2.
Structures of the HMOS examined in this review. (A) Neutral HMOS, (B) acidic nonfucosylated HMOS, (C) acidic fucosylated HMOS, (D) monomers and linkages.
Concentrations of oligosaccharides in human milk
Table 3 31–35 , 42 , 43 , 45 , 46 , 48 , 71 , 72 , 74 shows the concentrations of neutral oligosaccharides in milk samples of secretor mothers, as most data reported were for HMOS from secretor milks. In addition, the reference numbers of the studies, the numbers of mothers and samples, and the corresponding lactation periods are listed. Mean values and confidence intervals of the HMOS concentrations are shown separately for term and preterm milk samples. Four studies reported the HMOS concentrations determined in preterm milks,33,41,46,75 whereas 18 reported data for term milks. The total mean concentrations of 20 neutral HMOS from term milk and 17 neutral HMOS from preterm milk were 14.8 g/L and 11.6 g/L, respectively. The difference in mean values can be explained by the fact that 7 of the HMOS quantified were different and by an unusually high concentration of TF-LNH in term milks.32,34 In most cases, the amounts of individual HMOS seemed to reach similar levels, although mothers with term infants tended to exhibit higher concentrations of LNnH, LNFP III, LNFP V, LNDFH II, and TF-LNH than mothers with preterm infants. However, owing to the particularly high variation between the 4 studies reporting preterm milk data, analyses of variance could not be performed to confirm this. In order to systematically examine the effect of gestational age, additional studies that determine HMOS concentrations both in term and in preterm milks are required so that the confounding effects of the factor method of quantification are eliminated. Only 1 study met this requirement, but it does not specify the secretor status of the milk donors.75 Nine neutral HMOS from term milks, usually small-sized structures, were quantified in 5 or more studies. Mean concentrations of these compounds ranged between 0.14 g/L and 2.74 g/L, with the α1,2 fucosylated HMOS 2′-FL, LNFP I, and LNDFH I as well as the nonfucosylated HMOS LNT and LNnT being the dominant oligosaccharides in secretor milks. In addition, the confidence intervals were narrower than those for the corresponding HMOS from preterm milks. The high concentrations of the larger neutral HMOS DF-LNH II and TF-LNH in term milks were reported in only 1 or 2 studies each.32,34
Table 3.
Concentrations of neutral human milk oligosaccharides (HMOS) from secretor mothersa
| HMOS (ref. nos. in superscript) | Lactation (days) | No. of mothers/no. of samples | Mean concentration (g/L) | 95%CL (g/L) |
|---|---|---|---|---|
| Term studies | ||||
| 2′-FL31–35,42,43,45,71,72 | 0 to > 100 | 353/556 | 2.74 | 2.43–3.04 |
| 3-FLa31,32,34,42,43,45,48 | 0 to > 100 | 122/365 | 0.44 | 0.31–0.58 |
| DF-L31,35,42,43,45,74 | 0 to > 100 | 288/455 | 0.42 | 0.32–0.51 |
| LNT31,34,40,42,45,48,72,74 | 0 to 100 | 114/308 | 0.79 | 0.59–0.98 |
| LNnT31,34,35,42,48 | 0 to 100 | 184/372 | 0.74 | 0.36–1.12 |
| LNFP Ia31,32,34,35,40,42,43,45,48,72 | 0 to > 100 | 331/580 | 1.31 | 1.08–1.53 |
| LNFP II31,32,34,35,45,48,74 | 0 to 100 | 229/389 | 0.28 | 0.21–0.34 |
| LNFP III31,35,48 | 0 to 100 | 154/246 | 0.33 | 0.24–0.42 |
| LNFP V35,48 | 0 to 30 | 133/137 | 0.06 | −0.15 to 0.26 |
| LNFP VI48 | 0 to 30 | 4/8 | 0.01 | 0.00–0.02 |
| LNDFH Ia31,34,43,48,74 | 0 to > 100 | 61/214 | 0.80 | 0.66–0.94 |
| LNDFH II31,32,34,42,43,48 | 0 to > 100 | 76/319 | 0.14 | 0.10–0.18 |
| LNH31,34,72 | 0 to 100 | 49/209 | 0.09 | 0.06–0.13 |
| LNnH34 | 0 to 100 | 18/90 | 0.16 | 0.06–0.25 |
| F-LNH I31 | 0 to 100 | 21/109 | 0.20 | 0.08–0.33 |
| F-LNH II31,32,34 | 0 to 100 | 53/176 | 0.27 | 0.14–0.40 |
| DF-LNH I31 | 0 to 100 | 21/109 | 0.31 | 0.19–0.43 |
| DF-LNH II32,34 | 0 to 100 | 28/100 | 2.31 | 1.93–2.68 |
| DF-LNnH34 | 0 to 100 | 18/90 | 0.54 | 0.10–0.98 |
| TF-LNH32,34 | 0 to 100 | 28/100 | 2.84 | 2.60–3.07 |
| Sum of means | 14.78 | |||
| Preterm studies | ||||
| 2′-FL33,41,46 | 0–60 | 74/230 | 2.77 | 0.76–4.78 |
| 3-FL33,41,46 | 0–60 | 75/230 | 0.32 | 0.17–0.48 |
| DF-L33,41 | 0–60 | 54/190 | 0.41 | 0.17–0.65 |
| LNT33,41,46 | 0–60 | 75/356 | 1.04 | 0.39–1.68 |
| LNnT33,41,46 | 0–30 | 75/227 | 0.66 | 0.04–1.28 |
| LNFP I33,41,46 | 0–60 | 74/230 | 1.09 | 0.39–1.78 |
| LNFP II33,41,46 | 0–60 | 68/198 | 0.27 | 0.14–0.40 |
| LNFP III33,41,46 | 0–60 | 75/230 | 0.16 | 0.04–0.28 |
| LNFP V41 | 0–60 | 12/22 | 0.02 | 0.00–0.04 |
| LNDFH II33,41 | 0–60 | 47/168 | 0.07 | 0.00–0.14 |
| LNH33 | 0–30 | 42/168 | 0.06 | 0.04–0.08 |
| LNnH33 | 0–30 | 42/168 | 0.08 | 0.06–0.11 |
| F-LNH II33 | 0–30 | 41/168 | 0.33 | 0.26–0.41 |
| DF-LNH II33 | 0–30 | 35/140 | 2.70 | 2.54–2.86 |
| DF-para-LNH33 | 0–30 | 35/140 | 0.44 | 0.10–0.79 |
| DF-para-LNnH33 | 0–30 | 42/168 | 0.50 | −0.09 to 1.09 |
| TF-LNH33 | 0–30 | 35/140 | 0.65 | 0.21–1.08 |
| Sum of means | 11.57 | |||
Abbreviations: CL, confidence limit; ref. nos., reference numbers.
aHMOS exhibiting significant differences between the means of the lactation periods (see Tables S2-A, S2-B, and S2-C in the Supporting Information online).
