Abstract
On 14 December 2016, Professor Alan Fenwick OBE delivered the prestigious ‘Manson Lecture’ to the Royal Society for Tropical Medicine and Hygiene at the Royal Society in London. This paper, based on the Manson Lecture, presents the research carried out to study the epidemiology of schistosomiasis in Africa and test the various control tools as they were proposed from 1914 to date. Subsequently the development of national control programmes against schistosomiasis in Africa from 2000 towards the full national coverage now being delivered in many countries is discussed. In 2000 only Egypt in Africa was offering treatment to the infected and at-risk populations. By 2016 the World Health Assembly resolutions and the WHO NTD targets for 2020 are close to being achieved in some countries where schistosomiasis appears no longer to be ‘a public health problem’. However, in some areas in sub-Saharan Africa, continuous annual treatment is still needed because of remaining ‘hotspots’ where transmission seems to be stubbornly continuing. The author therefore questions whether the timescales to reach eliminations are realistic and whether the tools are available to reach those targets.
Introduction
Sir Patrick Manson (1844–1922) was an amazing man who developed and mostly proved hypotheses that completely changed the knowledge about several tropical diseases. He graduated as an MD in 1867 and moved to Formosa where he studied various tropical diseases while working in the Chinese Imperial Maritime Customs. He kept a careful diary and detailed notes of a number of diseases that he came across, such as elephantiasis (now known to be caused by filarial worms Brugia malayi and Wuchereria bancrofti), leprosy and Beriberi. Manson hypothesised that mosquitoes were responsible for the transmission of lymphatic filariasis (LF) and in 1878 he was ridiculed for this ‘absurd’ suggestion. He was of course proved to be correct. Ronald Ross is widely acknowledged as the individual responsible for showing that mosquitoes were the carriers of malaria, but in fact Sir Patrick Manson worked closely with Ross, but allowed Ross to take the credit when they were proved again to be correct.1 Manson in 1900 infected his son and a laboratory technician with malaria using a consignment of imported infected mosquitoes. He then sent two of his pupils to live in an endemic malaria area in Italy but kept them free of infection by having them sleep in a mosquito-proofed room while others sleeping in a non-mosquito proofed room became infected. Manson was involved in the discovery of many other pathogenic parasites and in the elucidation of their life cycles, including the fluke Paragonimus westermanni, Sparganum mansoni, Schistosoma mansoni and S. japonicum. He also described the filarial worms Loa loa, and Filaria perstans. Manson was an innovator; in a letter to the Lancet published in 1897 he stressed the need for tropical medicine teaching and his recommendations led to the foundation of the London School of Hygiene and Tropical Medicine (LSHTM) in 1899, some six months after the opening of the world's first tropical school in Liverpool. He was elected a fellow of the Royal Society in 1900, and he received a knighthood in 1903.2
Manson taught at LSHTM from its launch until his retirement in 1914, and he was medical adviser to the Colonial Office for nearly twenty years. In 1907, he was one of the founders of the Royal Society of Tropical Medicine and Hygiene (RSTMH) and was elected its first president. It is fitting that the RSTMH continue to remember and honour him both by awarding a medal in his name and supporting the annual Manson lecture.
Research into schistosomiasis from 1858–2016
From 1858, when Theodore Maximilian Bilharz reported finding live worms in the blood vessels of Egyptians on whom he was conducting a post mortem, to 1914 when Sir Patrick Manson retired, the schistosomiasis life cycle was finally described after the contribution of a number of medical personnel who were fascinated by the complicated worm which caused blood in the urine. It was not until 1914 that the role of freshwater snails as intermediate hosts was proven and at that time there was no known treatment for infection. The most celebrated helminthologist at the time was Robert Leiper. Born in Scotland, he qualified in medicine at Glasgow and was appointed to LSHTM by Manson in 1905. He had shown a fascination for the natural history of parasites and he was sent to China in spring 1914 with E. L. Atkinson to investigate the lifecycle of S. japonicum along the Yangtze River and then to Egypt where he was able to finally link schistosomes to snails.
