Abstract
Objective
To assess the relationship between the analgesic efficacy of extended-release paracetamol (ER-APAP) and brain blood oxygen level–dependent (BOLD) signal activation in response to painful stimulation measured by functional magnetic resonance imaging (fMRI) in patients with osteoarthritis of the knee.
Methods
This placebo-controlled, double-blind, crossover, randomized trial (N = 25) comprised three treatment periods in which patients received four doses of an eight-hour ER-APAP caplet (2 x 665 mg), four doses of matched placebo, and no treatment. Pain intensity of the knee was measured before and after painful stimulation at the knee with osteoarthritis and before and after fMRI.
Results
ER-APAP significantly reduced prestimulation osteoarthritis knee joint pain compared with baseline (P < 0.003) and placebo (P < 0.004). ER-APAP and placebo significantly reduced knee joint pain after stimulation (P = 0.014 and P = 0.032, respectively); however, pain reduction with ER-APAP was 35% greater than placebo. ER-APAP was associated with significant reductions in BOLD signal activation after stimulation compared with control in the sensory cortex (P = 0.002) and supramarginal gyrus (P = 0.003). Reduction in BOLD signal activation after stimulation for placebo was significantly greater than control in the subgenual prefrontal cortex (P < 0.001), frontal cortex (P < 0.001), insula (P < 0.003), and sensory cortex (P < 0.001).
Conclusions
ER-APAP had a significantly greater effect than placebo and no treatment in reducing knee pain, which was associated with reduced BOLD signal activations in pain pathways, including the sensory cortex and supramarginal gyrus. BOLD observations after placebo treatment may shed light on the role of the brain regions potentially involved in placebo response in clinical trials investigating pain therapies.
Keywords: Functional Magnetic Resonance Imaging (fMRI), Blood Oxygen Level–Dependent (BOLD), Brain activity, Paracetamol, Pain, Osteoarthritis
Introduction
Paracetamol (APAP) is an over-the-counter analgesic medicine used to manage mild to moderate pain associated with multiple acute and chronic pain disorders, including osteoarthritis (OA). Despite evidence that suggests that APAP works through multiple mechanisms in the central nervous system [1], the exact effects of this medication on neurologic activity in patients with OA are not yet characterized. Neuro-imaging measures showing the relationship between pain reduction in OA and changes in functional activities in the human brain may help elucidate the mechanism of action of APAP. Currently used self-reported pain measurements—such as a visual analog scale, numerical rating scale (NRS), and pain relief score—are subjective measurements that provide little understanding of the neurophysiologic processes underlying the pain associated with OA and therefore offer limited options for evaluating therapeutic efficacy [2,3].
Functional magnetic resonance imaging (fMRI) maps functional activity in the brain by indirect evaluation of changes in blood flow and oxygen levels in the capillary beds. Several fMRI neuroimaging studies have demonstrated that different patterns of brain activity occur in acute and chronic pain [4,5]. Acute pain has been shown to activate brain regions associated with nociceptive pain pathways, including the thalamus, primary and secondary somatosensory cortices, insula, and anterior cingulate cortex [4,6–9]. In clinical studies in patients with OA, painful mechanical stimulation of the affected knee joint has been associated with blood oxygen level–dependent (BOLD) responses measured by fMRI in the brain regions commonly involved in acute pain [4,6,10]. In pharmacologic investigations in healthy volunteers, analgesics such as ibuprofen-arginine, remifentanil, nalbuphine, naloxone, and naproxen have shown greater fMRI BOLD responses in pain-related brain regions compared with placebo [11–15]. One fMRI study in healthy volunteers receiving painful stimulation showed that APAP treatment was associated with reduced activation in the insula, anterior cingulate cortex, thalamus, and prefrontal cortices compared with placebo [16]. fMRI studies have also evaluated the placebo effect, showing that placebo can change brain activity in the thalamus, insula, anterior cingulate cortex, and prefrontal cortex in healthy volunteers [7]. These observations provide evidence that placebo alters the experience of pain through a different mechanism of action compared with active analgesics.
The present study aimed to correlate the efficacy of pain reduction with the intensity, location, and pattern of changes in BOLD signal in different regions of the brain using fMRI in response to noxious stimulation of the painful knee joint in subjects with OA after treatment with eight-hour extended-release (ER) APAP compared with placebo and no treatment.
Materials and Methods
Study Population
Eligible subjects were men and women age 45 years and older with a body mass index (BMI) of 17 to 35 kg/m2 and a diagnosis of OA based on radiologic and clinical findings as defined by American College of Rheumatology criteria affecting one or more knees for three or more months; if both knees were affected, the symptoms were required to be significantly worse in one knee. Exclusion criteria included women of childbearing potential or who were breastfeeding or any patient with a secondary cause of arthritis of the knee, lower extremity surgery (including arthroscopy) within six months before screening, prior injury to the index knee within 12 months before screening, disease of the spine, contraindication to MRI, history of asthma, and smoking more than 20 cigarettes per day.
The safety population included all subjects who received at least one dose of a treatment. The intent-to-treat population included all subjects who received at least one dose of a treatment and had at least one postbaseline efficacy assessment, and the per-protocol population included all subjects who fully complied with all study procedures.
The study protocol was approved by the institutional review board at the Hospital del Mar (Barcelona, Spain). It was conducted in accordance with requirements of the Declaration of Helsinki and is registered at www.clinicaltrials.gov (identifier: NCT01105936). All participants provided written informed consent before enrollment.
Study Design
This was a single-center, randomized, placebo-controlled, double-blind (for study drug and placebo), three-way crossover, phase IV study. After a screening visit, eligible subjects entered a washout period for OA pain medications for seven or more days (or five times the half-life, whichever was longer) before the first assessment and for the duration of the study. Subjects were randomly assigned to each of three study periods according to a randomization schedule generated by GlaxoSmithKline Consumer Healthcare (Parsippany, NJ, USA) using SAS (v. 9.2; SAS Institute, Cary, NC, USA): a period of treatment with four consecutive doses of 1,330 mg (2 × 665-mg caplets) ER-APAP (Panadol Extend; GlaxoSmithKline Consumer Healthcare, Warren, NJ, USA), another period of treatment with four consecutive doses of two caplets each of matched placebo, and another period during which no treatment was administered; the no-treatment period was given as the first or second study period only. Each treatment period was separated by a five- to 14-day washout period.