Table 4 31 , 33 , 34 , 40 , 45 , 46 , 71 , 72 lists the mean concentrations plus confidence intervals of 7 acidic HMOS from secretor mothers, reported in 1 to 6 studies. The sum of all mean concentrations of 7 acidic HMOS was 2.1 g/L for term milks (corresponding to the first 100 days postpartum) and 3.3 g/L for preterm milks (corresponding to the first 30 days postpartum). Generally, concentrations of acidic HMOS, particularly those of LST a and LST c, seem to be higher in preterm milks than in term milks. As was the case with neutral HMOS, this could not be confirmed by analyses of variance. In term milks, 6′-SL and DS-LNT were the dominant acidic HMOS.
Table 4.
Concentrations of acidic human milk oligosaccharides (HMOS) from secretor mothersa
| HMOS (ref. nos. in superscript) | Lactation (days) | No. of mothers/no. of samples | Mean concentration (g/L) | 95%CL (g/L) |
|---|---|---|---|---|
| Term studies | ||||
| 3′-SL31,34,40,72 | 0–100 | 51/217 | 0.19 | 0.14–0.24 |
| 6′-SL31,34,40,45,71,72 | 0–100 | 104/270 | 0.64 | 0.38–0.91 |
| LST a31,34 | 0–30 | 39/199 | 0.06 | 0.02–0.11 |
| LST b31,34,40 | 0–100 | 41/203 | 0.13 | 0.08–0.18 |
| LST ca31,34,72 | 0–100 | 49/191 | 0.25 | 0.13–0.38 |
| DS-LNT31,34 | 0–100 | 39/199 | 0.50 | 0.34–0.66 |
| FS-LNnH I34 | 0–100 | 18/90 | 0.36 | 0.21–0.51 |
| Sum of means | 2.13 | |||
| Preterm studies | ||||
| 3′-SL33,46 | 0–30 | 62/208 | 0.29 | 0.21–0.36 |
| 6′-SL33 | 0–30 | 42/168 | 0.66 | 0.25–1.08 |
| LST a33 | 0–30 | 42/168 | 0.29 | 0.13–0.44 |
| LST b33,46 | 0–30 | 62/208 | 0.13 | 0.06–0.19 |
| LST c33,46 | 0–30 | 62/208 | 0.71 | 0.24–1.17 |
| DS-LNT33,46 | 0–30 | 62/208 | 0.77 | 0.22–1.32 |
| FS-LNnH I33 | 0–30 | 42/168 | 0.44 | 0.27–0.62 |
| Sum of means | 3.29 | |||
Abbreviations: CL, confidence limit; ref. nos., reference numbers.
aHMOS exhibiting significant differences between the means of the lactation periods (see Table S2-D in the Supporting Information online).
Since the effect of secretor status on concentrations of acidic HMOS has not been examined in the literature31,33,71,72 and seems to be less important than the effect on neutral HMOS, data for HMOS without defined secretor status are presented in Table 5.31,33,34,39,40,44–47,70,72,73,75 In addition to data for the 7 acidic HMOS from term milk shown in Table 4, data for 4 fucosylated acidic HMOS were available. The major acidic HMOS were 6′-SL (10 studies) and DS-LNT (7 studies). To allow adequate evaluation of the mean concentration data, the number of studies that reported data for each HMOS is shown. In most cases, the corresponding mean values seem to be similar, independent of the secretor status of the donors. However, in term milks, the average concentrations of 6′-SL and LST b seem to be lower when 10 instead of 6 studies and when 6 instead of 3 studies reported data, respectively. These contradictory results can be explained by outliers detected by the software when a larger number of studies was included. Thus, 6′-SL concentrations reported by Thurl et al.31 and Goehring et al.71 and LST b values reported by Coppa et al.34 were eliminated.
Table 5.
Concentrations of acidic human milk oligosaccharides (HMOS) from lactating women, regardless of secretor statusa
| HMOS (ref. nos. in superscript) | Lactation (days) | No. of mothers/no. of samples | Mean concentration (g/L) | 95%CL (g/L) |
|---|---|---|---|---|
| Term studies | ||||
| 3′-SL31,34,39,40,44,45,47,70,72,73,75 | 0–100 | 200/509 | 0.16 | 0.12–0.19 |
| 6′-SLa34,39,40,44,45,47,70,72,73,75 | 0–100 | 179/400 | 0.35 | 0.29–0.42 |
| LST aa31,34,39,70,73 | 0–100 | 91/363 | 0.07 | 0.04–0.10 |
| LST b31,39,40,47,70,73 | 0–100 | 83/285 | 0.06 | 0.05–0.07 |
| LST c31,34,39,47,70,72,73 | 0–100 | 119/373 | 0.26 | 0.16–0.36 |
| DS-LNT31,34,39,44,47,70,73 | 0–100 | 111/395 | 0.54 | 0.37–0.71 |
| F-LST a47 | 11–30 | 3/3 | 0.02 | – |
| F-LST b70 | 0–4 | 20/60 | 0.08 | – |
| FS-LNH47 | 0–30 | 8/8 | 0.12 | −0.08 to 0.33 |
| FS-LNnH I34,47 | 0–100 | 26/98 | 0.29 | 0.14–0.44 |
| FDS-LNH II47 | 0–100 | 3/3 | 0.12 | – |
| Sum of means | 2.07 | |||
| Preterm studies | ||||
| 3′-SL33,46,75 | 0–30 | 114/350 | 0.24 | 0.20–0.28 |
| 6′-SL33,75 | 0–30 | 73/268 | 0.60 | 0.40–0.80 |
| LST a33 | 0–30 | 63/252 | 0.34 | 0.17–0.51 |
| LST b33,46 | 0–30 | 104/334 | 0.14 | 0.10–0.17 |
| LST c33,46 | 0–30 | 104/334 | 0.65 | 0.19–1.12 |
| DS-LNT33,46 | 0–30 | 104/334 | 0.92 | 0.26–1.58 |
| FS-LNnH I33 | 0–30 | 63/252 | 0.51 | 0.26–0.75 |
| Sum of means | 3.40 | |||
Abbreviation: CL, confidence limit; ref. nos., reference numbers.
aHMOS exhibiting significant differences between the means of the lactation periods (see Tables S2-E and S2-F in the Supporting Information online).
