Abstract
Context
Although a recent meta-analysis of randomized controlled trials showed that adoption of a vegetarian diet reduces plasma lipids, the association between vegetarian diets and long-term effects on plasma lipids has not been subjected to meta-analysis.
Objective
The aim was to conduct a systematic review and meta-analysis of observational studies and clinical trials that have examined associations between plant-based diets and plasma lipids.
Data Sources
MEDLINE, Web of Science, and the Cochrane Central Register of Controlled Trials were searched for articles published in English until June 2015.
Study Selection
The literature was searched for controlled trials and observational studies that investigated the effects of at least 4 weeks of a vegetarian diet on plasma lipids.
Data Extraction
Two reviewers independently extracted the study methodology and sample size, the baseline characteristics of the study population, and the concentrations and variance measures of plasma lipids. Mean differences in concentrations of plasma lipids between vegetarian and comparison diet groups were calculated. Data were pooled using a random-effects model.
Results
Of the 8385 studies identified, 30 observational studies and 19 clinical trials met the inclusion criteria (N = 1484; mean age, 48.6 years). Consumption of vegetarian diets was associated with lower mean concentrations of total cholesterol (−29.2 and −12.5 mg/dL, P < 0.001), low-density lipoprotein cholesterol (−22.9 and −12.2 mg/dL, P < 0.001), and high-density lipoprotein cholesterol (−3.6 and −3.4 mg/dL, P < 0.001), compared with consumption of omnivorous diets in observational studies and clinical trials, respectively. Triglyceride differences were −6.5 (P = 0.092) in observational studies and 5.8 mg/dL (P = 0.090) in intervention trials.
Conclusions
Plant-based diets are associated with decreased total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol, but not with decreased triglycerides.
Systematic Review Registration
PROSPERO number CRD42015023783. Available at: https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015023783.
Keywords: plant-based diets, plasma lipids, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, meta-analysis, systematic review
INTRODUCTION
Elevated blood concentrations of low-density lipoprotein cholesterol (LDL-C) are associated with increased risk of coronary heart disease. Although lowering LDL-C concentrations can reduce cardiovascular morbidity and mortality, hyperlipidemia is underdiagnosed and undertreated.1 A 10% increase in the prevalence of treatment for hyperlipidemia could prevent an estimated 8000 deaths per year.2 It has been further estimated that even modest steps, such as those proposed by the National Cholesterol Education Program Adult Treatment Panel 3 primary prevention guidelines, could prevent approximately 20 000 heart attacks and 10 000 deaths due to coronary heart disease and save almost $3 billion in heart disease-related medical costs per year.3 Although LDL-C has been the primary lipoprotein of concern, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides also play roles in heart disease risk, with TC, LDL-C, and triglycerides positively associated with risk and HDL-C possibly playing a protective role.4 Here, “plasma lipids” refers to the group of lipids including TC, LDL-C, HDL-C, and triglycerides.
Modifiable factors, including diet, weight, and exercise, may play significant roles in developing hyperlipidemia.5 Vegetarian diets are defined as diets that exclude meats; some vegetarian diets include dairy products and eggs. Vegetarian diets usually emphasize the consumption of fruits, vegetables, beans, and grains. Previous reviews have suggested that vegetarian diets are associated with lower plasma lipid concentrations.6,7 Although a recent meta-analysis of randomized controlled trials showed that adoption of a vegetarian diet reduces plasma lipids, long-term effects of vegetarian diets were not studied. To the best of knowledge, the association between vegetarian diets and long-term effects on plasma lipids has not been subjected to meta-analysis. Therefore, a meta-analysis of studies that have examined vegetarian diets’ relationship on plasma lipid concentrations was performed.
METHODS
Data sources and search strategy
The search strategy is shown in Table S1 in the Supporting Information online. The electronic databases MEDLINE, Web of Science, and the Cochrane Central Register of Controlled Trials were searched for English-language articles published from 1946 to June 2015, from 1900 to June 2015, and from 1966 to June 2015, respectively, and containing one or more of the keywords for vegetarian diets (“plant-based diet” or “diet, vegetarian” or “vegetarian diets” or “vegetarianism” or “diets vegan” or “vegan diets”) and for plasma lipids (“hyperlipidemia” or “cholesterol” or “low-density lipoprotein” or “high-density lipoprotein” or “triglyceride”). The reference lists of the retrieved articles were then reviewed to identify additional articles. This review was registered with the PROSPERO register of systematic reviews (registration no. CRD42015023783) and was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Study selection
Two reviewers (Y.Y. and S.M.L.) separately searched and retrieved abstracts for articles that met the following inclusion criteria: (1) participants aged over 20 years; (2) an intervention or exposure consisting of a vegetarian diet, defined as a diet that included meat less than once per month; a semivegetarian diet, defined as a diet that included meat more than once per month, but less than once per week; a vegan diet, defined as a diet that excluded all animal products; or a vegetarian diet that included some animal products as defined by the terms “lacto” (dairy products), “ovo” (eggs), or “pesco” (fish); (3) the collection of sufficient data to allow calculation of mean differences in total or LDL-C between participants who consumed a vegetarian diet and those who consumed a control diet; and (4) the use of a controlled trial or observational study design. The following exclusion criteria were applied: (1) article not an original paper; (2) lack of a comparison diet; (3) lack of continuous lipid data; (4) use of a duplicate sample; (5) small sample size (< 10); (6) animal studies; (7) trial duration of < 4 weeks; (8) article not in English; and (9) for observational studies, failure to adjust for sex and age. The PICOS (Participants, Intervention, Comparators, Outcomes, Study Design) criteria are shown in Table 1.10–39
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Criteria |
|---|---|
| Population | Adult humans, without regard to sex, race, or ethnicity |
| Intervention or exposure | Vegetarian or vegan diets |
| Comparator | Basis for comparison was preintervention total cholesterol, LDL-C, HDL-C, and triglyceride concentrations in the intervention group or the corresponding changes in an untreated comparison group, if available |
| Outcome | Primary outcomes: changes in LDL-CSecondary outcomes: changes in HDL-C, total cholesterol, triglycerides |
| Study design | Controlled trial or observational study design |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Data extraction and quality assessment
For each study, the following information was extracted: study methodology and sample size; baseline characteristics of the study population, including mean age, sex (proportion of men), use of antihyperlipidemic drugs, body mass index (BMI); diets examined and duration of their consumption; concentrations and variance measures of plasma lipids, including those measured in response to dietary interventions in clinical trials; adjustment factors for observational studies, and Jadad score for clinical trials.
Data synthesis and analysis
Mean differences in concentrations of plasma lipids (TC, LDL-C, HDL-C, triglycerides) between vegetarian and comparison diet groups were calculated. For intervention trials, the pooled standard error for the net difference in lipid concentrations was used or, when it was not given, estimated using the method of Follmann et al,8 assuming a correlation of 0.50 between the baseline and final plasma lipids values (parallel design) or between the intervention and the control period (crossover design) plasma lipid values. For studies comparing more than one exposure group or treatment arm, data were extracted from groups eating the fewest animal products, as this was deemed the best means of assessing the effects of vegetarian diets.
Using a random-effects model, which assigns a weight to each study on the basis of the study’s inverse variance, estimates of differences in plasma lipids associated with consumption of vegetarian diets were combined. Using the study as the unit of analysis, estimates were obtained for observational studies and controlled trials separately. Estimates of plasma lipid differences were presented as means and 95%CIs. Statistical significance was set to 2-sided P values < 0.05. Although triglyceride concentrations typically do not follow a normal distribution, inverse variances were calculated from original data because a previous simulation study showed that results were consistent across a range of underlying effect size distributions.9
Analyses stratified by type of vegetarian diet, country, sample size, age, sex, BMI, duration of diet, antihyperlipidemic medication use, and baseline lipid status were conducted separately for controlled trials and observational studies. A sensitivity analysis to assess the impact of each study on the combined effect was conducted by performing a 1-study removed analysis. To assess heterogeneity, calculations of I2 and meta-regression were done with subgroups, using the study as the unit of analysis.
To identify publication bias, funnel plots were created and examined, and to assess the relationship between sample size and effect size, Egger’s test was performed. The “trim and fill” method, which determines where missing studies are likely to appear, was used to adjust for publication bias. These analyses were done separately for controlled trials and observational studies and were conducted for the main outcomes of TC and LDL-C. All analyses were performed using Comprehensive Meta-Analysis, version 2 software (BioStat, Englewood, NJ, USA).