Subjects took the first of four doses of study medication under supervision at the clinic between 13:00 and 16:00 hours at the beginning of each period. They self-administered the second and third doses of study medication away from the clinic eight and 16 hours after the first dose and were given the fourth dose of study medication under supervision at the clinic approximately 24 hours after the first dose. Subjects underwent fMRI no earlier than two hours and no later than five hours after taking the fourth dose of ER-APAP or placebo and at the end of the no-treatment period. While lying down within the MRI scanner (Achieva 3.0T; Philips Healthcare Imaging Systems, Andover, MA, USA), subjects underwent pressure stimulation tests manipulating the patellofemoral joint of the index knee. Pressure was exerted using a pneumatic hose on the kneecap with the knee in an extended position. The exerted pressure was increased until the subject rated the pain in the range of 5 to 8 on an 11-point verbal rating scale (0 = no pain; 10 = extreme pain). The stimulus intensity used during the fMRI was established as the pressure that produced pain in the range of 5 to 8 when continuously applied over a period of 10 seconds. The stimulus intensity was determined at the beginning of the session and was applied while the subject was in the scanner. A new stimulus intensity measurement was determined for each study session. Therefore, during fMRI assessment, the stimulus intensity applied reflected each subject’s sensitivity to painful pressure in a basal situation before dosing.
Subjects rated their pain three times: before entering the scanner (prescan), after stimulation but before undergoing fMRI, and after fMRI (postscan). Functional MRI sequences consisted of gradient recalled acquisition in the steady state (time of repetition [TR] 2,000 ms; time of echo [TE] 35 ms; pulse angle 90°) within a field of view of 24 cm, with a 64 × 64-pixel matrix and a slice thickness of 4 mm (interslice gap = 1–1.5 mm). Twenty-two to 24 interleaved slices, parallel to the anterior–posterior commissure line, were acquired to cover the whole brain. The pain functional time series consisted of 180 consecutive image sets obtained over six minutes and 30 seconds. Acquisitions were preceded by four dummy images, allowing the pain MRI signal to reach equilibrium. The block paradigm was characterized by alternating 11 baseline periods of 20 seconds (plus a final baseline period of 30 seconds) and 11 painful stimulation periods of 10 seconds. Anatomical MRI examinations were also acquired for each individual, including a 22-slice two-dimensional inversion–recovery sequence (TR 2,614 ms; TE 22.8 ms; inversion time 750 ms) matching the functional acquisition and a three-dimensional fast spoiled gradient inversion–recuperation prepared sequence with 130 contiguous slices (TR 11.8 ms; TE 4.2 ms; flip angle 15°; field of view 30 cm; acquisition matrix 256 × 256 pixels; slice thickness 1.2 mm). Subjects continued in the study until they completed the randomized treatment sequence, with a minimum of five days and a maximum of 14 days between periods. Within seven to 14 days of a subject completing the third study treatment, the investigator or a designee followed up with the subject to perform safety assessments.
Efficacy Assessments and Measures
Subjects were evaluated for 1) treatment effect on pain intensity before pressure stimulation of the knee, 2) treatment effect on pain intensity after stimulation of the knee prescan and postscan, and 3) BOLD signal activation assessed by fMRI after treatment and stimulation of the knee. Pain intensity was measured using the 11-point NRS (ranging from 0 = no pain to 10 = extreme pain or pain as bad as you can imagine).
Treatment effect before stimulation was measured as the difference in pain intensity before and after treatment within each period. Treatment comparisons for this effect were based on the differences of changes in pain intensity before and after treatment between the three treatment arms. Treatment effect after stimulation was measured as the difference between pain intensity before treatment, after stimulation, and pain intensity after treatment, after stimulation. Assessment of pain intensity after treatment, after stimulation, was performed before and after MRI scan.
Safety
Safety was assessed based on adverse events (AEs) reported by all subjects after treatment with study medication.
Statistical Analysis
No formal sample size calculations were performed for this study as there were no existing data on BOLD response to stimuli in OA patients treated with ER-APAP. A sample size of 25 to 30 subjects was chosen based on a similar GlaxoSmithKline pain study using fMRI and was considered large enough to capture the effect of ER-APAP compared with placebo and no treatment.
Imaging data for the BOLD responses were processed and analyzed using Statistical Parametric Mapping software (SPM5; The Wellcome Department of Imaging Neuroscience, London, UK) run in MATLAB v. 7.0 (The MathWorks Inc, Natick, MA, USA) on a Microsoft Windows platform. Image preprocessing involved motion correction, spatial normalization, and smoothing using a Gaussian filter (full width, half-maximum, 8 mm). Data were normalized to the standard SPM5–echo planar imaging template and resliced to 2 mm isotropic resolution in Montreal Neurological Institute (MNI) space. A repeated-measures paired one-tailed Student’s t test (SPM model) was used for each cluster comparing any two treatments across the cohort of 25 subjects. A voxel threshold of a P value of less than 0.01 and a cluster-extent-based threshold of 50 or more contiguous voxels were used as criteria to consider a cluster significant. Although a P value of 0.01 represents a liberal cluster-defining primary threshold, this threshold was chosen in order to increase the sensitivity to weak and diffuse signals. This was particularly important for this proof-of-principle study with a relatively small sample size and an expected relatively low power. On the other hand, cluster-extent-based thresholding of 50 or more contiguous voxels contributes to multiple comparisons correction [17]. Only treatment comparisons with a display threshold at a voxel P value of less than 0.01 are presented.