J. B. Christopherson (1868–1955) was the first person to develop a drug against schistosomiasis. In 1902 Christopherson became physician to the Governor-General of the Sudan; in 1904, he became Director of Medical Services to the Sudan Government, and in 1909 and became Director of the Civil Hospitals at Khartoum and Omdurman. After World War One, in 1918, he returned to the UK and worked in the bilharzia clinic of the Ministry of Pensions.
It was in 1918, he published that antimony potassium tartrate was an effective drug in the treatment of bilharzia.3,4 Although it had severe side effects, this treatment, which involved the use of antimony derivatives, was the state of the art drug until the 1950s, and indeed the next generation of drugs also contained antimony and were in use until the 1970s.
Schistosomiasis in Egypt and Sudan
From the 1920s until World War Two in 1939, work on schistosomiasis was concentrated on the Nile River as The Sudan and Egypt were annually flooded by the surging rivers (The Blue and White Niles in Sudan and the main Nile in Northern Sudan and Egypt). The area between the Blue and White Niles to the South of Khartoum (known as the Gezira), and the Nile Delta to the north of Cairo, became the most fertile of areas as silt from the flooding river annually fertilised both areas. However, the irrigation canals dug in the Gezira and the Nile Delta proved to be excellent habitats for the Biomphalaria and Bulinus snails, which are the intermediate hosts of S. mansoni and S. haematobium, respectively.
The first efforts at control were aimed at preventing schistosomiasis being introduced into the newly constructed ‘Gezira’ irrigation scheme. The canals were dug by imported Egyptian labour and these labourers were screened at the border and treated if found to be infected. Sadly, these measures were not effective and by the 1940s the prevalence and intensity of schistosomiasis in the Gezira irrigated area of over 1 million acres criss-crossed by slow moving gravity fed canals full of host snails was very high indeed.5,6
After World War Two (from 1940–1970), there was a period of intensive activity against schistosomiasis in the Middle East and East Africa. In 1963 the ‘Egypt 49’ project was launched to eliminate schistosomiasis from the Nile Delta.7 All the canals in the Delta were treated with molluscicide (copper sulphate was the primary molluscicide used) to try and eliminate snails, and concurrently mass drug administration (MDA) to the human residents by injection of an antimony drug was carried out in villages in the Nile Delta. Sadly, an unexpected consequence of this public health intervention has been the spread of hepatitis C in Egypt as 14 daily injections were needed and there was not the hygienic cleaning of needles that would be used today. The Hepatitis C sero-positivity of people in Egypt is very much higher than anywhere else in the world.8,9
Meanwhile a new irrigation scheme was constructed, in the basin known as the Fayoum oasis, and this area was subjected to a massive control effort in the late 1960s with treatment of the canals with Bayluscide (niclosamide), which was supported by the German Government. Bayluscide was a chemical developed by Bayer that was proved in the laboratory to be an efficient molluscicide. In the Fayoum, which was an irrigation scheme fed by a single water source from the Nile, the hypothesis was that if all snails could be killed by intensive spraying of the canals with Bayluscide there would be no new cases of schistosomiasis in humans. This snail control was carried out using ‘Unimogs’—vehicles driven along the canal banks from which the Bayluscide was sprayed into the snail infested water. The snail control was accompanied by treatment of the human population with the then new CIBA drug Ambilhar, which was much safer than the antimony derivatives. Evaluations of the Fayoum project were published by Bell and later by Gryseels but showed a disappointing result, indicating that snail control was not as easy as had been expected and Ambilhar was perhaps not as effective as had been hoped.10,11