Effect of secretor status
Secretor mothers regularly produced HMOS with fucose residues bound in α1,2 linkages to terminal galactose units of various oligosaccharides. In contrast, nonsecretor women usually did not produce these HMOS. However, Hong et al.72 and van Niekerk et al.46 reported very low concentrations of the α1,2 fucosylated compound LNFP I in milk samples from nonsecretor mothers. In addition, Hong et al.72 found a relatively high mean value of 2′-FL (0.45 g/L) for 10 nonsecretor mothers. They attributed this extraordinarily high content to 2 nonsecretor mothers who produced amounts of the α1,2 fucosylated structure 2′-FL that were similar to amounts produced by secretor donors.72 In their study, secretor status was determined on the basis of serological tests. Since the Leb epitope was shown to be weakly expressed at birth,76 it is possible that the secretor status was incorrectly assigned in some cases.76,77
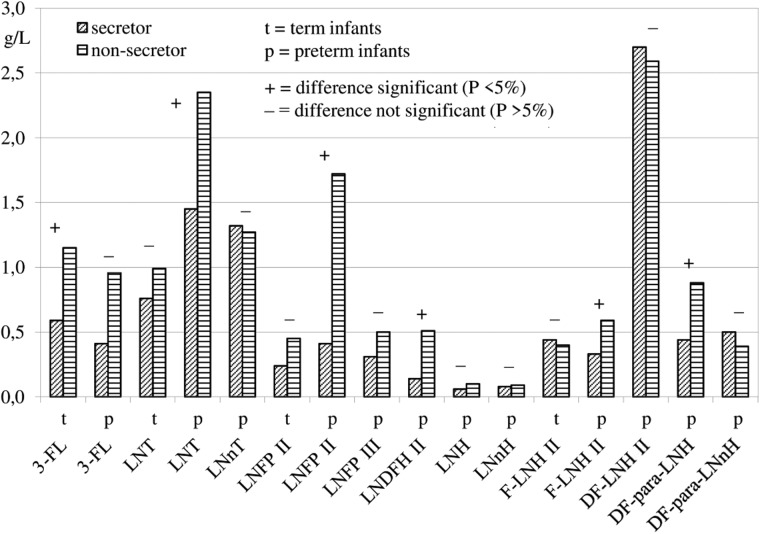
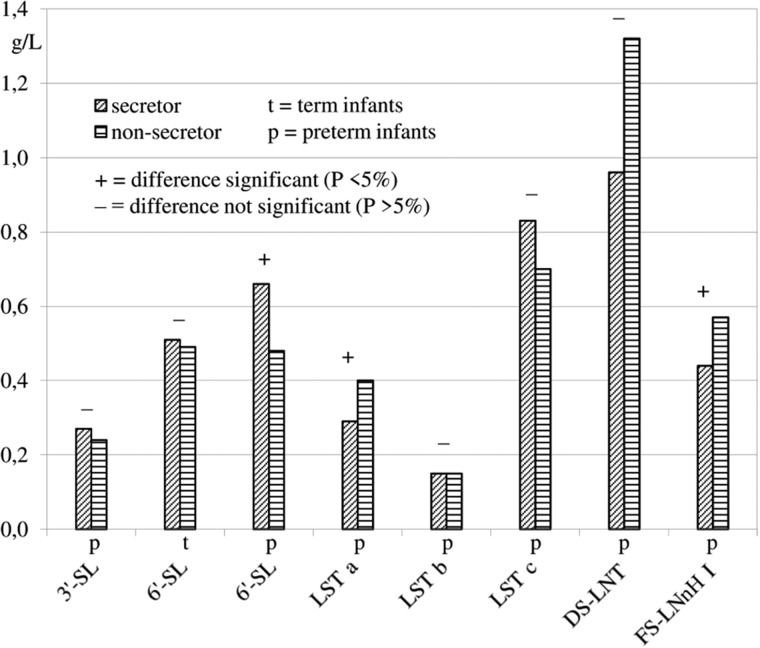
The effect of the secretor status was tested for all HMOS except the above-mentioned α1,2 fucose-containing structures that usually are absent in milks of nonsecretor mothers. To avoid a possible influence of the quantification methods, only data from studies reporting HMOS concentrations from both secretor and nonsecretor mothers in a given lactation period were used for the analyses of variance. Statistical comparisons, usually based on 1 to 3 studies, were possible with 4 neutral HMOS from term milks and 12 neutral HMOS from preterm milks (Figure 3). Concentrations of 3-FL in term milks and of LNT, LNFP II, LNDFH II, F-LNH II, and DF-para-LNH in preterm milks were significantly higher in milks from nonsecretor mothers than in milks from secretor mothers. In addition, concentrations of the acidic HMOS 6′-SL in term and preterm milks and of 6 other acidic HMOS from preterm milks could be compared (Figure 4). Concentrations of 6′-SL, LST a, and FS-LNnH I in preterm milks were significantly different between secretor and nonsecretor mothers, but no general tendency toward higher concentrations of acidic HMOS in milks from nonsecretor mothers was detectable.
Figure 3.
Comparison of neutral HMOS concentrations in milks from secretor and nonsecretor mothers.
Figure 4.
Comparison of acidic HMOS concentrations in milks from secretor and nonsecretor mothers.
Influence of lactation periods
In order to examine the effect of the time of lactation on HMOS concentrations, the time postpartum was classified into 6 lactation periods, as described above (see Design of the systematic review in Methods). The large volume of data on concentrations of HMOS is provided in the Supporting Information online (see Tables S2-A through S2-F and Tables S3, S4, and S5). Hereby, the concentrations of HMOS are listed separately for mothers with term and preterm infants, considering the lactation periods. The influence of the lactation period was tested in secretor mothers because these data on concentrations were readily available. No significant effect of the lactation period on any of the 13 HMOS from preterm milks was detected. Twenty-two HMOS from term milks could be tested. Concentrations of the neutral, ie, nonsialylated, HMOS 3-FL increased significantly (at least 2-fold) during the first 3 and 4 months postpartum (see Table S2-A in the Supporting Information online). Similarly, the concentration of LNFP III rose, but not significantly (see Table S3 in the Supporting Information online). During the course of lactation, the neutral α1,2 fucosylated HMOS LNFP I decreased approximately 2-fold (see Table S2-B in the Supporting Information online), and the acidic nonfucosylated HMOS LST c decreased approximately 4-fold (see Table S2-D in the Supporting Information online). Further significant differences were detected with the neutral HMOS LNDFH I (see Table S2-C in the Supporting Information online). In addition, concentrations of the neutral HMOS 2′-FL, DF-L, LNFP II, F-LNH II, and DF-LNH II and the acidic HMOS 3′-SL, 6′-SL, and LST a in term milks decreased during the course of lactation, although not significantly (see Tables S3 and S4 in the Supporting Information online). Concentrations of the 2 acidic HMOS 6′-SL and LST a declined significantly during the first 100 days postpartum when the secretor status of mothers was not considered (see Tables S2-E and S2-F in the Supporting Information online). This outcome could be explained by the additional studies that were taken into account in these cases (Tables 4 and 5).