Treatment effect on pain (change in pain intensity: pretreatment – post-treatment), treatment effect on pressure stimulation before fMRI scan (change in pain intensity: pretreatment after stimulation – post-treatment after stimulation before MRI scan), and treatment effect on pressure stimulation after MRI scan (change in pain intensity: pretreatment after stimulation – post-treatment after stimulation after fMRI scan) were analyzed using analysis of covariance (ANCOVA) in a mixed effects model, with treatment as a fixed effect and subjects as a random effect. Period and baseline pain intensity (NRS score at prescreening) were used as covariates. A t test (H0: least squares [LS] mean change = 0) was performed for the mean change from baseline of pain intensity calculated across subjects within each treatment to determine if the effect of treatment was significant compared with baseline (pretreatment). Differences between treatment effects (changes in pain intensity before and after treatment or changes in pain intensity pretreatment after stimulation with post-treatment after stimulation) were compared by multiple comparisons in Proc Mixed (SAS v. 9.2) at a P value of 0.05. All subjects who received treatment were included in the analysis and reporting of safety data.
Results
Study Population
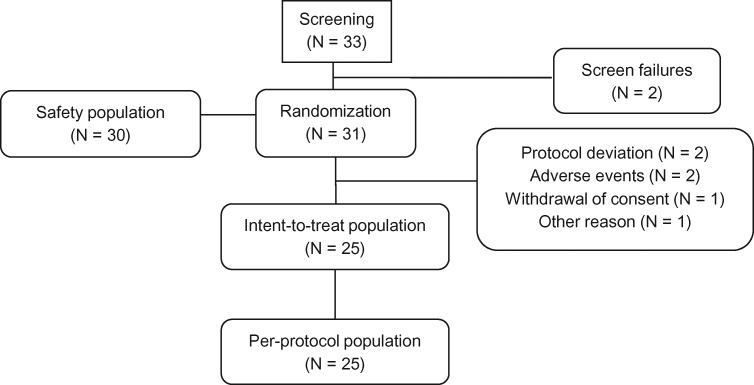
A total of 33 subjects were screened and 31 were randomized, of which 30 were included in the safety population and 25 were evaluable and complied with all study procedures. Of the six subjects excluded from the analyses, two discontinued because of AEs (not treatment related), two were excluded for protocol deviations (use of prohibited medications), one withdrew consent, and one withdrew for other reasons (Figure 1). The pain intensity analysis included data from 20 subjects in the ER-APAP period, 23 subjects in the placebo period, and 23 subjects in the no-treatment period. The fMRI analyses included data from 18 subjects. The study was conducted between September 2010 and August 2011.
Figure 1.
Subject disposition.
Demographic and baseline characteristics of the safety population are summarized in Table 1. The mean age of subjects in the safety population was 68.5 years, and the majority of subjects were female (86.7%). The mean baseline knee pain intensity was 6.6 (SD = 0.95).
Table 1.
Baseline and demographic characteristics
| Characteristic | Safety Population (N = 30) |
|---|---|
| Baseline pain intensity score (NRS), mean (SD) | 6.6 (0.95) |
| Gender, No. (%) | |
| Male | 4 (13.3) |
| Female | 26 (86.7) |
| Race, No. (%) | |
| White | 30 (100) |
| Age, mean (SD), y | 68.5 (7.97) |
| BMI, mean (SD), kg/m2 | 28.7 (4.2) |
BMI = body mass index; NRS = numerical rating scale; SD = standard deviation.
Pain Intensity Assessments
Prestimulation
ER-APAP significantly reduced pain intensity (P = 0.003) (Table 2), as measured by NRS scores compared with baseline (pretreatment), while placebo and no treatment failed to produce any significant effect in reducing pain intensity compared with pretreatment. The effect of ER-APAP in reducing pain intensity (measured as change from baseline) was significantly greater than that of placebo (P = 0.004) and borderline significant compared with no treatment (P = 0.051). No significant differences were reported in change in pain intensity between placebo and no treatment (Table 2).
Table 2.
Effect of treatment on knee joint pain prior to painful stimulation
| Statistics | ER-APAP | Placebo | No Treatment | ER-APAP vs Placebo | ER-APAP vs No Treatment | Placebo vs No Treatment |
|---|---|---|---|---|---|---|
| LS mean | 1.24* | −0.29* | 0.10* | 1.53† | 1.14† | −0.39† |
| 95% CI | 0.45 to 2.02‡ | −1.02 to 0.44‡ | −0.71 to 0.90‡ | 0.53 to 2.53§ | −0.01 to 2.29§ | −1.49 to 0.71§ |
| P | 0.003¶ | NS¶ | NS¶ | 0.004‖ | 0.051‖ | NS‖ |
ANCOVA = analysis of covariance; CI = confidence interval; ER-APAP = extended-release paracetamol; LS = least squares; NS = not significant.
LS mean change from baseline in pain intensity. Change from baseline was calculated for each subject as difference of pretreatment pain intensity before stimulation with post-treatment pain intensity before stimulation.
Difference between treatment LS mean change from baseline in pain intensity derived from ANCOVA, with period and baseline pain intensity as covariates and treatment as a factor.
95% CI for LS mean change from baseline in pain intensity within each treatment group.
95% CI for between-treatment difference of LS mean change from baseline in pain intensity.
P value associated with t test (H0: LS mean of change from baseline of pain intensity within each treatment group = 0).
P value from multiple comparisons of LS mean change from baseline of pain intensity between treatments.
After Stimulation, Prescan
ER-APAP had a significant effect in reducing pain from mechanical stimulation in the knee joint. Pain intensity following stimulation after four doses of ER-APAP treatment was significantly lower than pain intensity after stimulation but before ER-APAP treatment (P = 0.014) (Table 3). Placebo also had a significant effect in reducing pain after knee joint stimulation prescan (P = 0.032) (Table 3). The effect of ER-APAP was 35% greater than that of placebo; however, this effect was not statistically significant.
Table 3.