RESULTS
Search results
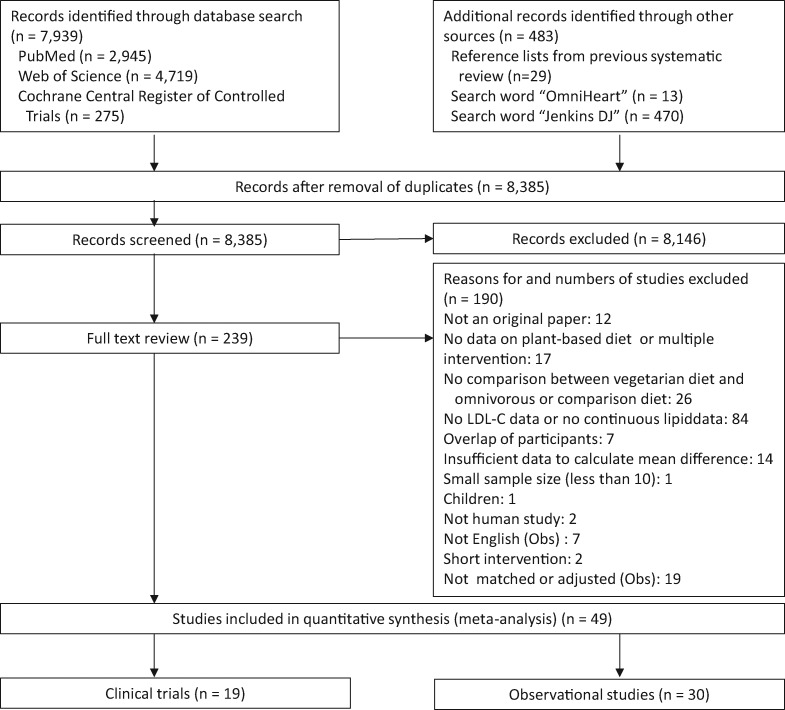
The search strategy led to the retrieval of 8385 studies, of which 30 observational studies10–39 and 19 clinical trials40–58 met the inclusion criteria (Figure 1).
Figure 1.
Flow diagram of the literature search process. Abbreviations: LDL-C, low-density lipoprotein cholesterol; Obs, observational study.
Study characteristics and quality
Observational studies.
The 30 observational studies (Table 240–58) included 10 143 participants (median sample size, 74.5; range, 13–3424) with a mean age of 40.6 years (range, 23.8–71.8 years). Each of the 30 observational studies used a cross-sectional design. In 23 of these studies, participants had been following vegetarian diets for more than 1 year.10–12,14–19,22–24,26–36,38 Eight studies focused on vegan diets,11,23,24,29,32,33,35,38 12 on lacto-ovo-vegetarian diets,15,17,19,21,25–28,30,31,37,39 and 10 on mixed diet types (vegan, lacto, lacto-ovo, pesco, and/or semivegetarian).10,12–14,16,18,20,22,34,36 The matched or adjusted factors in each study are shown in Table 2.
Table 2.
Study design and population characteristics of observational studies of plant-based diets and plasma lipids
| Reference, country | Study design | Matched factors | N | Mean age (y) | Percent male | Mean BMI (kg/m2) or mean weight (kg) | Mean baseline plasma lipids (mg/dL) |
Percent using medication | Duration of vegetarian diets | Exposure | Control | Comorbidities | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | LDL-C | HDL-C | TG | ||||||||||||
| Sacks et al (1975),10 USA | CS | Age, sex | 230 | 44.0 | 62.9 | 65.5 kg | 155.0 | 95.5 | 46.0 | 72.5 | Strongly discouraged using medication | 38 mo | Pesco | Omnivorous | |
| Burslem et al (1978),11 USA | CS | Age, sex | 134 | 27.3 | 37.0 | NR | 161.6 | 103.3 | 45.9 | 85.4 | 0 | 5.2 y | Vegan | Omnivorous | No metabolic diseases |
| Male, 20–30 y | CS | Age, sex | 45 | 20–30 | 100 | NR | 161.7 | 103.3 | 43.8 | 90.1 | 0 | 5.2 y | Vegan | Omnivorous | No metabolic diseases |
| Female, 20–30 y | CS | Age, sex | 56 | 20–30 | 0.0 | NR | 156.4 | 99.9 | 47.0 | 81.4 | 0 | 5.2 y | Vegan | Omnivorous | No metabolic diseases |
| Male, 30–40 y | CS | Age, sex | 15 | 30–40 | 100 | NR | 164.5 | 102.3 | 43.3 | 94.3 | 0 | 5.2 y | Vegan | Omnivorous | No metabolic diseases |
| Female, 30–40 y | CS | Age, sex | 18 | 30–40 | 0.0 | NR | 175.1 | 114.8 | 50.1 | 78.3 | 0 | 5.2 y | Vegan | Omnivorous | No metabolic diseases |
| Huijbregts et al (1980),39 the Netherlands | CS | Age, sex, weight | 14 | 18–26 | 100 | 69.9 kg | 176.9 | 106.5 | 55.3 | 100.1 | NR | NR | Lacto-ovo | Omnivorous | Healthy |
| Nestel et al (1981),37 Australia | CS | Age, sex, weight | 13 | 28.5 | 100 | 63.9 kg | 163.1 | 103.6 | 41.8 | 100.4 | NR | NR | Lacto-ovo | Omnivorous | NR |
| Knuiman & West (1982),23 the Netherlands | CS | Age, sex | 27 | 33.8 | 100 | 23.0 | 172.3 | 98.1 | 42.9 | NR | NR | 4 y | Vegan | Omnivorous | NR |
| Liebman & Bazzarre (1983),21 USA | CS | Age, sex, height, weight, exercise level, alcohol consumption, smoking | 54 | 30.7 | 100 | 23.3 | 187.0 | 120.0 | 43.7 | 85.3 | 0 | > 6 mo | Lacto-ovo | Omnivorous | No hyperlipidemia, CHD, angina, hypertension, or diabetes |
| Roshanai & Sanders (1984),24 UK | CS | Age, sex | 47 | NR | 48.9 | 22.0 | 151.6 | 87.1 | 52.9 | 58.3 | NR | NR | Vegan | Omnivorous | NR |
| Male | 23 | NR | 100 | 23.0 | 159.2 | 96.1 | 51.2 | 60.1 | |||||||
| Female | 24 | NR | 0.0 | 21.0 | 144.2 | 78.5 | 54.5 | 56.7 | |||||||
| Fisher et al (1986),12 USA | CS | Age, sex | 50 | 20–47 | 44.0 | NR | 156.5 | 105.3 | 45.5 | 96.0 | NR | Vegan 9 y; lacto-ovo 7.7 y | Vegan/lacto-ovo | Omnivorous | NR |
| Nieman et al (1989),30 USA | CS | Age, sex, religion | 37 | 71.8 | 0.0 | 23.3 | 229.1 | 139.5 | 64.6 | 123.5 | 0 | 47 y | Lacto-ovo | Omnivorous (low fat) | No stroke, hypertension, diabetes, cancer, or CHD |
| Sanders & Roshanai (1992),29 UK | CS | Age, sex | 40 | 32.3 | 50.0 | 21.9 | 157.0 | 90.4 | 54.4 | 61.3 | 0 | 12 y | Vegan | Omnivorous | Healthy (not receiving any treatment) |
| Male | 20 | 32.5 | 100 | 22.7 | 160.3 | 96.5 | 51.2 | 62.4 | |||||||
| Female | 20 | 32.0 | 0.0 | 21.2 | 153.7 | 84.3 | 57.6 | 60.2 | |||||||
| Krajcovicova-Kudlackova et al (1994),34 Slovakia | CS | Age, sex, geographical region | 109 | 23.8 | 50.5 | 21.7 | 183.0 | 111.9 | 51.3 | 99.9 | NR | Males 2.4 y; females 2.8 y | Lacto-ovo/lacto | Omnivorous | Healthy |
| Male | 55 | 24.0 | 100 | 22.6 | 185.5 | 114.4 | 50.8 | 102.6 | |||||||
| Female | 54 | 23.6 | 0.0 | 20.7 | 180.3 | 109.4 | 51.8 | 97.2 | |||||||
| Harman & Parnell (1998),13 New Zealand | CS | Age, sex | 47 | 42.8 | 48.9 | 24.9 | 196.1 | 127.4 | 49.4 | 99.5 | NR | NR | Lacto/vegan | Omnivorous | NR |
| Male | 23 | 44.7 | 100 | 25.2 | 197.0 | 129.3 | 46.4 | 106.3 | |||||||