DISCUSSION
During the 1980s and early 1990s, many quantitative data on oligosaccharide fractions in human milk were published. These data were obtained mainly by use of the separation techniques gel permeation chromatography and low-resolution liquid chromatography.78–82 The application of more advanced chromatographic and electrophoretic techniques like high-performance liquid chromatography–UV, high-pH anion-exchange chromatography with pulsed amperometric detection, capillary electrophoresis with UV detection, and capillary gel electrophoresis with laser-induced fluorescence detection after 1995 allowed the quantification of individual oligosaccharides in their native form or after derivatization.24–29 Recently, a variety of promising mass spectrometric methods, along with nuclear magnetic resonance techniques, have emerged.13,30,45,48,63,72,83–85 The growing information on oligosaccharide structures, quantities, and biological functions has been examined in numerous review articles.2,6,7,20,21,23,86–89 In recent years, several reviews have also described the analytical methods applied for quantification and structural analysis of HMOS.1,90–93 Some of the reviews mentioned above, as well as some original studies, summarize data on HMOS concentrations without providing a comprehensive overview.1,47,48,54,89 To the best of knowledge, this is the first systematic review of quantitative data for individual HMOS. Data on concentrations are reported, and the influence of 2 biological parameters—secretor status and lactation period—is examined.
Variations in HMOS data
Mean values and confidence intervals of a variety of neutral and acidic HMOS could be calculated from the studies selected. On the basis of the available literature and because of the strict study and data selection criteria applied in this review, only data from secretor mothers with term infants were available from several different studies. For this review, HMOS for which data were available from 5 or more studies, eg, 2′-FL, 6′-SL, LNDFH II, were considered particularly relevant. The high concentrations of the relatively large structures DF-LNH II and TF-LNH were reported by 2 studies, both performed by the same working group that applied a similar quantification method.32,34 These high concentrations, which strongly influenced the sum values of HMOS, need to be confirmed by other research groups. Data processing revealed wide variations in HMOS concentrations, which is reflected in the relatively wide limits of confidence shown in Tables 3, 4, and 5. In particular, results obtained by different working groups analyzing HMOS varied considerably. For example, the concentration of LNnT exhibiting a type 2 structure reported by Coppa et al.32,34 exceeded that detected by Thurl et al.31 by at least 4-fold. As a consequence, although the results of this review show concentrations of type 1 structures to be approximately twice that of type 2 structures (Tables 3 and 4), the clear dominance of type 1 structures reported1,31 could not be confirmed. It is hypothesized that the predominance of type 1 structures coevolved with the dominance of bifidobacteria in infants’ guts, as exoenzymes produced by bifidobacteria preferentially degrade oligosaccharide type 1 structures.94 In the case of the acidic compound 6′-SL, unusually high concentrations reported in 1 study31 were detected as outliers when compared with concentrations reported in most other studies.34,39,40,47,70 This finding has biological implications, since acidic HMOS, including 6′-SL, for example, are thought to modulate immunologic responses in the infants.22
The variation in HMOS concentrations was also attributed to biological parameters like gestational age, secretor status, lactation period, or general biological variability. The influence of gestational age on the composition of human milk, eg, on lipids and proteins, has been discussed in pediatrics for decades.36 Although the data analyses with term and preterm milks were conducted separately in this review, no clear effects of gestational age on HMOS concentrations were found. The different sums of total neutral HMOS might be attributable to the different compounds quantified and by an unusually high concentration of TF-LNH in term milks.32,34 Concentrations of acidic HMOS may have been higher in preterm milks than in term milks because preterm samples were obtained during the early lactation period of 30 days, while term samples were obtained during the period of 100 days. Some data in this review as well as some from published studies show that concentrations of acidic HMOS, in particular, decrease significantly during the first 3 months postpartum.31,34,39
This overview confirms the general finding that secretor mothers produce high amounts of α1,2 fucosylated HMOS compared with nonsecretor mothers,2,15,16,32,33 whose milk either contain nondetectable or very low concentrations.46,72 The prevalence of α1,2 fucosylated HMOS in secretor milks likely has biological consequences for infants. These compounds significantly reduced the incidence of diarrhea associated with enterotoxigenic Escherichia coli, Campylobacter jejuni, or caliciviruses,60,62 thus possibly conferring an evolutionary advantage for infants of secretor mothers. The indirect effects of the secretor status on HMOS lacking α1,2 fucoses were also examined. An influence of the secretor status on HMOS concentrations was found in 9 of 24 comparative tests (1 test with term, 8 tests with preterm milks). All 6 tests of neutral HMOS that had significant results showed that nonsecretor mothers produced higher concentrations than secretor mothers, which is in accordance with findings reported in the literature.31,33,45 Probably owing to the lack of the α1,2 fucose-transferring secretor enzyme, milks from nonsecretor mothers contain higher amounts of the remaining nonfucosylated as well as the α1,3-, and α1,4-fucosylated HMOS. Since very few studies were available reporting HMOS data from both secretor and nonsecretor mothers, all results—whether significant or not—should be evaluated with care. Different results cannot be ruled out when more studies become available.