Effect of treatment on knee pain induced by painful stimulation before and after fMRI scan
| Statistics | ER-APAP | Placebo | No Treatment | ER-APAP vs Placebo | ER-APAP vs No Treatment | Placebo vs No Treatment |
|---|---|---|---|---|---|---|
| Prescan | ||||||
| LS mean | 0.6* | 0.43* | 0* | 0.12† | 0.59† | 0.48† |
| 95% CI | 0.13 to 1.06‡ | 0.04 to 0.91‡ | −0.5 to 0.5‡ | −0.47 to 0.70§ | −0.08 to 1.26§ | −0.17 to 1.12§ |
| P | 0.014¶ | 0.032¶ | NS¶ | NS‖ | NS‖ | NS‖ |
| Postscan | ||||||
| LS mean | 0.55^ | −0.17^ | −0.34^ | 0.72** | 0.88** | 0.16** |
| 95% CI | −0.3 to 1.39†† | −1.0 to 0.62†† | −1.2 to 0.5†† | −0.16 to 1.6‡‡ | −0.13 to 1.9‡‡ | −0.8 to 1.14‡‡ |
| P | NS§§ | NS§§ | NS§§ | NS¶¶ | NS¶¶ | NS¶¶ |
ANCOVA = analysis of covariance; CI = confidence interval; ER-APAP = extended-release paracetamol; fMRI = functional magnetic resonance imaging; LS = least squares; NRS = numerical rating scale; NS = not significant.
LS mean change in pain intensity after stimulation/before fMRI scan. Change in pain intensity after stimulation/before fMRI scan was calculated for each subject as difference between pain intensity before treatment/after stimulation with pain intensity after treatment, after stimulation, and before fMRI scan.
Between-treatment differences of LS mean change in pain intensity after stimulation but before fMRI scan derived from ANCOVA, with treatment as a fixed effect and subjects as a random effect and period and baseline pain intensity (NRS score at prescreening) as covariates.
95% CI for LS mean change in pain intensity after stimulation/before fMRI scan within each treatment group.
95% CI for between-treatment differences in LS mean change in pain intensity after stimulation/before fMRI scan.
P value associated with t test (H0: LS mean change in pain intensity after stimulation/before fMRI scan = 0) within each treatment group.
P value from multiple treatment comparisons of LS mean change in pain intensity after stimulation/before fMRI scan.
^LS mean change in pain intensity after stimulation/after fMRI scan. Change in pain intensity after stimulation/after fMRI scan was calculated for each subject as difference between pain intensity before treatment/after stimulation with pain intensity after treatment, after stimulation, and after fMRI scan.
Between-treatment difference in LS mean change in pain intensity after stimulation/after fMRI scan derived from ANCOVA, with treatment as a fixed effect and subjects as a random effect. Period and baseline pain intensity (NRS score at prescreening) were used as covariates.
95% CI for LS mean change in pain intensity after stimulation/after fMRI scan within each treatment group.
95% CI for between-treatment differences in LS mean change in pain intensity after stimulation/after fMRI scan.
P value associated with t test (H0: LS mean change in pain intensity after stimulation/after fMRI scan = 0) within each treatment group.
P value from multiple treatment comparisons of LS mean change in pain intensity after stimulation/after fMRI scan.
After Stimulation, Postscan
ER-APAP had a greater effect than placebo and no treatment in reducing pain from mechanical stimulation measured after MRI scan. The mean reduction of pain intensity (pain intensity pretreatment after stimulation – pain intensity post-treatment after stimulation) was 0.55 when subjects were treated with ER-APAP, −0.17 when they were treated with placebo, and −0.34 when they did not have any treatment. However, differences between the effect of ER-APAP and the effects of placebo and no treatment were not significant.
BOLD Response Assessment
BOLD Signal Patterns: Overviews in Different Levels of the Whole Brain
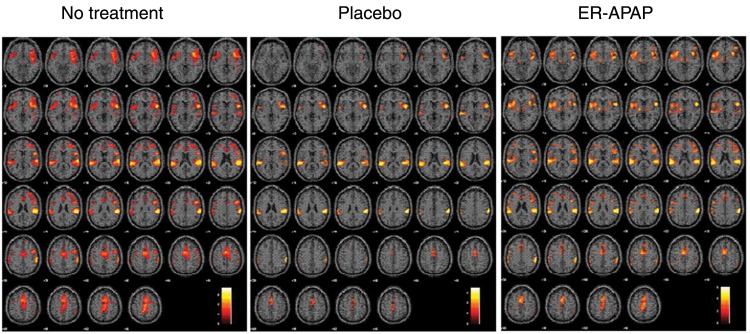
BOLD signal activation patterns were observed in multiple brain regions after painful stimulation following no treatment, placebo, and ER-APAP treatment in the whole brain. Visual inspection shows that similar, but less extensive, BOLD activation patterns were observed following the placebo and ER-APAP treatment sessions as compared with the no-treatment session (Figure 2). The detailed comparison of the BOLD signal activation between the different treatment sessions and the possible clinical meanings will be discussed in the Results and Discussion sections.
Figure 2.
The blood oxygen level–dependent signal activation patterns following no treatment, placebo treatment, and extended-release paracetamol (ER-APAP) treatment, respectively, in the different levels of the whole brain after painful stimulation at the subject’s knee joint.
ER-APAP vs No Treatment
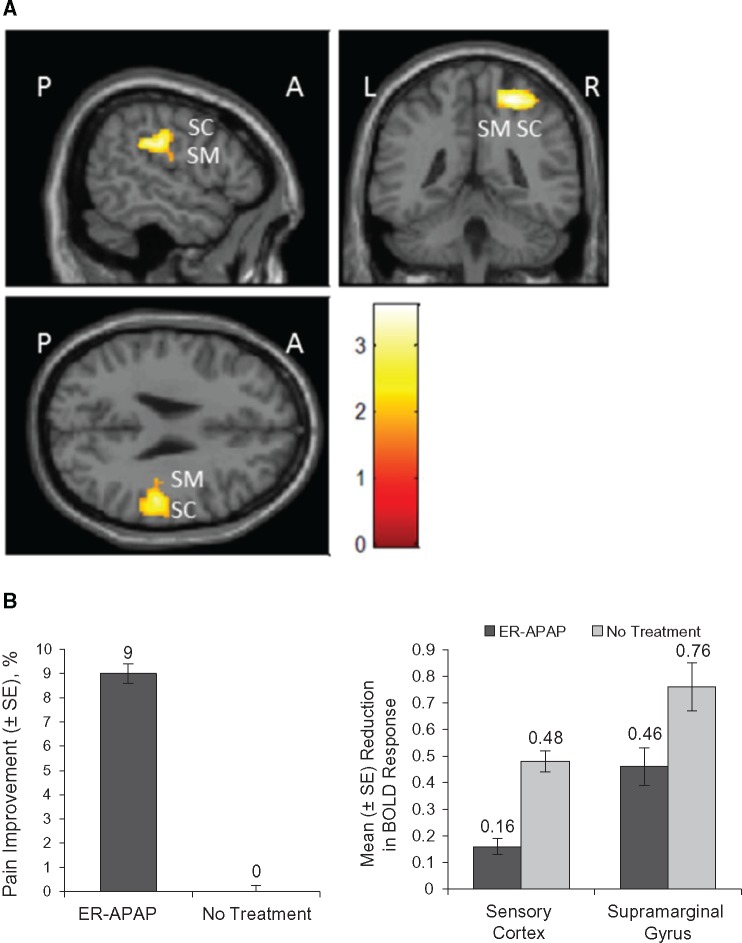
Comparison images of the BOLD signal activation were observed in multiple brain regions after stimulation (Figure 3A). ER-APAP had a significant effect in reducing BOLD signal activation caused by painful stimulation compared with no treatment in the sensory cortex (P = 0.002) and the supramarginal gyrus (P = 0.003) (Table 4; Figure 3B). The reduction in BOLD response in these brain regions was concomitant with the significant effect of ER-APAP in reducing pain intensity after stimulation compared with no treatment (Figure 3B).