| Female | 24 | 41.0 | 0.0 | 24.7 | 195.3 | 125.7 | 52.2 | 93.0 | |||||||
| Li et al (1999),25 Australia | CS | Sex | 74 | 25.3 | 0.0 | 22.5 | 166.0 | 91.9 | 59.9 | 84.0 | NR | > 6 mo | Lacto-ovo | Omnivorous | Healthy |
| Richter et al (1999),28 Germany | CS | Age, sex | 95 | 36.4 | 37.5 | NR | 200.5 | 124.9 | 51.8 | 109.9 | NR | > 2 y | Lacto-ovo | Omnivorous | No diabetes, gout, hypo- or hyperthyreosis, or disease of liver and kidney |
| Male | 37 | 42.0 | 100 | NR | 205.0 | 129.9 | 46.4 | 130.5 | |||||||
| Female | 58 | 33.0 | 0.0 | NR | 197.7 | 121.7 | 55.2 | 96.8 | |||||||
| Lee et al (2000),15 Hong Kong | CS | Age, sex, BMI | 193 | 40.0 | 36.8 | 23.7 | 183.2 | 113.6 | 49.6 | 95.2 | NR | > 1 y | Lacto-ovo | Omnivorous | Healthy |
| Lu et al (2000),16 Taiwan | CS | Age, sex | 109 | 38.6 | 48.6 | 21.5 | 171.9 | 109.4 | 50.7 | 83.6 | NR | > 2 y | Vegan/lacto | Omnivorous | No liver disease, diabetes, or hypertension |
| Male | 53 | 38.0 | 100 | 21.9 | 169.0 | 113.1 | 43.4 | 88.6 | |||||||
| Female | 56 | 39.2 | 0.0 | 21.2 | 171.5 | 103.9 | 56.7 | 77.3 | |||||||
| Lin et al (2001),27 Taiwan | CS | Age, sex | 40 | 57.5 | 50.0 | 24.0 | 164.0 | 118.0 | 47.0 | 97.0 | 0 | > 1 y | Lacto-ovo | Omnivorous | No hypertension, diabetes, hyperlipoproteinemia, or overt vascular disease |
| Goff et al (2005),35 UK | CS | Age, sex, BMI | 46 | 35.5 | 46.9 | 23.1 | 153.7 | 88.1 | 49.3 | 79.4 | 0 | > 3 y | Vegan | Omnivorous | No diabetes, CHD, or metabolic disorder |
| Fu et al (2008),17 Taiwan | CS | Age, sex | 70 | 55.1 | 0.0 | 23.3 | 188.8 | 123.8 | 49.9 | 78.1 | 0 | > 2 y (mean, 7.9 y) | Lacto-ovo | Omnivorous | Healthy |
| Teixeira et al (2007),14 Brazil | CS | Age, sex, ethnicity, socioeconomic class | 201 | 47.0 | 47.8 | 25.3 | 207.7 | 136.0 | 45.5 | 141.7 | NR | > 5 y (mean, 19 y) | Lacto-ovo/ vegan/pesco/ lacto | Omnivorous | |
| Karabudak et al (2008),36 Turkey | CS | Age, sex, BMI | 52 | 28.2 | 0.0 | 21.7 | 164.3 | 88.9 | 54.1 | 88.6 | 0 | > 2 y | Semi-/lacto-ovo/lacto | Omnivorous | Healthy |
| Chen et al (2011),31 Taiwan | CS | Sex | 363 | 51.9 | 0.0 | 23.1 | 187.0 | 122.5 | 59.0 | 90.5 | 0 | > 1 y | Lacto-ovo | Omnivorous | No diabetes, dyslipidemia, hypertension, cerebrovascular disease, chronic gingivitis, connective tissue disease, coronary artery disease, or fever |
| Fernandes Dourado et al (2011),26 Brazil | CS | Age, sex | 87 | 40.0 | 58.6 | 24.3 | 191.4 | 125.0 | 41.6 | 127.3 | 0 | > 1 y (mean, 16 y) | Lacto-ovo | Omnivorous | No temporary or permanent physical impairments or chronic disease in those who took medications that might influence the lipid profile |
| Yang et al (2011),19 China | CS | Age, sex | 300 | 33.3 | 100 | 23.9 | 177.2 | 108.8 | 45.1 | 109.6 | NR | > 1 y (mean, 10.4 y) | Lacto-ovo | Omnivorous | No renal disease, cancer, diabetes, heart disease, or hypertension |
| Kim et al (2012),18 Korea | CS | Age, sex | 75 | 49.2 | 50.7 | 22.6 | 181.5 | 109.1 | 48.7 | 123.8 | 0 | > 15 y (mean, 24.6 y) | Vegan/lacto-ovo | Omnivorous | Healthy |
| Gojda et al (2013),38 Czech Republic | CS | Age, sex, BMI, ethnicity, physical activity, energy intake | 21 | 28.4 | 57.1 | 22.7 | 147.8 | 77.5 | 58.5 | 60.5 | 0 | > 3 y (mean, 8.05 y) | Vegan | Omnivorous | Healthy |
| Jung et al (2013),20 Korea | CS | Age, sex | 296 | 52.9 | 53.4 | 24.1 | 207.0 | 131.7 | 55.2 | 141.6 | NR | NR | Vegan/lacto/ovo/lacto-ovo | Omnivorous | Metabolic syndrome (vegetarian, 30.4%, control, 17.6%) |
| Chiang et al (2013),22 Taiwan | CS | Age, sex | 706 | 56.4 | 0.0 | 23.3 | 189.9 | 123.7 | 57.2 | 107.2 | 0.4 | >1 y | Lacto-ovo/lacto/ovo/vegan | Omnivorous | No systemic diseases such as cancer, heart failure, uremia, and liver cirrhosis or acute illness such as acute myocardial infarction |
| Huang et al (2014),32 Taiwan | CS | Sex, pre- or postmenopausal | 3424 | 43.2 | 0.0 | NR | 184.5 | 114.6 | 59.2 | 111.9 | 0 | > 1 y | Vegan | Omnivorous | NR |
| Jian et al (2015),33 Taiwan | CS | Sex | 3189 | 43.4 | 100 | NR | 181.5 | 116.2 | 51.5 | 141.8 | 0 | > 1 y | Vegan | Omnivorous | NR |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CS, cross-sectional; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NR, not reported; TC, total cholesterol; TG, triglycerides.
Clinical trials.
Nineteen clinical trials were identified (Table 3). These trials included a total of 1484 participants (median sample size = 58; range, 11–291) with a mean age of 48.6 years (range, 21–65 years). All were open (nonmasked) trials. The mean duration was 25.5 weeks. Eighteen were randomized controlled trials.40–50,52–58 Vegan diets were examined in 9,41,45–47,49,51–54 lacto-vegetarian diets in 2,40,48 and lacto-ovo-vegetarian diets in 8.42–44,50,55–58 Fourteen studies used a parallel design,41–43,46,48–55,57,58 while 5 used a crossover design.40,44,45,47,56 Baseline plasma lipid concentrations for each trial are shown in Table 3.
Table 3.