Surprisingly, the influence of the time of lactation on HMOS concentrations was significant for only 4 HMOS out of 22 tested in term milks from secretor mothers, although this had already been reported in several studies.31,34,35,39,43 This result can be explained by the wide variations in findings within the studies and the even greater variations between the studies. As reported previously,31,34 LNFP I concentrations decreased significantly during the first months of lactation. However, a decrease in the major HMOS 2′-FL and a general tendency that α1,2 fucosylation and the activity of the secretor enzyme decline during the course of lactation could not be convincingly demonstrated, in contrast to findings in other studies.31,34,43 It is hypothesized that the large interlaboratory differences in findings prevented the detection of this effect. In contrast, the concentration of 3-FL was shown to increase during lactation. This outcome, already reported in some studies,31,35,43 can be explained by differing activities of the Lewis enzyme and other fucosyltransferases during the course of lactation.31 Two of the major HMOS, 3-FL and LNFP II, were shown to specifically block a fucose-binding lectin of the human pathogen Pseudomonas aeruginosa, thus possibly protecting newborns against infection.95
Future HMOS studies
A variety of biological factors influence or could influence HMOS concentrations. Thus, future HMOS studies should precisely define the milk sampling procedures, such as time postpartum, Lewis blood groups of the donors (at least the secretor status), gestational age, ethnicity, and techniques of milk sampling. In addition, there is an initial report that sialylated HMOS might be influenced by the nutritional status of the mother.96 Further studies on the effect of the maternal diet on HMOS are needed.
A variety of chromatographic, electrophoretic, and spectrometric methods of quantification were used in the studies analyzed in this review, which likely influenced the concentrations reported. In recent years, mass spectrometry and nuclear magnetic resonance methods emerged and were judged by several researchers to be superior to the established methods.63,92 However, because of the complexity of overlapping signals of the same monosaccharide residue in very similar chemical environments, quantitative data obtained by nuclear magnetic resonance should be evaluated with care.93,97 Data on absolute HMOS concentrations obtained with mass spectrometry or nuclear magnetic resonance are rare. In most cases, methods were described, but the number of samples analyzed was small and was not well defined biologically.28,30,53,63,65 Currently, there are no studies that compare the performance of the different quantification techniques. In the case of chromatographic methods, the application of internal standards is recommended. With mass spectrometric methods, the use of the corresponding standard substances should be obligatory, and universal calibration should not be applied. Hong et al.72 reported some differences in results when the 2 methods of quantification were compared. Ideally, interlaboratory tests should be conducted with several standardized human milk samples, with homogenized pooled human milk preparations from secretor and Lewis-positive donors representing the most complex matrix. Several laboratories should participate, all applying the major techniques: high-performance liquid chromatography, high-pH anion-exchange chromatography, capillary electrophoresis, capillary gel electrophoresis, mass spectrometry, and nuclear magnetic resonance. Moreover, several laboratories should examine the human milk samples in parallel, using different methods. As was recently shown for a large set of glycoprotein glycans,98 an adapted approach and effort could also be applied to the quantification of HMOS.
This review focused on the concentrations of HMOS. However, the biological effects of HMOS are related to the daily amounts ingested by infants during the entire period of breastfeeding. Therefore, the concentration values would have to be multiplied by the milk volume or milk mass consumed. Average milk intakes reported in the literature could be used for purposes of estimation. It is generally assumed that, after the first month postpartum, infants drink about 700 mL of milk per day.99,100 Data on HMOS concentrations and the daily amounts consumed are a prerequisite for evaluating the possible multiple biological functions of HMOS or oligosaccharide subgroups, eg, antiadhesive or prebiotic effects.
Detailed knowledge about HMOS structures and quantities is of more than just academic interest. For about 15 years, certain oligosaccharides, particularly mixtures of short-chain galactooligosaccharides and long-chain fructooligosaccharides (9:1) that mimic the complexity of size and the biological effects of HMOS, have been added to infant formulas and are currently recommended if exclusive breastfeeding is not possible. More recently, studies in newborn infants showed that the HMOS 2′-FL, whether combined with short-chain galactooligosaccharides or not, is safe and tolerated as a formula supplement.101,102 Moreover, infants fed 2′-FL-fortified formula showed immune biomarkers similar to those detected in breastfed infants.103
Most recent studies
New studies reporting concentrations of HMOS are continuously being published. An update of the literature search from August 27, 2015, corresponding to the deadline of this review, until December 14, 2016, yielded 231 records. After duplicate publications were eliminated, 135 reports were screened. Ten promising studies were examined in detail,104–113 but 9 did not meet the strict inclusion criteria applied in this review (see Table S6 in the Supporting Information online). One article113 reported concentrations of 3 neutral HMOS.
CONCLUSION
Data on the concentrations of oligosaccharides in human milk, as presented in this review, are a prerequisite for understanding the biological functions of HMOS and should help guide further developments in infant and maternal nutrition. Nevertheless, more detailed information on the composition of human milk is needed. Further studies with well-defined human milk samples (ie, documentation of lactation period, Lewis blood group, gestational age of the mothers, etc) using state-of-the art quantification methods will likely reveal valuable information about the composition and quantity of HMOS.
Supplementary Material
Acknowledgments
The authors thank Niclas Jünemann and Jane Schröder, Fulda University of Applied Sciences, Germany, for editing the manuscript; and Paul Rigby, Danone Nutricia Research, Utrecht, the Netherlands, for revising the language.
Author contributions. BS, GB and ST initiated this new perspective in face of the emerging HMOS science. GB and ST designed the structure of the review article. CM performed the literature search. CM and GB screened the search results. GB and ST selected the articles on basis of the full-texts and extracted the data. MM performed the data analyses. ST wrote the paper. BS provided key suggestions with regard to the concept and content of the manuscript. GB, BS, MM, CM, and ST contributed to the discussion of the results and to the revision of the manuscript, respectively.