Figure 3.
ER-APAP vs no treatment. A) The combined functional magnetic resonance images of BOLD activation after painful stimulation at the knee joint following treatment with ER-APAP vs no treatment. B) Left: mean change from baseline (%) in pain intensity after pressure stimulation following treatment with ER-APAP and no treatment. P = 0.014 for comparison of pretreatment pain after stimulation and post-treatment pain after stimulation prescan for ER-APAP; P = 0.99 for comparison of pain intensity after stimulation at the beginning and end of the no-treatment period prescan. Right: reduction of BOLD response after painful stimulation at the knee joint following treatment with ER-APAP and no treatment. P = 0.002, sensory cortex; P = 0.003, supramarginal gyrus. A = anterior; BOLD = blood oxygen level–dependent; ER-APAP = extended-release paracetamol; L = left side; P = posterior; R = right side; SC = sensory cortex; SE = standard error of mean; SM = supramarginal gyrus.
Table 4.
Effect of treatment on brain blood oxygen level–dependent response after painful stimulation in OA knee joint
| Treatment/Brain Region | Cluster Size* | MNI† Coordinates X, Y, Z | Mean‡ Difference | P§ |
|---|---|---|---|---|
| ER-APAP vs no treatment | ||||
| Sensory cortex* | 112 | 24, −44, 62 | −0.3153 | 0.002 |
| Supramarginal gyrus | 134 | 56, −32, 30 | −0.2955 | 0.003 |
| Placebo vs ER-APAP | ||||
| Thalamus | 343 | −6, −4, 12 | −1.1651 | 0.003 |
| Placebo vs no treatment | ||||
| Subgenual prefrontal cortex | 116 | 6, 28, 0 | −0.4541 | <0.001 |
| Frontal cortex | 305 | 38, 26, 26 | −0.3031 | <0.001 |
| Insula | 63 | 40, 26, −10 | −0.5008 | 0.003 |
| Sensory cortex | 176 | 36, −44, 62 | −0.5502 | <0.001 |
ER-APAP = extended-release paracetamol; MNI = Montreal Neurological Institute; OA = osteoarthritis.
Cluster size representing the number of continuous voxels forming an anatomical unit with intensity exceeding a preselected cluster threshold (≥50).
Maximum peak coordinates for the specific cluster as defined by the MNI template.
Mean of difference is calculated as mean of differences of MRI numeric values between first and second named treatment for each subject.
P < 0.01 and a cluster extension threshold of 50 or more continuous voxels were used as criteria to consider a result significant. P values were associated with a paired one-tailed t test.
Placebo vs No Treatment
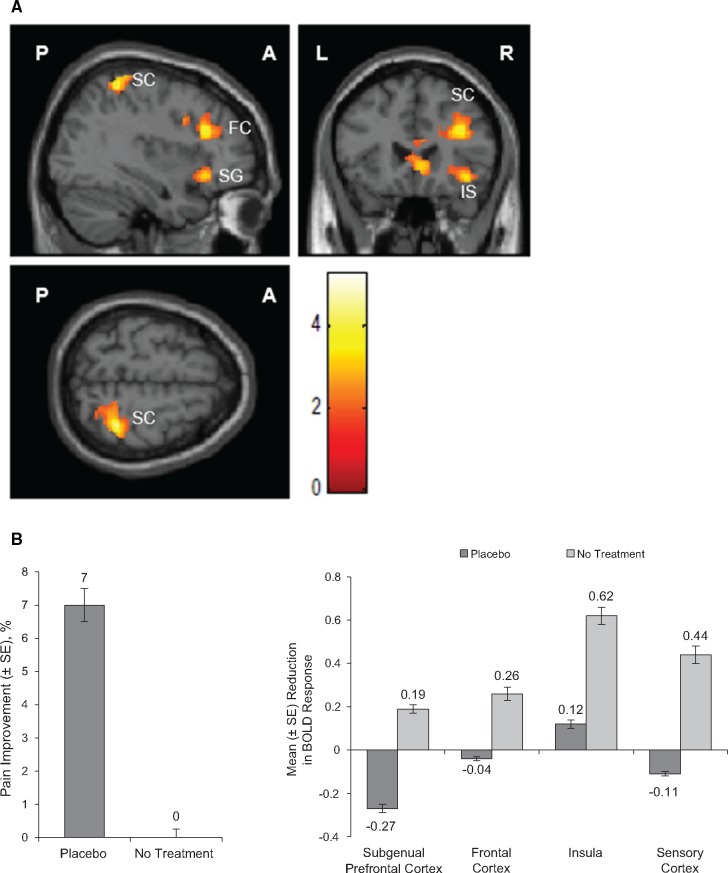
Reductions in BOLD signal activation in multiple brain regions were observed when subjects were in the placebo period compared with the no-treatment period in combined fMRI images (Figure 4A;Table 4). Placebo significantly reduced BOLD response after stimulation compared with no treatment in the subgenual prefrontal cortex (P < 0.001), frontal cortex (P < 0.001), insula (P = 0.003), and sensory cortex (P < 0.001) (Table 4; Figure 4B). The reduction in BOLD response was concomitant with the significant effect of placebo in reducing pain intensity after stimulation compared with no treatment (Figure 4B).