Study design and population characteristics of clinical trials of plant-based diets and plasma lipids
| Reference, country | Study design and duration | Jadad score | N | Mean age (y) | Percent male | Mean BMI (kg/m2) | Mean baseline plasma lipids (mg/dL) |
Medication use | Intervention diet | Control diet | Comorbidities | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | LDL-C | HDL-C | TG | |||||||||||
| Kestin et al (1989),44 Australia | RCT (CO), 6 wk | 2 | 26 | 44.0 | 100 | 25.5 | 234.7 | 157.8 | 56.5 | 113.4 | None | Lacto-ovo | Omnivorous | Not on hyperlipoproteinemia or hypertension medication |
| Ling et al (1992),54 Finland | RCT (PL), 4 wk | 2 | 18 | 42.8 | 22.2 | 26.6 | 213.3 | 141.5 | 50.1 | 102.3 | NR | Vegan | Omnivorous | 2 coronary heart disease, 1 obesity, 1 hypertension |
| Ornish et al (1998),42 USA | RCT (PL), 48 wk | 3 | 35 | 59.3 | 91.4 | 27.1 | 234.9 | 153.5 | 45.3 | 225.9 | None | Ornish (low-fat lacto-ovo) | Omnivorous | Coronary heart disease |
| Nicholson et al (1999),53 USA | RCT (PL), 12 wk | 2 | 11 | 54.3 | 54.5 | NR | 207.6 | NR | 44.0 | 193.2 | 36.4 % | Low-fat vegan | Omnivorous | Non–insulin-dependent diabetes mellitus |
| Barnard et al (2000),45 USA | RCT (CO), 8 wk | 3 | 35 | 36.1 | 0 | 25.5 | 163.0 | 97.0 | 49.0 | 81.0 | None | Low-fat vegan | Omnivorous | Healthy premenopausal women |
| Agren et al (2001),46 Finland | RCT (PL), 12 wk | 2 | 29 | 50.8 | 3.4 | 24.3 | 190.3 | 126.8 | 45.5 | 89.7 | None | Vegan | Omnivorous | Rheumatoid arthritis |
| Dansinger et al (2005),58 USA | RCT (PL), 48 wk | 3 | 80 | 49.0 | 50.0 | 35.0 | 217.5 | 139.0 | 46.0 | 164.0 | Mean of 2.4 medications per person | Ornish (low-fat lacto-ovo) | Calorie restriction | Presence of at least 1 of the metabolic cardiac risk factors |
| Gardner et al (2005),43 USA | RCT (PL), 4 wk | 3 | 120 | 48.5 | 50.0 | 26.5 | 224.3 | 148.9 | 48.3 | 128.5 | None | Lacto-ovo | Omnivorous (low-fat) | No heart disease or diabetes |
| de Mello et al (2006),40 Brazil | RCT (CO), 4 wk | 2 | 17 | 59.0 | 82.4 | 26.2 | 206.5 | 132.3 | 45.2 | 139.1 | None | Lacto (low-protein) | Omnivorous | T2D |
| Aldana et al (2007),55 USA | RCT (PL), 48 wk | 2 | 93 | 61.6 | 56.3 | 31.0 | 170.2 | 95.4 | 43.4 | 157.1 | Yes, unknown percentage | Ornish (low-fat lacto-ovo) | Omnivorous | Coronary heart disease |
| Burke et al (2007),50 USA | RCT (PL), 72 wk | 2 | 176 | 44.0 | 13.1 | 34.0 | 204.0 | NR | NR | 134.0 | None | Lacto-ovo (calorie- and fat-restricted) | Omnivorous (calorie- and fat-restricted) | Overweight and obese |
| Gardner et al (2007),57 USA | RCT (PL), 48 wk | 2 | 155 | 41.0 | 0.0 | 31.5 | NR | 107.4 | 50.5 | 118.5 | None | Ornish (low-fat lacto-ovo) | Calorie restriction | Overweight in premenopause |
| Elkan et al (2008),41 Sweden | RCT (PL), 12 wk | 2 | 58 | 50.3 | 10.3 | 24.0 | 191.7 | 118.1 | 52.3 | 97.4 | None | Vegan | Omnivorous | Rheumatoid arthritis |