Funding/support. This review article received no further grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Excluded studies reporting quantitative HMOS data
Table S2-A Concentration of 3-FL during the course of lactation (secretor mothers with term infants)
Table S2-B Concentration of LNFP I during the course of lactation (secretor mothers with term infants)
Table S2-C Concentration of LNDFH I during the course of lactation (secretor mothers with term infants)
Table S2-D Concentration of LST c during the course of lactation (secretor mothers with term infants)
Table S2-E Concentration of 6′-SL during the course of lactation (term infants)
Table S2-F Concentration of LST a during the course of lactation (term infants)
Table S3 Concentrations of neutral HMOS from secretor mothers during the course of lactation
Table S4 Concentrations of acidic HMOS from secretor mothers during the course of lactation
Table S5 Concentrations of acidic HMOS during the course of lactation regardless of secretor status of the mothers
Table S6 Most recent studies reporting quantitative HMOS data
Table S7 PRISMA 2009 checklist
References
- 1. Urashima T, Asakuma S, Leo F. et al. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr. 2012;3:473S–482S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur J Biochem. 1992;209:483–501. [DOI] [PubMed] [Google Scholar]
- 3. Kuhn R, Baer HH. Die Konstitution der Lacto-N-tetraose [in German]. Chem Ber. 1956;89:504–511. [Google Scholar]
- 4. Montreuil J, Mullet S. Étude des variations des constituants glucidiques du lait de femme au cours de la lactation [in French]. Bull Soc Chim Biol (Paris). 1960;42:365–377. [PubMed] [Google Scholar]
- 5. Bruntz R, Dabrowski U, Dabrowski J. et al. Fucose-containing oligosaccharides from human milk from a donor of blood group 0 Lea nonsecretor. Biol Chem Hoppe Seyler. 1988;369:257–274. [PubMed] [Google Scholar]
- 6. Boehm G, Stahl B. Oligosaccharides. In: Mattila-Sandholm T, Saarela M, eds. Functional Dairy Products. Cambridge, UK: Woodhead Publishing; 2003:203–243. [Google Scholar]
- 7. Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Urashima T, Fukuda K, Kitaoka M. et al. Milk oligosaccharides. In: Gordon NS, ed. Oligosaccharides: Sources, Properties, and Applications. New York, NY: Nova Science Publishers; 2011:1–88. [Google Scholar]
- 9. Wu S, Tao N, German JB. et al. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu S, Grimm R, German JB. et al. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Y, Lasanajak Y, Song X. et al. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol Cell Proteomics. 2014;13:2944–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashline DJ, Yu Y, Lasanajak Y. et al. Structural characterization by multistage mass spectrometry (MSn) of human milk glycans recognized by human rotaviruses. Mol Cell Proteomics. 2014;13:2961–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stahl B, Thurl S, Zeng JR. et al. Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem. 1994;223:218–226. [DOI] [PubMed] [Google Scholar]
- 14. Shen L, Grollman EF, Ginsburg V. An enzymatic basis for secretor status and blood group substance specificity in humans. Proc Natl Acad Sci USA. 1968;59:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobata A. Milk glycoproteins and oligosaccharides. In: Horowitz M, Pigman W, eds. The Glycoconjugates. New York, NY: Academic Press; 1977:423–440. [Google Scholar]
- 16. Thurl S, Henker J, Siegel M. et al. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–799. [DOI] [PubMed] [Google Scholar]
- 17. Engfer MB, Stahl B, Finke B. et al. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–1596. [DOI] [PubMed] [Google Scholar]
- 18. Gnoth MJ, Kunz C, Kinne-Saffran E. et al. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–3020. [DOI] [PubMed] [Google Scholar]
- 19. György P, Norris RF, Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48:193–201. [DOI] [PubMed] [Google Scholar]
- 20. Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87(13 suppl):26–34. [DOI] [PubMed] [Google Scholar]
- 21. Chichlowski M, German JB, Lebrilla CB. et al. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol. 2011;2:331–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eiwegger T, Stahl B, Haidl P. et al. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–1188. [DOI] [PubMed] [Google Scholar]
- 23. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaturvedi P, Warren CD, Ruiz-Palacios GM. et al. Milk oligosaccharide profiles by reversed-phase HPLC of their perbenzoylated derivatives. Anal Biochem. 1997;251:89–97. [DOI] [PubMed] [Google Scholar]
- 25. Thurl S, Müller-Werner B, Sawatzki G. Quantification of individual oligosaccharide compounds from human milk using high-pH anion-exchange chromatography. Anal Biochem. 1996;235:202–206. [DOI] [PubMed] [Google Scholar]
- 26. Kunz C, Rudloff S, Hintelmann A. et al. High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides. J Chromatogr B Biomed Sci Appl. 1996;685:211–221. [DOI] [PubMed] [Google Scholar]
- 27. Shen Z, Warren CD, Newburg DS. High-performance capillary electrophoresis of sialylated oligosaccharides of human milk. Anal Biochem. 2000;279:37–45. [DOI] [PubMed] [Google Scholar]
- 28. Albrecht S, Schols HA, van den Heuvel EGHM. et al. CE-LIF-MSn profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis. 2010;31:1264–1273. [DOI] [PubMed] [Google Scholar]
- 29. Kottler R, Mank M, Hennig R. et al. Development of a high-throughput glycoanalysis method for the characterization of oligosaccharides in human milk utilizing multiplexed capillary gel electrophoresis with laser-induced fluorescence detection. Electrophoresis. 2013;34:2323–2336. [DOI] [PubMed] [Google Scholar]
- 30. Ninonuevo MR, Park Y, Yin H. et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–7480. [DOI] [PubMed] [Google Scholar]
- 31. Thurl S, Munzert M, Henker J. et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–1271. [DOI] [PubMed] [Google Scholar]
- 32. Coppa GV, Gabrielli O, Zampini L. et al. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. J Pediatr Gastroenterol Nutr. 2011;53:80–87. [DOI] [PubMed] [Google Scholar]
- 33. Gabrielli O, Zampini L, Galeazzi T. et al. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128:e1520–e1531. [DOI] [PubMed] [Google Scholar]
- 34. Coppa GV, Pierani P, Zampini L. et al. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999;88:89–94. [DOI] [PubMed] [Google Scholar]
- 35. Erney RM, Malone WT, Skelding MB. et al. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr. 2000;30:181–192. [DOI] [PubMed] [Google Scholar]
- 36. Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216 doi:10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Leoz MLA, Gaerlan SC, Strum JS. et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res. 2012;11:4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumiyoshi W, Urashima T, Nakamura T. et al. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br J Nutr. 2003;89:61–69. [DOI] [PubMed] [Google Scholar]