Figure 4.
Placebo vs no treatment. A) The combined functional magnetic resonance images of BOLD activation after painful stimulation at the knee joint following treatment with placebo vs no treatment. B) Left: mean change from baseline (%) in pain intensity after pressure stimulation following placebo treatment and no treatment. P = 0.03 for comparison of pretreatment pain intensity after stimulation and post-treatment pain intensity after stimulation prescan for placebo treatment; P = 0.99 for comparison of pain intensity after stimulation at the beginning and end of the no-treatment period prescan. Right: reduction of BOLD response after painful stimulation at the knee joint following treatment with placebo and no treatment. P < 0.001, subgenual prefrontal cortex; P < 0.001, frontal cortex; P = 0.003, insula; P < 0.001, sensory cortex. A = anterior; BOLD = blood oxygen level–dependent; ER-APAP = extended-release paracetamol; FC = frontal cortex; IS = insula; L = left side; P = posterior; R = right side; SC = sensory cortex; SE = standard error of mean; SG = subgenual prefrontal cortex.
ER-APAP vs Placebo
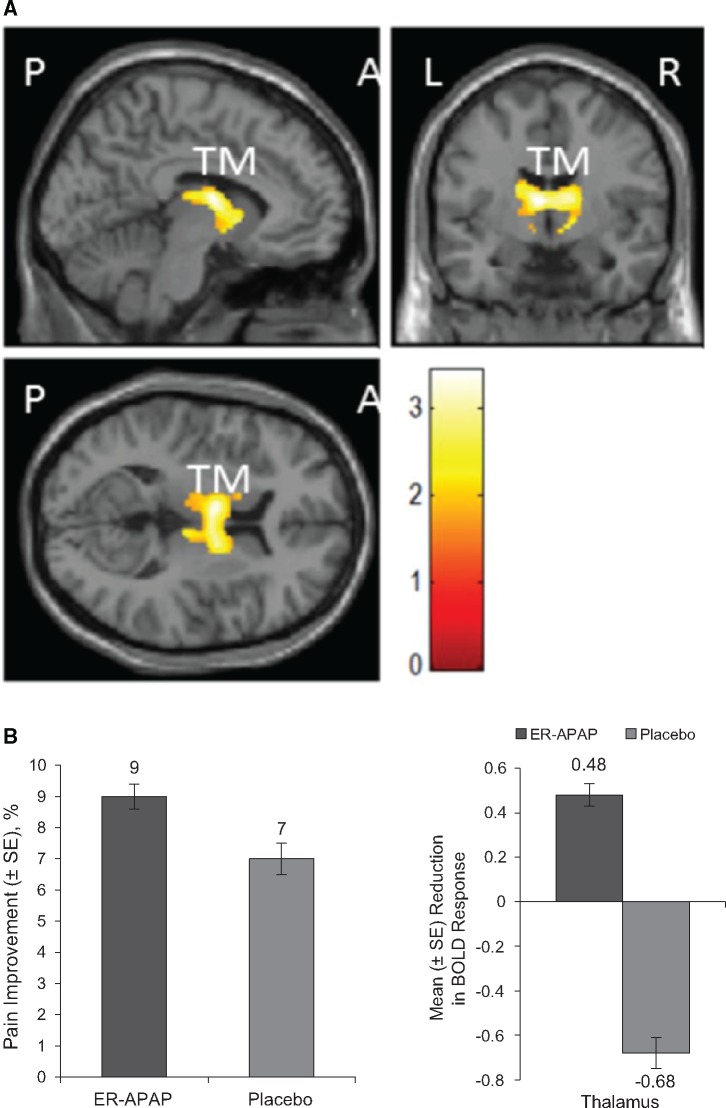
Placebo showed a significantly greater reduction of BOLD signal activation in the thalamus region after stimulation compared with ER-APAP in combined fMRI images (P < 0.003) (Figure 5A;Table 4); despite the fact that ER-APAP demonstrated a greater effect than placebo in reducing pain intensity after stimulation (Figure 5B), ER-APAP maintained BOLD signal activation at certain levels in only parts of the thalamic nuclei (the ventral anterior nucleus of the thalamus and/or the ventral lateral nucleus of the thalamus) and not the entire thalamus region.
Figure 5.
ER-APAP vs placebo. A) Combined functional magnetic resonance images of BOLD activation after painful stimulation at the knee joint following treatment with ER-APAP vs placebo. B) Left: mean change from baseline (%) in pain intensity after pressure stimulation following treatment with ER-APAP and placebo. P = 0.014 for comparison of pretreatment pain intensity after stimulation and post-treatment pain intensity after stimulation prescan for ER-APAP; P = 0.03 for comparison of pretreatment pain intensity after stimulation and post-treatment pain intensity after stimulation prescan for placebo. Right: reduction of BOLD response after painful stimulation at the knee joint following treatment with ER-APAP and placebo. P = 0.003, thalamus. A = anterior; BOLD = blood oxygen level–dependent; ER-APAP = extended-release paracetamol; L = left side; P = posterior; R = right side; SE = standard error of the mean; TM = thalamus.
Safety
A total of eight AEs were reported by six (20%) subjects. Of these, three AEs were observed with ER-APAP (anxiety, dental caries, and lumbar spine pain), three with placebo (anxiety, diarrhea, and headache), and two during the no-treatment period (claustrophobia and laceration). Six of the AEs observed were mild, and two were moderate in intensity (i.e., dental caries and bone pain). None of the AEs observed were related to treatment. Two patients discontinued due to AEs: One had moderate lumbar spine pain during the ER-APAP period, and the other had mild claustrophobia during the no-treatment period.