| Barnard et al (2009),49 USA | RCT (PL), 74 wk | 3 | 99 | 55.6 | 39.4 | 34.9 | 193.0 | 111.1 | 51.0 | 153.2 | 54.5 % | Low-fat vegan | ADA diet | T2D |
| Miller et al (2009),56 USA | RCT (CO), 4 wk | 2 | 18 | 30.6 | 50.0 | 22.6 | 184.9 | 107.2 | 62.2 | 78.1 | None | Ornish (low-fat lacto-ovo) | Mediterranean South Beach | Healthy (no history of metabolic, hepatic, renal, or systemic disease) |
| Ferdowsian et al (2010),51 USA | CT (PL), 22 wk | 1 | 107 | 21–65 | 17.7 | NR | 186.5 | 105.4 | 51.8 | 147.2 | Yes, unknown percentage | Low-fat vegan | Omnivorous | BMI ≥ 25 and/or T2D |
| Kahleova et al (2013),48 Czech Republic | RCT (PL), 24 wk | 2 | 74 | 56.2 | 47.3 | 35.1 | 166.3 | 98.8 | 41.8 | 186.0 | 51.4 % | Lacto | EASD diet | T2D |
| Mishra et al (2013),52 USA | RCT (PL), 18 wk | 3 | 291 | 45.2 | 17.2 | 35.0 | 187.6 | 108.2 | 55.2 | 121.4 | NR | Low-fat vegan | Omnivorous | BMI ≥ 25 and/or T2D |
| Bunner et al (2014),47 USA | RCT (CO), 16 wk | 3 | 42 | 45.7 | 7.1 | 27.6 | 187.1 | 106.0 | 61.5 | 96.1 | NR | Low-fat vegan | Omnivorous | Migraine |
Abbreviations: ADA, American Diabetes Association; BMI, body mass index; CT, clinical trial; CO, crossover; EASD, European Association for the Study of Diabetes; NR, not reported; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PL, parallel; RCT, randomized controlled trial; TC, total cholesterol; TG, triglycerides; T2D, type 2 diabetes.
Pooled effects of vegetarian diets on plasma lipids.
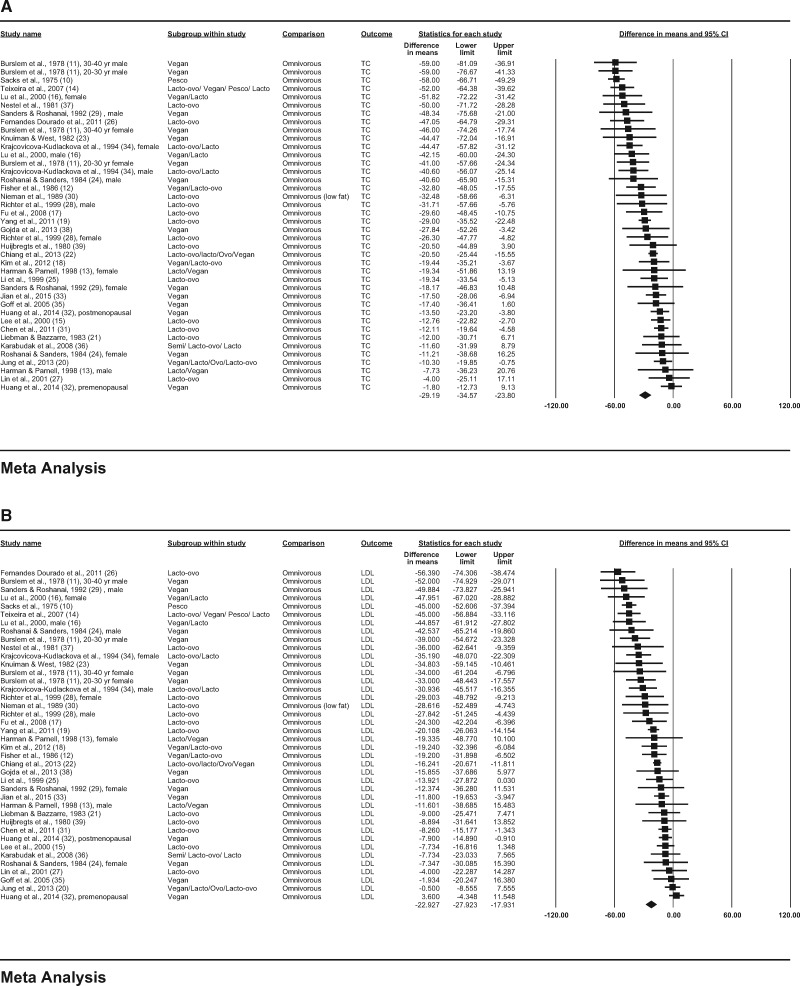
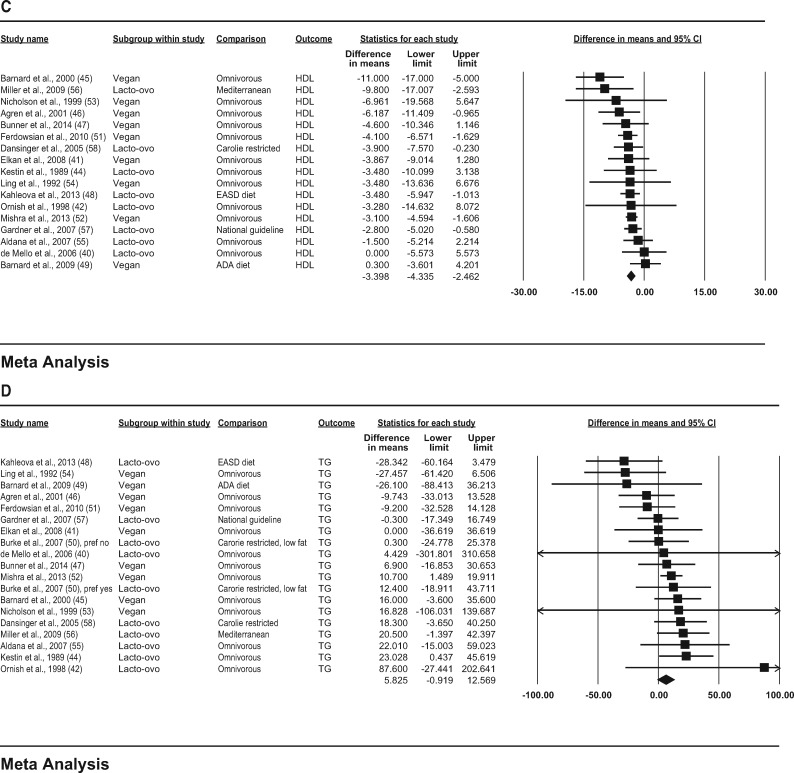
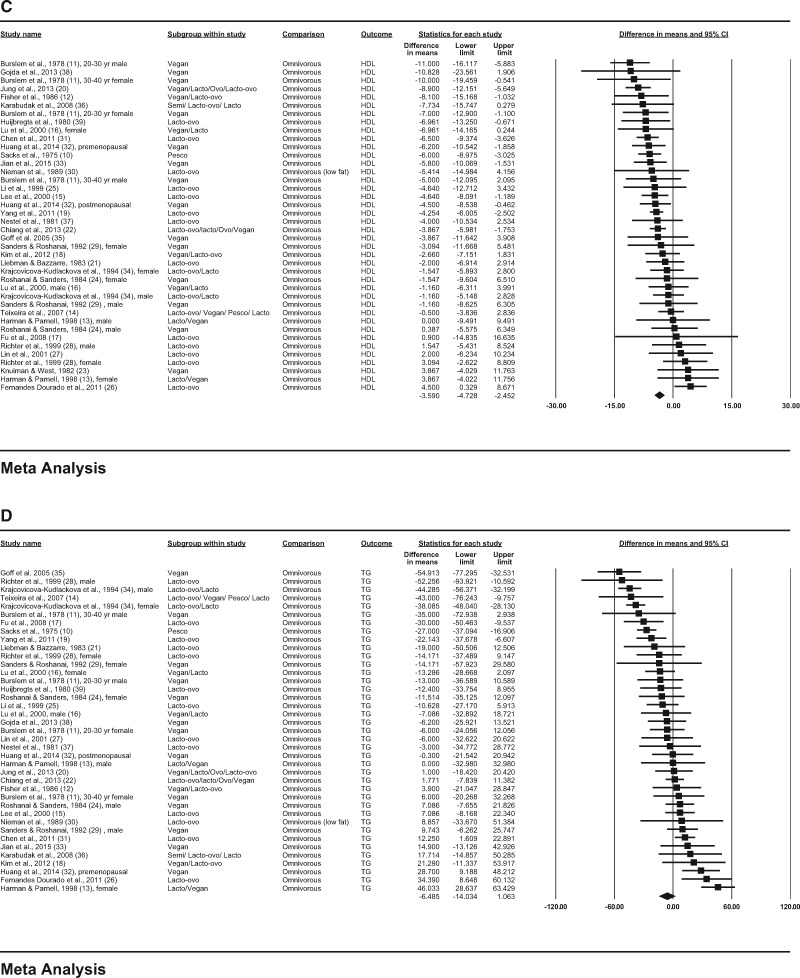
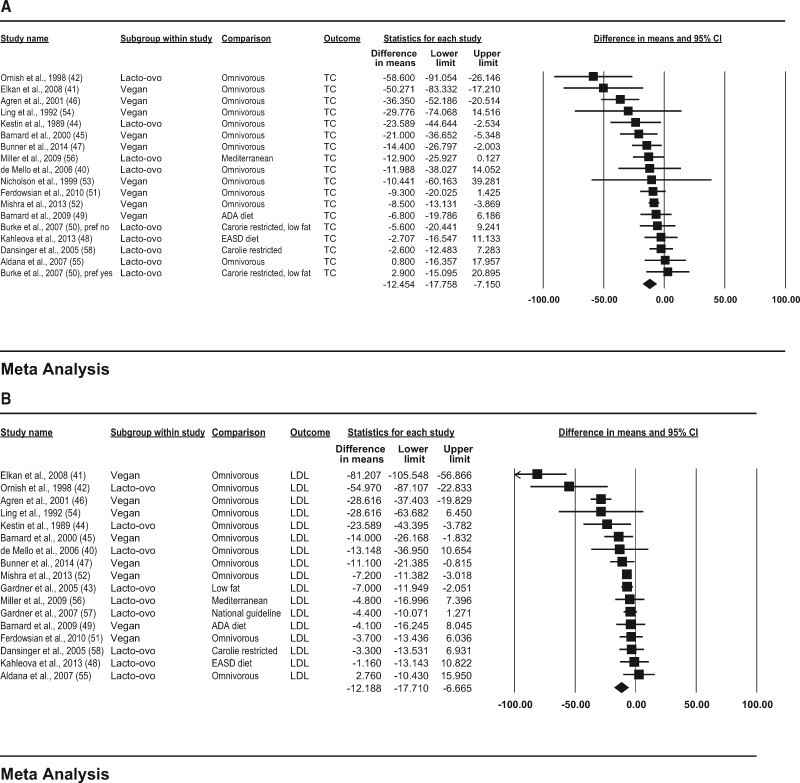
In the observational studies, consumption of vegetarian diets was associated with lower mean concentrations of TC (−29.2 mg/dL; 95%CI, −34.6, −23.8; P < 0.001; I2 = 81.4; P for heterogeneity < 0.001); LDL-C (−22.9 mg/dL; 95%CI, −27.9, −17.9; P < 0.001; I2 = 83.3; P for heterogeneity < 0.001); HDL-C (−3.6 mg/dL; 95%CI, −4.7, −2.5; P < 0.001; I2 = 49.7; P for heterogeneity < 0.001); and triglycerides (−6.5 mg/dL; 95%CI, −14.0, 1.1; P = 0.092; I2 = 83.0; P for heterogeneity < 0.001) compared with consumption of omnivorous diets (Figure 2A–D).
Figure 2.
Pooled plasma lipid responses to vegetarian diets in observational studies. Effects on (A) TC (total cholesterol), (B) LDL-C (low-density lipoprotein cholesterol),
Figure 3.
(C) HDL-C (high-density lipoprotein cholesterol), and (D) triglycerides are depicted as squares; error bars indicate 95%CIs. Meta-analysis yielded pooled estimates of TC (−12.5 mg/dL; 95%CI, −17.8, −7.2; P < 0.001); LDL-C (−12.2 mg/dL; 95%CI, −17.7, −6.7; P < 0.001); HDL-C (−3.4 mg/dL; 95%CI, −4.3, −2.5; P < 0.001); and triglycerides (5.8 mg/dL; 95%CI, −0.9, 12.6; P = 0.090), which are depicted as black diamonds. Vegan diets were defined as those that omitted all animal products; vegetarian diets may include some animal products, as indicated by the terms lacto (dairy products) and ovo (eggs). Reference numbers of studies are shown in parentheses.