- 39. Sumiyoshi W, Urashima T, Nakamura T. et al. Sialyl oligosaccharides in the milk of Japanese women: changes in concentration during the course of lactation. J Appl Glycosci. 2003;50:461–467. [Google Scholar]
- 40. Kunz C, Rudloff S, Schad W. et al. Lactose-derived oligosaccharides in the milk of elephants: comparison with human milk. Br J Nutr. 1999;82:391–399. [DOI] [PubMed] [Google Scholar]
- 41. Nakhla T, Fu D, Zopf D. et al. Neutral oligosaccharide content of preterm human milk. Br J Nutr. 1999;82:361–367. [DOI] [PubMed] [Google Scholar]
- 42. Asakuma S, Urashima T, Akahori M. et al. Variation of major neutral oligosaccharides levels in human colostrum. Eur J Clin Nutr. 2008;62:488–494. [DOI] [PubMed] [Google Scholar]
- 43. Chaturvedi P, Warren CD, Altaye M. et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–372. [DOI] [PubMed] [Google Scholar]
- 44. Martín-Sosa S, Martín MJ, García-Pardo LA. et al. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J Dairy Sci. 2003;86:52–59. [DOI] [PubMed] [Google Scholar]
- 45. Smilowitz JT, O'Sullivan A, Barile D. et al. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. 2013;143:1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Niekerk E, Autran CA, Nel DG. et al. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr. 2014;144:1227–1233. [DOI] [PubMed] [Google Scholar]
- 47. Bao Y, Zhu L, Newburg DS. Simultaneous quantification of sialyloligosaccharides from human milk by capillary electrophoresis. Anal Biochem. 2007;370:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bao Y, Chen C, Newburg DS. Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Anal Biochem. 2013;433:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Littel RC, Milliken GA, Stroup WW. et al. SAS® System for Mixed Models. 1st ed.Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 50. Albrecht S, Schols HA, van den Heuvel EGHM. et al. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;346:2540–2550. [DOI] [PubMed] [Google Scholar]
- 51. Bao Y, Newburg DS. Capillary electrophoresis of acidic oligosaccharides from human milk. Electrophoresis. 2008;29:2508–2515. [DOI] [PubMed] [Google Scholar]
- 52. Bode L, Kuhn L, Kim HY. et al. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. 2012;96:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galeotti F, Coppa GV, Zampini L. et al. On-line high-performance liquid chromatography-fluorescence detection-electrospray ionization-mass spectrometry profiling of human milk oligosaccharides derivatized with 2-aminoacridone. Anal Biochem. 2012;430:97–104. [DOI] [PubMed] [Google Scholar]
- 54. Galeotti F, Coppa GV, Zampini L. et al. Capillary electrophoresis separation of human milk neutral and acidic oligosaccharides derivatized with 2-aminoacridone. Electrophoresis. 2014;35:811–818. [DOI] [PubMed] [Google Scholar]
- 55. Hunt KM, Preuss J, Nissan C. et al. Human milk oligosaccharides promote the growth of staphylococci. Appl Environ Microbiol. 2012;78:4763–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuhn L, Kim H-Y, Hsiao L. et al. Oligosaccharide composition of breast milk influences survival of uninfected children born to HIV-infected mothers in Lusaka, Zambia. J Nutr. 2015;145:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leo F, Asakuma S, Nakamura T. et al. Improved determination of milk oligosaccharides using a single derivatization with anthranilic acid and separation by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2009;1216:1520–1523. [DOI] [PubMed] [Google Scholar]
- 58. Marx C, Bridge R, Wolf AK. et al. Human milk oligosaccharide composition differs between donor milk and mother's own milk in the NICU. J Hum Lact. 2014;30:54–61. [DOI] [PubMed] [Google Scholar]
- 59. Monti L, Cattaneo TMP, Orlandi M. et al. Capillary electrophoresis of sialylated oligosaccharides in milk from different species. J Chromatogr A. 2015;1409:288–291. [DOI] [PubMed] [Google Scholar]
- 60. Morrow AL, Ruiz-Palacios GM, Altaye M. et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145:297–303. [DOI] [PubMed] [Google Scholar]
- 61. Musumeci M, Simpore J, D'Agata A. et al. Oligosaccharides in colostrum of Italian and Burkinabe women. J Pediatr Gastroenterol Nutr. 2006;43:372–378. [DOI] [PubMed] [Google Scholar]
- 62. Newburg DS, Ruiz-Palacios GM, Altaye M. et al. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. 2004;14:253–263. [DOI] [PubMed] [Google Scholar]
- 63. Ninonuevo MR, Perkins PD, Francis J. et al. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J Agric Food Chem. 2008;56:618–626. [DOI] [PubMed] [Google Scholar]
- 64. Sabharwal H, Sjöblad S, Lundblad A. Sialylated oligosaccharides in human milk and feces of preterm, full-term, and weaning infants. J Pediatr Gastroenterol Nutr. 1991;12:480–484. [DOI] [PubMed] [Google Scholar]
- 65. Sakaguchi Y, Hayama T, Yoshida H. et al. Liquid chromatography/tandem mass spectrometry with fluorous derivatization method for selective analysis of sialyl oligosaccharides. Rapid Commun Mass Spectrom. 2014;28:2481–2489. [DOI] [PubMed] [Google Scholar]
- 66. Sjögren YM, Duchen K, Lindh F. et al. Neutral oligosaccharides in colostrum in relation to maternal allergy and allergy development in children up to 18 months of age. Pediatr Allergy Immunol. 2007;18:20–26. [DOI] [PubMed] [Google Scholar]
- 67. Song JF, Weng MQ, Wu SM. et al. Analysis of neutral saccharides in human milk derivatized with 2-aminoacridone by capillary electrophoresis with laser-induced fluorescence detection. Anal Biochem. 2002;304:126–129. [DOI] [PubMed] [Google Scholar]
- 68. Totten SM, Zivkovic AM, Wu S. et al. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012;11:6124–6133. [DOI] [PubMed] [Google Scholar]
- 69. Wang M, Li M, Wu S. et al. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. 2015;60:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Asakuma S, Akahori M, Kimura K. et al. Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Biosci Biotechnol Biochem. 2007;71:1447–1451. [DOI] [PubMed] [Google Scholar]
- 71. Goehring KC, Kennedy AD, Prieto PA. et al. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One. 2014;9:e101692 doi:10.1371/journal.pone.0101692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hong Q, Ruhaak LR, Totten SM. et al. Label-free absolute quantitation of oligosaccharides using multiple reaction monitoring. Anal Chem. 2014;86:2640–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leo F, Asakuma S, Fukuda K. et al. Determination of sialyl and neutral oligosaccharide levels in transition and mature milks of Samoan women, using anthranilic derivatization followed by reverse phase high performance liquid chromatography. Biosci Biotechnol Biochem. 2010;74:298–303. [DOI] [PubMed] [Google Scholar]
- 74. Olivares M, Albrecht S, Palma G de. et al. Human milk composition differs in healthy mothers and mothers with celiac disease. Eur J Nutr. 2015;54:119–128. [DOI] [PubMed] [Google Scholar]
- 75. Spevacek AR, Smilowitz JT, Chin EL. et al. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J Nutr. 2015;145:1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hammar L, Mansson S, Rohr T. et al. Lewis phenotype of erythrocytes and Leb -active glycolipid in serum of pregnant women. Vox Sang. 1981;40:27–33. [DOI] [PubMed] [Google Scholar]