Discussion
The results of this study confirm the results of previous studies demonstrating the effectiveness of eight-hour ER-APAP in reducing OA joint pain [18–20]. The results also demonstrate that ER-APAP had a significant effect in reducing pain from mechanical stimulation in the knee joint that was expressed more clearly before than after the MRI scan. There was a significant correlation between specific brain activity patterns and the efficacy of pain reduction. The effect of ER-APAP in reducing pain caused by mechanical stimulation was greater than that of placebo and no treatment, and that correlated with the reduction of the BOLD signal from fMRI in brain regions of pain pathways, such as the supramarginal gyrus and sensory cortex. The sensory cortex is the highest sensory center in the nociceptive pain pathways; therefore, the reduction of BOLD signal activation by ER-APAP in this region was not unexpected. This study is the first to demonstrate an association between changes in neurologic activity and APAP treatment in the supramarginal gyrus in patients with OA knee pain. The supramarginal gyrus is not only the part of the somatosensory association cortex involved in the perception and processing of speech and language [21], but it has also been shown to be involved in the prediction and perception of physical pain [22,23]. It also interprets certain sensory data and is involved in the perception of space and limb location [24,25]. Although the inter-relationship between supramarginal gyrus activity, nociceptive pain in OA patients, and APAP treatment is not fully understood, these results may provide insight into the potential mechanism of action of APAP in efferent pain pathways. A previous study in healthy volunteers showed reduced pain-related BOLD signal activation in the insula, anterior cingulate cortex, thalamus, and prefrontal cortices during APAP treatment, suggesting that the inhibitory effects on these regions of the brain may also contribute to the antinociceptive effect of APAP [16].
BOLD signal changes also occurred in the thalamus region. There was a significant reduction in brain activation in this region with placebo treatment compared with ER-APAP. This result was somewhat unexpected, given the known pain-relieving actions of ER-APAP and the patterns of thalamic activation in response to pain signals, as the thalamus receives signals from the spinal cord and sends the signals to the sensory cortex for interpretation [7,26–28]. Because no reduction of the BOLD signal in the thalamus region was reported when comparing ER-APAP with no treatment, the reduction of brain activity in the thalamus region when comparing placebo with ER-APAP may be the effect of the placebo alone. Reduced thalamic activity under placebo conditions is consistent with theories hypothesizing that afferent sensory pain transmission is inhibited by placebo [7,29]. Previous fMRI studies have shown reduced activity in the thalamus during painful stimuli under blinded placebo conditions compared with control [7,30,31]. The response to placebo in pain-related regions of the brain has been shown to vary with the degree of connectivity between the thalamus and the dorsolateral prefrontal cortex [32]. It is interesting that subjects in the current study maintained BOLD signals at certain levels in only the ventral anterior nucleus of the thalamus and/or ventral lateral nucleus of the thalamus but not the dorsal thalamic region. The clinical meaning of this observation is not completely clear. However, because the ventral anterior nucleus of the thalamus lies on the pathway between the corpus striatum and the motor areas of the frontal cortex, it might influence the activities of the motor cortex. The ventral lateral nucleus of the thalamus has connections similar to those of the ventral anterior nucleus of the thalamus, with major input from the cerebellum and minor input from the red nucleus. Its projections pass to the motor and premotor regions of the cerebral cortex. Therefore, it is very possible that placebo treatment in this study reduces more motor activities compared with ER-APAP treatment. APAP may block pain signals not only from the spinal cord through the thalamus into the sensory cortex (afferent pathway), but also from the motor cortex to the motor neurons in the spinal cord and then to peripheral tissues via the thalamus (efferent pathway). Further studies would be necessary to support this hypothesis.
In most clinical efficacy studies, subjects given a placebo treatment will have perceived an actual improvement in their medical condition, such as pain. One study demonstrated that placebo can also have a surprisingly positive effect on patients who know that the given treatment is without active drug compared with patients who did not receive treatment [33]. The current study found that, when compared with the no-treatment arm, placebo reduced BOLD signal activation in several brain regions, including the subgenual prefrontal cortex, frontal cortex, insula, and sensory cortex. Previous neuroimaging studies have shown an association of placebo treatment with activation in these and other limbic brain regions, including the anterior cingulate, prefrontal, orbitofrontal and insular cortices, nucleus accumbens, amygdala, and brainstem periaqueductal gray matter [34–37]. The perception of pain requires the integration of sensory, cognitive, and affective information. Placebo analgesia likely results partly from afferent inhibition of a nociceptive signal, in addition to interactions of an affective–cognitive network with input from both hemispheres [37]. High placebo response has been linked with activity of dopaminergic and mu-opioid systems in reward and motivation centers of the brain (nucleus accumbens), while anti-analgesic nocebo response has been linked with decreased activity of these same systems. This link between the limbic system and positive analgesic placebo response may arise from descending inhibition through the periaqueductal gray matter, inhibiting spinal nociceptive reflexes [35], while the expectations of anti-analgesic nocebos act in the opposite way to block this effect [38]. The results presented in this study are consistent with this hypothesis.
The fMRI results presented in this study are based on a cluster-defining primary threshold (P = 0.01) that represents a liberal approach as compared with more standard thresholds of 0.005 and 0.001. Such an approach bears the risk of inflated false-positive rates and may pose large clusters spanning multiple anatomical regions. Eklund et al. [39] demonstrated that a cluster-defining threshold with a P value of 0.001 had much better family-wise error control than a cluster-defining threshold with a P value of 0.01. However, we chose the more liberal approach in order to increase sensitivity to weak and diffuse signals, considering the fact that this study was expected to have low power due to the relatively small sample size of 18 subjects. The overall goal of this proof-of-principle study was to explore as many regions of interest as possible that subsequently could be further validated and consolidated in a larger study with adequate sample size.
Conclusions
ER-APAP was effective in reducing knee pain from OA, consistent with results of previous clinical trials. The reduction in pain intensity from mechanical stimulation with ER-APAP was associated with reduced BOLD signal activation in brain regions involved in nociceptive pain pathways, specifically in the sensory cortex and supramarginal gyrus. A reduction of BOLD signal activation with placebo compared with ER-APAP was observed in certain nuclei of the thalamus. In contrast, placebo reduced BOLD signal activation in several other brain regions when compared with no treatment, including the subgenual prefrontal cortex, frontal cortex, insula, and sensory cortex. Most of these brain regions are either part of the limbic system or work closely with this system for emotional, cognitive, or psychological functions, suggesting a potential mechanism of the placebo effect in the brain. This study provides further evidence that changes in BOLD signal activation measured by fMRI are associated with pain reduction and could potentially be used as an objective end point for pain clinical trials.