In the clinical trials, consumption of vegetarian diets was associated with a mean reduction in TC (−12.5 mg/dL; 95%CI, −17.8, −7.2; P < 0.001; I2 = 54.8; P for heterogeneity = 0.003); LDL-C (−12.2 mg/dL; 95%CI, −17.7, −6.7; P < 0.001; I2 = 79.2; P for heterogeneity < 0.001); and HDL-C (−3.4 mg/dL; 95%CI, −4.3, −2.5; P < 0.001; I2 = 8.5; P for heterogeneity = 0.354) and a nonsignificant increase in triglyceride concentration (5.8 mg/dL; 95%CI, −0.9, 12.6; P = 0.090; I2 = 22.5; P for heterogeneity = 0.182), compared with consumption of omnivorous diets (Figure 3A–D).
Figure 2.
(C) HDL-C (high-density lipoprotein cholesterol), and (D) triglycerides are depicted as squares; error bars indicate 95%CIs. Meta-analysis yielded pooled estimates of TC (−29.2 mg/dL; 95%CI, −34.6, −23.8; P < 0.001); LDL (−22.9 mg/dL; 95%CI, −27.9, −17.9; P < 0.001); HDL-C (−3.6 mg/dL; 95%CI, −4.7, −2.5; P < 0.001); and triglycerides (−6.5 mg/dL; 95%CI, −14.0, 1.1; P = 0.092), which are depicted as black diamonds. Vegan diets were defined as those that omitted all animal products; vegetarian diets may include some animal products, as indicated by the terms lacto (dairy products), ovo (eggs), and pesco (fish). Reference numbers of studies are shown in parentheses.
Figure 3.
Pooled plasma lipid responses to vegetarian diets in clinical trials. Effects on (A) TC (total cholesterol), (B) LDL-C (low-density lipoprotein cholesterol),
Subgroup analysis and meta-regression.
Pooled changes in plasma lipids associated with consumption of vegetarian diets in planned strata for observational studies and clinical trials are summarized in Tables S2 and S3 in the Supporting Information online.
In observational studies, heterogeneity was statistically significant for TC, LDL-C, HDL-C, and triglycerides. Subgroup analysis in observational studies revealed that vegetarian effect size for TC and LDL-C was statistically larger with vegan than with lacto-ovo vegetarian diets; in studies conducted in North or South America; and in younger age groups (< 50 vs > 50 years). Moreover, LDL-C concentrations were lower in studies with smaller sample sizes (< 100). Meta-regression in observational studies also revealed that younger age was associated with lower values for TC (0.44, P < 0.001) and LDL-C (0.31, P = 0.002). In addition, TC and LDL-C in vegetarian groups were lower in studies with smaller sample sizes (slope 0.006, P < 0.001 for TC; slope 0.006, P < 0.001 for LDL-C), larger percentages of male participants (slope −0.14, P < 0.001; slope −0.11, P < 0.001), and lower overall mean plasma lipids for all participants, vegetarian and nonvegetarian (slope 0.41, P < 0.001 for TC; slope 0.30, P < 0.001 for LDL-C).
In clinical trials, the reductions of TC and LDL-C were greater in the BMI subgroup 18.5 to 25 kg/m2 than in other subgroups. Meta-regression also revealed that smaller BMI was associated with larger TC (slope 1.49, P < 0.001) or LDL-C (slope 1.02, P < 0.001) reductions with vegetarian diets. Participants who did not use lipid-lowering medication showed larger reductions in TC and LDL-C than participants who used them. Vegan diets were associated with larger LDL-C reductions than lacto-ovo vegetarian diets. Smaller sample size was associated with greater LDL-C reductions in the subgroup analysis and greater reductions of both TC and LDL-C in meta-regression analysis (slope 0.03, P = 0.050; and slope 0.03, P = 0.015, respectively).
Sensitivity analysis.
In the 1-study removed analysis, results were largely unchanged, with plasma lipid differences between vegetarian and comparison groups ranging from −30.0 to −28.0 mg/dL for TC and from −23.74 to −21.96 mg/dL for LDL-C in observational studies (P < 0.001 in all cases) and from −13.5 to −10.4 mg/dL for TC and from −13.2 to −9.2 mg/dL for LDL-C in clinical trials (all results were P < 0.001).
Publication bias.
Funnel plot outcomes revealed that larger trials reporting large reductions in TC were possibly overrepresented in observational studies. A few studies showing a smaller effect size were absent in the middle right side (see Figure S1A in the Supporting Information online). Egger’s test could not confirm this impression (P = 0.133). Trim-and-fill method outcomes suggested that 7 studies were missing, and their addition would have changed the overall effect on TC to −23.8 mg/dL (95%CI, −29.6, −18.0).
Funnel plot outcomes for the clinical trials suggested that smaller trials that reported large reductions in TC were overrepresented (see Figure S1B in the Supporting Information online). If publication bias did not exist, study results would be symmetrically displayed about the mean effect size; studies showing smaller lipid reductions were missing in the bottom right side. Egger’s test could not confirm this impression (P = 0.069). Trim-and-fill method outcomes suggested that 4 trials might have been missing, and their addition would have changed the overall effect on TC from −12.5 mg/dL to −8.57 mg/dL (95%CI, −14.79, −2.35).
DISCUSSION
This meta-analysis of 30 observational studies and 19 controlled trials shows that, compared with consumption of omnivorous diets, consumption of vegetarian diets is associated with lower TC, LDL-C, and HDL-C concentrations but not with differences in triglyceride concentrations. The meta-analysis shows overall differences in TC of −29.2 mg/dL in observational studies and −12.5 mg/dL in clinical trials and differences in LDL-C of −22.9 mg/dL in observational studies and −12.2 mg/dL in clinical trials. High-density lipoprotein cholesterol was also lower in vegetarian groups than in omnivorous groups, although the degree of difference was relatively modest (−3.6 mg/dL in observational studies and −3.4 mg/dL in clinical trials). Subgroup analysis indicated that younger age (< 50 years), male sex, lower baseline plasma lipids, and lower BMI were associated with greater reductions in TC and LDL-C.
The findings of the current study are consistent with those of previous reviews,6,7 and the present analysis extends these findings to include a meta-analysis of observational study data. While observational studies present a higher risk of bias compared with clinical trials, they also reflect long-term effects of vegetarian diets on plasma lipids that are not apparent in most clinical trials. Those who have followed vegetarian dietary patterns for longer periods may have healthier body compositions as well as better adherence to a vegetarian diet, both of which may have an effect on blood lipids. In addition, this study presents the raw mean difference for each endpoint, which is useful when the measure is meaningful either inherently or because of widespread use.59
For context, a previous meta-analysis showed that, on average, statin use reduced LDL-C concentrations by 70 mg/dL (1.8 mmol), with considerable variation depending on statin type.60 The results of the present analysis showed that diet alone reduced LDL-C by 22.9 mg/dL in observational studies and by 12.2 mg/dL in clinical trials. While dietary changes may not be as powerful as statins in reducing plasma lipids, dietary and pharmacologic interventions are not mutually exclusive. They can work together, and, in some cases, dietary practices can obviate the need for medications. Because side effects may interfere with medication compliance and may preclude statin use for certain patients, dietary options have some intrinsic advantages.
Vegetarian diets are typically lower in saturated fatty acids and cholesterol, compared with omnivorous diets. In 3 large cohort studies that included large numbers of vegetarian participants (Adventist Health Study 2 cohort, European Prospective Investigation into Cancer and Nutrition (EPIC)-Oxford study, and UK Women’s Study), intakes of saturated fatty acid and cholesterol were lower in vegetarians than in omnivorous participants, with strict vegetarians having the lowest intakes of both.61 The subgroup analysis in the present study showed that a vegan diet had larger effects on plasma lipids than a lacto-ovo vegetarian diet. The observed effects of plant-based diets on plasma lipids are likely to be, in large part, the result of differences in saturated fatty acid intake and, to a lesser extent, cholesterol intake.62,63 The role of saturated fat intake in cardiovascular outcomes has been questioned recently, in part due to heterogeneity in meta-analyses.64 This issue is beyond the scope of the present article, which is limited to the effect of diet on blood lipid concentrations.
The effects of changes in dietary cholesterol on serum cholesterol decline as baseline dietary cholesterol increases.65 Hopkins’s analysis indicated that hepatic cholesterol overload may be the primary basis for the observed weak response to increasing dietary cholesterol in the context of a high baseline concentration.65 However, according to the subgroup analysis in the present study, a lower baseline plasma lipid concentration was related to a greater reduction of TC and LDL-C in plasma by vegetarian diets in clinical trials.