- 77. Thurl S, Henker J, Siegel M. et al. Lewis blood groups of breastfeeding women tested serologically and by chromatographic analysis of human milk oligosaccharides. Michwiss Milk Sci Int. 1998;53:127–129. [Google Scholar]
- 78. Viverge D, Grimmonprez L, Cassanas G. et al. Variations of lactose and oligosaccharides in milk from women of blood types secretor A or H, secretor Lewis, and secretor H/nonsecretor Lewis during the course of lactation. Ann Nutr Metab. 1985;29:1–11. [DOI] [PubMed] [Google Scholar]
- 79. Viverge D, Grimmonprez L, Cassanas G. et al. Discriminant carbohydrate components of human milk according to donor secretor types. J Pediatr Gastroenterol Nutr. 1990;11:365–370. [DOI] [PubMed] [Google Scholar]
- 80. Coppa GV, Gabrielli O, Pierani P. et al. Qualitative and quantitative studies of carbohydrates of human colostrum and mature milk. Ital J Pediatr. 1991;17:303–307. [Google Scholar]
- 81. Coppa GV, Gabrielli O, Pierani P. et al. L. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91:637–641. [PubMed] [Google Scholar]
- 82. Thurl S, Henker J, Taut H. et al. Variations of neutral oligosaccharides and lactose in human milk during the feeding. Z Ernährungswiss. 1993;32:262–269. [DOI] [PubMed] [Google Scholar]
- 83. Stahl B, Steup M, Karas M. et al. Analysis of neutral oligosaccharides by matrix-assisted laser desorption ionization mass spectrometry. Anal Chem. 1991;63:1463–1466. [Google Scholar]
- 84. Pfenninger A, Karas M, Finke B. et al. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 1: methodology). J Am Soc Mass Spectrom. 2002;13:1331–1340. [DOI] [PubMed] [Google Scholar]
- 85. Pfenninger A, Karas M, Finke B. et al. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 2: application to isomeric mixtures). J Am Soc Mass Spectrom. 2002;13:1341–1348. [DOI] [PubMed] [Google Scholar]
- 86. Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137(3 suppl):847S–849S. [DOI] [PubMed] [Google Scholar]
- 87. Zivkovic AM, German JB, Lebrilla CB. et al. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA. 2011;108(suppl 1):4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 2012;3:383S–391S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Castanys-Munoz E, Martin MJ, Prieto PA. 2'-fucosyllactose: an abundant, genetically determined soluble glycan present in human milk. Nutr Rev. 2013;71:773–789. [DOI] [PubMed] [Google Scholar]
- 90. Ninonuevo MR, Lebrilla CB. Mass spectrometric methods for analysis of oligosaccharides in human milk. Nutr Rev. 2009;67(suppl 2):S216–S226. [DOI] [PubMed] [Google Scholar]
- 91. Ruhaak LR, Lebrilla CB. Advances in analysis of human milk oligosaccharides. Adv Nutr. 2012;3:406S–414S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruhaak LR, Lebrilla CB. Analysis and role of oligosaccharides in milk. BMB Rep. 2012;45:442–451. [DOI] [PubMed] [Google Scholar]
- 93. Mantovani V, Galeotti F, Maccari F. et al. Recent advances on separation and characterization of human milk oligosaccharides. Electrophoresis. 2016;37:1514–1524. [DOI] [PubMed] [Google Scholar]
- 94. Urashima T, Fukuda K, Messer M. Evolution of milk oligosaccharides and lactose: a hypothesis. Animal. 2012;6:369–374. [DOI] [PubMed] [Google Scholar]
- 95. Perret S, Sabin C, Dumon C. et al. Structural basis for the interaction between human milk oligosaccharides and the bacterial lectin PA-IIL of Pseudomonas aeruginosa. Biochem J. 2005;389 (pt 2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Charbonneau MR, O'Donnell D, Blanton LV. et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164:859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van Leeuwen SS, Schoemaker RJW, Gerwig GJ. et al. Rapid milk group classification by 1H NMR analysis of Le and H epitopes in human milk oligosaccharide donor samples. Glycobiology. 2014;24:728–739. [DOI] [PubMed] [Google Scholar]
- 98. Huffman JE, Pucic-Bakovic M, Klaric L. et al. Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol Cell Proteomics. 2014;13:1598–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neville MC, Keller R, Seacat J. et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375–1386. [DOI] [PubMed] [Google Scholar]
- 100. Neville MC, Allen JC, Archer PC. et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54:81–92. [DOI] [PubMed] [Google Scholar]
- 101. Marriage BJ, Buck RH, Goehring KC. et al. Infants fed a lower calorie formula with 2'-FL show growth and 2'-FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr. 2015;61:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Puccio G, Alliet P, Cajozzo C. et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr. 2017;64:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Goehring KC, Marriage BJ, Oliver JS. et al. Similar to those who are breastfed, infants fed a formula containing 2'-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr. 2016;146:2559–2566. [DOI] [PubMed] [Google Scholar]
- 104. Alderete TL, Autran C, Brekke BE. et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102:1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Austin S, De Castro CA, Benet T. et al. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients. 2016;8 doi:10.3390/nu8060346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Austin S, De Castro CA, Benet T. et al. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers [abstract]. J Pediatr Gastroenterol Nutr. 2016;63(suppl 2):S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Eckhardt E, Austin S, Nembrini C. et al. Levels of and interrelations between major human milk oligosaccharides in breast milk from the life cohort [abstract]. J Pediatr Gastroenterol Nutr. 2016;63(suppl 2):S36.27380598 [Google Scholar]
- 108. Kunz C, Meyer C, Collado MC. et al. Influence of gestational age, secretor and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr. 2017;64:789–798. [DOI] [PubMed] [Google Scholar]
- 109. Newburg DS, Chen C, Cline A. et al. Acidic human milk oligosaccharides vary across Chinese, Mexican, and American populations and over lactation [abstract]. J Pediatr Gastroenterol Nutr. 2016;63(suppl 2):S35. [Google Scholar]
- 110. Perrin MT, Fogleman AD, Newburg DS. et al. A longitudinal study of human milk composition in the second year postpartum: implications for human milk banking. Matern Child Nutr. 2017;13:e12239 doi:10.1111/mcn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sakwinska O, Austin S, Moine D. et al. Microbiota and correlations with HMO content in breast milk from Chinese lactating mothers [abstract]. J Pediatr Gastroenterol Nutr. 2016;63(suppl 2):S108 doi:10.1371/journal.pone.0160856 [Google Scholar]
- 112. Underwood MA, Gaerlan S, de Leoz MLA. et al. Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr Res. 2015;78:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xu G, Davis JC, Goonatilleke E. et al. Absolute quantitation of human milk oligosaccharides reveals phenotypic variations during lactation. J Nutr. 2017;147:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.