In the 1970s, field trials were carried out to determine whether either or both of two molluscicides, niclosamide (Bayluscide) or N-tritlyl morpholine (Frescon), were cost effective as snail control chemicals.12 Trials in Sudan showed that niclosamide as a wettable powder or as an emulsifiable concentrate was effective in stagnant or slow moving water, but suffered from the disadvantage of being lethal to other aquatic organisms, including fish.13 A more important constraint developed in the 1970s when the price of oil rocketed upwards, making niclosamide too expensive for widespread and regular use. Meanwhile Frescon, a purpose-designed chemical from Shell Chemicals, was tested as a drip feed mollusicicide and also it was sprayed into the Gezira canal network from crop spraying aircraft.12 Frescon was effective against snails but did not kill the snail eggs and so repopulation after treatment was rapid.14 Again price became a constraint and, by the 1980s, snail control using chemicals was mostly discarded as a regular control tool in favour of regular repeated treatment of the infected and at-risk human population using another new drug, praziquantel. Praziquantel was a safe and effective drug, which is still the main control tool available today.15,16
A number of people had investigated the possibility of using plant extracts as a molluscicide to avoid the widespread environmental effect of the chemical molluscicides, and Dr Aklilu Lemma in Ethiopia had discovered that the plant Phytolacca dodecandra (known as Endod) was an effective molluscicide. His plan was to plant this shrub along the banks of canals in Ethiopia so that the fruit would fall in the water naturally and kill the snails.17 Despite his efforts and the establishment of an ‘Endod farm’ in Zambia, this and other plant molluscicides did not prove to be an effective long-term snail control measure.18
From 1966 onwards there were a number of candidate drugs with which to treat humans infected with the schistosome worms. Hycanthone, Ambilhar and metrifonate were all drugs, which initially appeared to be promising, but each presented with drawbacks that emerged as they were used more widely. Hycanthone was administered as an injection and seemed ideal but as more and more people were treated a lethal side effect emerged with some individuals suffering from liver necriosis and death. Hycanthone was withdrawn after a campaign led by Dr Bueding who first recognised the dangers of this drug. Ambilhar and metrifonate proved to be more effective against urinary schistosomiasis than against S. mansoni, but Ambilhar, a CIBA Geigy drug, was recognised as causing hallucinations and instability in certain patients, and like hycanthone was eventually withdrawn. Metrifonate proved to be highly effective against S. haematobium and safe but suffered from the drawback that three doses were required one week apart and the logistical difficulties in getting rural populations to follow this regimen was considered to be a major constraint which killed sales once praziquantel was marketed. Finally, the answer to all physicians and public health personnel's dreams came with the development by Bayer of praziquantel during the 1970s.15
Research in East Africa and on St Lucia
During the 1950s and 1960s there was a generation of schistosomiasis researchers who mostly lived in East Africa and were supported by the British Government. Drs Andrew Davis, Norman Crossland, Robert Sturrock, Peter Jordan, Michael Prentice, Gerry Webbe, Ray Foster, Fergus McCullough (even Alan Fenwick). A lone American Dr Joe Cook completed the team.19
In the late 1960s some of the schistosomiasis people left East Africa, Dr Davis moved from Tanga to WHO, Dr Webbe from Mwanza to the LSHTM, Drs Jordan and Sturrock all went to St Lucia, where they were joined by Dr Cook and later by Michael Prentice (from Uganda). Dr Crossland joined Shell in UK and Dr Foster joined Pfizer. A major step forward towards finally providing the answer to treatment against schistosomiasis was taken by Dr Andrew Davis at WHO when he sponsored multi-centred trials of praziquantel that showed that a single oral dose of 40 mg/kg was the optimal dose with the proviso that all individuals offered treatment needed to have consumed some food shortly before taking the drug. Since the 1970s, praziquantel has been the drug of choice and it remains so, with fortunately little evidence of resistance having developed.