This meta-regression and subgroup analysis showed that the duration of adherence to a vegetarian diet did not modulate the observed effects of the diet. However, younger age was associated with lower TC and LDL-C, suggesting that an effect of diet duration may play a role. Additionally, the present analysis could not adjust for dietary compliance. Further studies are needed to clarify the relation between the duration of vegetarian diets and its effect on plasma lipids.
In this study, HDL-C concentrations were also significantly lower in the context of vegetarian diets than in omnivorous diets. Although some studies have suggested that HDL-C concentrations are inversely associated with coronary heart disease,66 recent studies have shown that interventions that increase HDL-C do not reduce the risk of coronary heart disease67 and that genetic variants that raise HDL-C do not necessarily reduce the risk of coronary heart disease.68
Due to their range of health benefits, vegetarian diets are specifically mentioned in the 2015–2020 Dietary Guidelines for Americans69 as 1 of 3 noteworthy healthful diet patterns. As demonstrated in this study, improved lipid profiles are among these benefits. Moreover, the range of plant-derived foods is enormous, including simple fruits, vegetables, beans, and whole grains as well as products that are processed and prepared with a variety of additional ingredients. The lipid-lowering effect of a plant-based diet can be maximized by selection of specific foods. In a randomized trial of a so-called portfolio diet that included foods rich in soluble fiber, soy protein, plant sterols, and almonds, an LDL-C reduction of 28.6% was observed in 4 weeks.70 The strengths of the present meta-analysis include a substantial sample size that lends confidence to these findings and allowed subgroup analyses in specific population groups. In addition, the focus of the meta-analysis on food consumption as opposed to supplements or other artificial interventions makes the findings applicable to the public.
An important limitation is heterogeneity. Meta-regression and subgroup analyses showed that sex, age, baseline plasma lipids, type of vegetarian diets, sample size, and BMI may be key reasons for this heterogeneity. Still, lower TC and LDL-C concentrations were seen in all subgroups. In addition, all observational studies used cross-sectional rather than prospective designs, a limitation that is somewhat alleviated by the inclusion of randomized clinical trials. Lastly, although all observational studies included in this study adjusted for age and sex, some did not adjust for other possible confounders such as BMI or physical activity level. Further studies are needed to explore the possible mechanisms by which vegetarian diets influence plasma lipids. The results of this meta-analysis suggest a strong association between consumption of vegetarian diets and lower plasma lipid concentrations.
CONCLUSION
Consumption of vegetarian diets, particularly vegan diets, is associated with lower levels of plasma lipids, which could offer individuals and healthcare professionals an effective option for reducing the risk of heart disease or other chronic conditions. Although not all clinicians have the training or time to confidently guide patients toward healthful vegetarian diets, registered dietitians can provide the services necessary to assist patients in making this transition.
Supplementary Material
Acknowledgments
Funding/support. No external funding supported this work.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article available at the publisher’s website.
Table S1 Search strategy
Table S2 Subgroup analysis on plasma total cholesterol and low-density lipoprotein cholesterol for clinical trials
Table S3 Subgroup analysis on plasma high-density lipoprotein cholesterol and triglyceride for clinical trials
Figure S1 Funnel plot of comparison of weight and differences in mean total cholesterol associated with consumption of vegetarian diets. Funnel plot of study weights against change in TC in (A) observational studies and (B) clinical trials. TC results in individual studies are depicted as circles scattered around the pooled TC estimate. The trim-and-fill method indicates that 7 observational studies and 4 trials might have been missing owing to publication bias. After adjustment for putative missing data, the overall differences for TC changed to −23.8 mg/dL (95%CI, −29.6 to −18.0) in observational studies and −8.57 mg/dL (95%CI, −14.79 to −2.35) in clinical trials.
References
- 1. Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302:2104–2110. [DOI] [PubMed] [Google Scholar]
- 2. Farley TA, Dalal MA, Mostashari F et al. . Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–609. [DOI] [PubMed] [Google Scholar]
- 3. Pletcher MJ, Lazar L, Bibbins-Domingo K et al. . Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–254. [DOI] [PubMed] [Google Scholar]
- 4. Lemieux I, Lamarche B, Couillard C et al. . Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–2692. [DOI] [PubMed] [Google Scholar]
- 5. Stone NJ, Robinson JG, Lichtenstein AH et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 6. Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104:947–956. [DOI] [PubMed] [Google Scholar]
- 7. Wang F, Zheng J, Yang B et al. . Effects of vegetarian diets on blood lipids: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002408 doi:10.1161/JAHA.115.002408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Follmann D, Elliott P, Suh I et al. . Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. [DOI] [PubMed] [Google Scholar]
- 9. Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a comparison between DerSimonian-Laird and restricted maximum likelihood. Stat Methods Med Res. 2012;21:657–659. [DOI] [PubMed] [Google Scholar]
- 10. Sacks FM, Castelli WP, Donner A et al. . Plasma lipids and lipoproteins in vegetarians and controls. N Engl J Med. 1975;292:1148–1151. [DOI] [PubMed] [Google Scholar]
- 11. Burslem J, Schonfeld G, Howald MA et al. . Plasma apoprotein and lipoprotein lipid levels in vegetarians. Metabolism. 1978;27:711–719. [DOI] [PubMed] [Google Scholar]
- 12. Fisher M, Levine PH, Weiner B et al. . The effect of vegetarian diets on plasma-lipid and platelet levels. Arch Intern Med. 1986;146:1193–1197. [PubMed] [Google Scholar]
- 13. Harman SK, Parnell WR. The nutritional health of New Zealand vegetarian and non-vegetarian Seventh-day Adventists: selected vitamin, mineral and lipid levels. N Z Med J. 1998;111:91–94. [PubMed] [Google Scholar]
- 14. Moreira de Almeida Teixeira RdC, Bisi Molina MdC, Zandonade E, et al. Cardiovascular risk in vegetarians and omnivores: a comparative study. Arq Bras Cardiol. 2007;89:237–244. [DOI] [PubMed] [Google Scholar]
- 15. Lee HY, Woo J, Chen ZY et al. . Serum fatty acid, lipid profile and dietary intake of Hong Kong Chinese omnivores and vegetarians. Eur J Clin Nutr. 2000;54:768–773. [DOI] [PubMed] [Google Scholar]
- 16. Lu SC, Wu WH, Lee CA et al. . LDL of Taiwanese vegetarians are less oxidizable than those of omnivores. J Nutr. 2000;130:1591–1596. [DOI] [PubMed] [Google Scholar]
- 17. Fu C-H, Yang CCH, Lin C-L et al. . Alteration of cardiovascular autonomic functions by vegetarian diets in postmenopausal women is related to LDL cholesterol levels. Chin J Physiol. 2008;51:100–105. [PubMed] [Google Scholar]
- 18. Kim MK, Cho SW, Park YK. Long-term vegetarians have low oxidative stress, body fat, and cholesterol levels. Nutr Res Pract. 2012;6:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang S-Y, Zhang H-J, Sun S-Y et al. . Relationship of carotid intima-media thickness and duration of vegetarian diet in Chinese male vegetarians. Nutr Metabol (London). 2011;8:63 doi: 10.1186/1743-7075-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung JG, Kang HW, Hahn SJ et al. . Vegetarianism as a protective factor for reflux esophagitis: a retrospective, cross-sectional study between Buddhist priests and general population. Dig Dis Sci. 2013;58:2244–2252. [DOI] [PubMed] [Google Scholar]
- 21. Liebman M, Bazzarre TL. Plasma lipids of vegetarian and nonvegetarian males: effects of egg consumption. Am J Clin Nutr. 1983;38:612–619. [DOI] [PubMed] [Google Scholar]
- 22. Chiang J-K, Lin Y-L, Chen C-L et al. . Reduced risk for metabolic syndrome and insulin resistance associated with ovo-lacto-vegetarian behavior in female Buddhists: a case-control study. PLoS One. 2013;8 doi:10.1371/journal.pone.007179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knuiman JT, West CE. W. The concentration of cholesterol in serum and in various serum lipoproteins in macrobiotic, vegetarian and non-vegetarian men and boys. Atherosclerosis. 1982;43:71–82. [DOI] [PubMed] [Google Scholar]