Acknowledgments
We thank James Otto and Kenneth Reed of GlaxoSmithKline Consumer Healthcare for assistance with preparation of the manuscript and David Stevens for his contribution to the design of the study. The authors also acknowledge Dr. Steven Cooper for his critical review of this manuscript and the staff of the Rheumatology Department at the Hospital del Mar for conducting the study.
References
- 1. Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009;121:269–80. [PubMed] [Google Scholar]
- 2. Flor H, Turk DC.. Chronic Pain: An Integrated Biobehavioral Approach. Seattle, WA: IASP Press; 2011. [Google Scholar]
- 3. Wanigasekera V, Mezue M, Andersson J, Kong Y, Tracey I.. Disambiguating pharmacodynamic efficacy from behavior with neuroimaging: Implications for analgesic drug development. Anesthesiology 2016;1241:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK.. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;94:463–84. [DOI] [PubMed] [Google Scholar]
- 5. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013;136(pt 9):2751–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ 3rd, Willis WD Jr.. Diencephalic mechanisms of pain sensation. Brain Res 1985;3563:217–96. [DOI] [PubMed] [Google Scholar]
- 7. Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004;3035661:1162–7. [DOI] [PubMed] [Google Scholar]
- 8. Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci 1999;3541387:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peyron R, Laurent B, Garcia-Larrea L.. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000;305:263–88. [DOI] [PubMed] [Google Scholar]
- 10. Baliki MN, Geha PY, Jabakhanji R, et al. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008;4:47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delli Pizzi S, Mantini D, Ferretti A, et al. Pharmacological functional MRI assessment of the effect of ibuprofen-arginine in painful conditions. Int J Immunopathol Pharmacol 2010;233:927–35. [DOI] [PubMed] [Google Scholar]
- 12. Wise RG, Rogers R, Painter D, et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage 2002;164:999–1014. [DOI] [PubMed] [Google Scholar]
- 13. Gear R, Becerra L, Upadhyay J, et al. Pain facilitation brain regions activated by nalbuphine are revealed by pharmacological fMRI. PLoS One 2013;81:e50169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor JJ, Borckardt JJ, Canterberry M, et al. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology 2013;387:1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gimenez M, Pujol J, Ali Z, et al. Naproxen effects on brain response to painful pressure stimulation in patients with knee osteoarthritis: A double-blind, randomized, placebo-controlled, single-dose study. J Rheumatol 2014;4111:2240–8. [DOI] [PubMed] [Google Scholar]
- 16. Pickering G, Kastler A, Macian N, et al. The brain signature of paracetamol in healthy volunteers: A double-blind randomized trial. Drug Des Devel Ther 2015;9:3853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woo CW, Krishnan A, Wager TD.. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage 2014;91:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bacon TH, Hole JG, North M, Burnett I.. Analgesic efficacy of sustained release paracetamol in patients with osteoarthritis of the knee. Br J Clin Pharmacol 2002;536:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson M, Marangou A, Russo MA, et al. Patient preference for sustained-release versus standard paracetamol (acetaminophen): A multicentre, randomized, open-label, two-way crossover study in subjects with knee osteoarthritis. J Int Med Res 2009;375:1321–35. [DOI] [PubMed] [Google Scholar]
- 20. Altman RD, Zinsenheim JR, Temple AR, Schweinle JE.. Three-month efficacy and safety of acetaminophen extended-release for osteoarthritis pain of the hip or knee: A randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2007;154:454–61. [DOI] [PubMed] [Google Scholar]
- 21. Gazzaniga MS. The Cognitive Neurosciences, 4th edition Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 22. Wager TD, Atlas LY, Lindquist MA, et al. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;36815:1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamm C, Nusbaum HC, Meltzoff AN, Decety J.. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One 2007;212:e1292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carlson NR. Structure of the nervous system In: Carlson NR, ed. Physiology of Behavior, 11 edition.New York, NY: Pearson; 2013:66–98. [Google Scholar]
- 25. Reed CL, Caselli RJ.. The nature of tactile agnosia: A case study. Neuropsychologia 1994;325:527–39. [DOI] [PubMed] [Google Scholar]
- 26. Yen CT, Lu PL.. Thalamus and pain. Acta Anaesthesiol Taiwan 2013;512:73–80. [DOI] [PubMed] [Google Scholar]
- 27. Sprenger C, Finsterbusch J, Buchel C.. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: A combined spino-cortical FMRI study. J Neurosci 2015;3510:4248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen KB, Kaptchuk TJ, Chen X, et al. A neural mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cereb Cortex 2015;2510:3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melzack R, Wall PD.. Pain mechanisms: A new theory. Science 1965;1503699:971–9. [DOI] [PubMed] [Google Scholar]
- 30. Geuter S, Eippert F, Hindi Attar C, Buchel C.. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 2013;67:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME.. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 2007;127(1–2):63–72. [DOI] [PubMed] [Google Scholar]
- 32. Sevel LS, O'Shea AM, Letzen JE, et al. Effective connectivity predicts future placebo analgesic response: A dynamic causal modeling study of pain processing in healthy controls. Neuroimage 2015;110:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS One 2010;512:e15591.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oken BS. Placebo effects: Clinical aspects and neurobiology. Brain 2008;131(pt 11):2812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott DJ, Stohler CS, Egnatuk CM, et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 2008;652:220–31. [DOI] [PubMed] [Google Scholar]
- 36. Lidstone SC, Stoessl AJ.. Understanding the placebo effect: Contributions from neuroimaging. Mol Imaging Biol 2007;94:176–85. [DOI] [PubMed] [Google Scholar]
- 37. Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM.. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage 2007;384:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goffaux P, Redmond WJ, Rainville P, Marchand S.. Descending analgesia—when the spine echoes what the brain expects. Pain 2007;130(1–2):137–43. [DOI] [PubMed] [Google Scholar]
- 39. Eklund A, Nichols TE, Knutsson H.. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016;11328:7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]