- 24. Roshanai F, Sanders TA. Assessment of fatty acid intakes in vegans and omnivores. Hum Nutr Appl Nutr. 1984;38:345–354. [PubMed] [Google Scholar]
- 25. Li D, Ball M, Bartlett M. Lipoprotein(a), essential fatty acid status and lipoprotein lipids in female Australian vegetarians. Clin Sci (London). 1999;97:175–181. [PubMed] [Google Scholar]
- 26. Fernandes Dourado K, de Arruda Camara e Siqueira Campos F, Sakugava Shinohara NK. Relation between dietary and circulating lipids in lacto-ovo vegetarians. Nutr Hosp. 2011;26:959–964. [DOI] [PubMed] [Google Scholar]
- 27. Lin CL, Fang TC, Gueng MK. Vascular dilatory functions of ovo-lactovegetarians compared with omnivores. Atherosclerosis. 2001;158:247–251. [DOI] [PubMed] [Google Scholar]
- 28. Richter V, Purschwitz K, Bohusch A et al. . Lipoproteins and other clinical-chemistry parameters under the conditions of lacto-ovo-vegetarian nutrition. Nutr Res. 1999;19:545–554. [Google Scholar]
- 29. Sanders TA, Roshanai F. Platelet phospholipid fatty-acid composition and function in vegans compared with age-matched and sex-matched omnivore controls. Eur J Clin Nutr. 1992;46:823–831. [PubMed] [Google Scholar]
- 30. Nieman DC, Underwood BC, Sherman KM et al. . Dietary status of Seventh-Day Adventist vegetarian and non-vegetarian elderly women. J Am Diet Assoc. 1989;89:1763–1769. [PubMed] [Google Scholar]
- 31. Chen C-W, Lin C-T, Lin Y-L et al. . Taiwanese female vegetarians have lower lipoprotein-associated phospholipase A2 compared with omnivores. Yonsei Med J. 2011;52:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y-W, Jian Z-H, Chang H-C et al. . Vegan diet and blood lipid profiles: a cross-sectional study of pre and postmenopausal women. BMC Womens Health. 2014;14 doi:10.1186/1472-6874-14-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jian Z-H, Chiang Y-C, Lung C-C et al. . Vegetarian diet and cholesterol and TAG levels by gender. Public Health Nutr. 2015;18:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krajcovicova-Kudlackova M, Simoncic R, Bederova A et al. . Selected parameters of lipid-metabolism in young vegetarians. Ann Nutr Metabol. 1994;38:331–335. [DOI] [PubMed] [Google Scholar]
- 35. Goff LM, Bell JD, So PW et al. . Veganism and its relationship with insulin resistance and intramyocellular lipid. Eur J Clin Nutr. 2005;59:291–298. [DOI] [PubMed] [Google Scholar]
- 36. Karabudak E, Kiziltan G, Cigerim N. A comparison of some of the cardiovascular risk factors in vegetarian and omnivorous Turkish females. J Human Nutr Diet. 2008;21:13–22. [DOI] [PubMed] [Google Scholar]
- 37. Nestel PJ, Billington T, Smith B. Low density and high density lipoprotein kinetics and sterol balance in vegetarians. Metabolism. 1981;30:941–945. [DOI] [PubMed] [Google Scholar]
- 38. Gojda J, Patkova J, Jacek M et al. . Higher insulin sensitivity in vegans is not associated with higher mitochondrial density. Eur J Clin Nutr. 2013;67:1310–1315. [DOI] [PubMed] [Google Scholar]
- 39. Huijbregts AW, Van Schaik A, Van Berge-Henegouwen GP et al. . Serum lipids, biliary lipid composition, and bile acid metabolism in vegetarians as compared to normal controls. Eur J Clin Invest. 1980;10:443–449. [DOI] [PubMed] [Google Scholar]
- 40. de Mello VDF, Zelmanovitz T, Perassolo MS et al. . Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr. 2006;83:1032–1038. [DOI] [PubMed] [Google Scholar]
- 41. Elkan AC, Sjöberg B, Kolsrud B et al. . Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Therapy. 2008;10:R34. doi:10.1186/ar2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ornish D, Scherwitz LW, Billings JH et al. . Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. [DOI] [PubMed] [Google Scholar]
- 43. Gardner CD, Coulston A, Chatterjee L et al. . The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults—a randomized trial. Ann Intern Med. 2005;142:725–733. [DOI] [PubMed] [Google Scholar]
- 44. Kestin M, Rouse IL, Correll RA et al. . Cardiovascular disease risk factors in free-living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr. 1989;50:280–287. [DOI] [PubMed] [Google Scholar]
- 45. Barnard ND, Scialli AR, Bertron P et al. . Effectiveness of a low-fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am J Cardiol. 2000;85:969–972. [DOI] [PubMed] [Google Scholar]
- 46. Agren JJ, Tvrzicka E, Nenonen MT et al. . Divergent changes in serum sterols during a strict uncooked vegan diet in patients with rheumatoid arthritis. Br J Nutr. 2001;85:137–139. [DOI] [PubMed] [Google Scholar]
- 47. Bunner AE, Agarwal U, Gonzales JF et al. . Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain. 2014;15:69 doi:10.1186/1129-2377-15-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kahleova H, Matoulek M, Bratova M et al. . Vegetarian diet-induced increase in linoleic acid in serum phospholipids is associated with improved insulin sensitivity in subjects with type 2 diabetes. Nutr Diabetes. 2013;3:e75 doi:10.1038/nutd.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barnard ND, Cohen J, Jenkins DJ et al. . A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89:1588s–1596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burke LE, Hudson AG, Warziski MT et al. . Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: a randomized clinical trial. Am J Clin Nutr. 2007;86:588–596. [DOI] [PubMed] [Google Scholar]
- 51. Ferdowsian HR, Barnard ND, Hoover VJ et al. . A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot. 2010;24:384–387. [DOI] [PubMed] [Google Scholar]
- 52. Mishra S, Xu J, Agarwal U et al. . A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr. 2013;67:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nicholson AS, Sklar M, Barnard ND et al. . Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med. 1999;29:87–91. [DOI] [PubMed] [Google Scholar]
- 54. Ling WH, Laitinen M, Hanninen O. Shifting from conventional diet to an uncooked vegan diet reversibly alters serum lipid and apolipoprotein levels. Nutr Res. 1992;122:1431–1440. [DOI] [PubMed] [Google Scholar]
- 55. Aldana SG, Greenlaw R, Salberg A et al. . The effects of an intensive lifestyle modification program on carotid artery intima-media thickness: a randomized trial. Am J Health Promot. 2007;21:510–516. [DOI] [PubMed] [Google Scholar]
- 56. Miller M, Beach V, Sorkin JD et al. . Comparative effects of three popular diets on lipids, endothelial function, and C-reactive protein during weight maintenance. J Am Diet Assoc. 2009;109:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gardner CD, Kiazand A, Alhassan S et al. . Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women. JAMA. 2007;297:969–977. [DOI] [PubMed] [Google Scholar]
- 58. Dansinger ML, Gleason JA, Griffith JL et al. . Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction. JAMA. 2005;293:43–53. [DOI] [PubMed] [Google Scholar]
- 59. Michael B, Larry VH, Julian PT. Introduction to Meta-analysis. Chichester, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 60. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423 doi:https://doi.org/10.1136/bmj.326.7404.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Appleby PN, Key TJ. The long-term health of vegetarians and vegans. Proc Nutr Soc. 2016;75:287–293. [DOI] [PubMed] [Google Scholar]
- 62. Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism. 1965;14:776–787. [DOI] [PubMed] [Google Scholar]
- 63. Hegsted DM. Serum-cholesterol response to dietary cholesterol: a re-evaluation. Am J Clin Nutr. 1986;44:299–305. [DOI] [PubMed] [Google Scholar]
- 64. de Souza RJ, Mente A, Maroleanu A et al. . Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978 doi: https://doi.org/10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hopkins PN. Effects of dietary cholesterol on serum cholesterol: a meta-analysis and review. Am J Clin Nutr. 1992;55:1060–1070. [DOI] [PubMed] [Google Scholar]
- 66. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N et al. . Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Keene D, Price C, Shun-Shin MJ et al. . Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379 doi:10.1136/bmj.g4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Frikke-Schmidt R, Nordestgaard BG, Stene MC et al. . Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. [DOI] [PubMed] [Google Scholar]
- 69. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed.http://health.gov/dietaryguidelines/2015/guidelines/. Published 2015. Accessed February 16, 2017. [Google Scholar]
- 70. Jenkins DJ, Kendall CW, Marchie A et al. . Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.