Meanwhile Drs Jordan, Sturrock and Cook on the Caribbean island of St Lucia designed a study to evaluate the contributions to control from molluscicides, chemotherapy, health education and water supplies all implemented separately in physically isolated but similar valleys on the island.20,21
In the Gezira Scheme in the Sudan, molluscicide and praziquantel research was carried out from 1971 though to 1978 and then the Blue Nile Health Project was launched in 1978 funded by WHO, and the American and British Governments. This Project led by Dr Ahmed Gaddal was the first integrated programme to control both malaria and schistosomiasis, and was most successful in controlling two diseases that had been out of control for several years.22
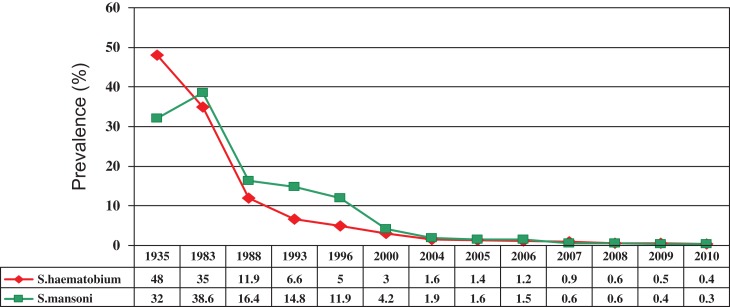
In 1988, Egypt was funded by the American Government and the World Bank to carry out in parallel a research programme (The Schistosomiasis Research Project [SRP]) and a national schistosomiasis control programme delivering praziquantel funded by the World Bank. The SRP—a collaboration between Egyptian and US scientists—led to many peer-reviewed publications on snail control, epidemiology, advocacy, testing for resistance to praziquantel and vaccine development. Both of these programmes ran for 14 years, with World Bank funding being used to purchase and deliver praziquantel throughout the Nile Delta, the Fayoum Irrigation Project and Upper Egypt. During the first period of 6 years the safety of praziquantel was recognised by the Ministry of Health who then felt able to allow MDA to replace the previous edict that only those diagnosed as infected could be given treatment. Additionally, Egypt began to manufacture its own praziquantel at a competitive price of around 7 US cents per tablet. The implementation of regular annual treatment led to a significant year by year reduction in prevalence of schistosomiasis (Figure 1).23
Figure 1.
The successful control of schistosomiasis in Egypt.
Schistosomiasis in West Africa
Meanwhile, rivers in West Africa were being dammed to provide water for irrigation and hydro-electricity, and in both Senegal and Ghana massive outbreaks of schistosomiasis followed the change in the environment to meet these needs. The creation of favourable snail habitats, and an influx of people led to an upsurge in transmission of schistosomiasis in previously unexposed people. In Senegal the development of the outbreak was followed by researchers in the Antwerp School of Tropical Medicine, and their results showed just how devastating the combination of an influx of people not previously exposed to schistosomiasis and new water bodies, which provided ideal snail habitats, could lead to what can only be descried as a serious epidemic. The Edna McConnell Clark Foundation meanwhile funded a research programme on Lake Volta in Ghana that led to some important sociological studies into human behaviour and transmission of schistosomiasis on a newly created man-made lake.24
The corporate drug donation programmes
The donation of drugs to treat almost 1 billion people against neglected tropical disease (NTDs) in 2017 is a massive contribution by several pharmaceutical companies, which will lead to elimination of seven NTDs as a public health problem by 2020 or shortly afterwards. But how did it come about?
The first significant drug donation happened in 1986. The CEO of Merck in USA (Merck Sharp Dom [MSD] in Europe), Dr Roy Vagelos, was informed by his scientific staff William Campbell and Mike Fisher that they had discovered that an annual dose of Ivermectin, a deworming tablet used in the veterinary field, could keep infected people free of larvae from onchocerciasis. These larvae, produced by adult worms living in nodules in the human population of mostly West Africa near fast flowing rivers, cause horrific itching and subsequently cause river blindness. MSD offered to assist the WHO and the World Bank campaign against the disease in West Africa by donating the drug to be delivered to all the communities at risk for as long as needed. This strategy replaced the previous control efforts that had targeted the larvae of the black fly vectors by spraying of DDT into all fast-moving rivers in West Africa at a high cost and of course a damaging effect on many other creatures in the environment.25
It was because the governments in the endemic region and the infected patients were ‘so poor’ that Roy Vagelos conceived this massive donation programme and felt compelled to convince the board of Merck to donate the drug to governments and patients at no cost for the treatment of onchocerciasis until the disease was eliminated. That donation continues to date—but it is no longer the only drug donation programme as other companies have followed this lead.26
Since 1986 until the present day, ivermectin has reached many millions of people. The public health campaign was successful and now river blindness is no longer a major public health issue in previously endemic areas of Africa and is eliminated from most of South America. Since 1986 about 1.5 billion tablets have been donated globally to control river blindness. In 2016, William Campbell was awarded the Nobel Prize for his contribution to tropical disease control.
A second drug donation was made some 12 years later but along the same lines as that by MSD, by GSK when it was realised that albendazole would prevent the larvae of filariasis from circulating in the human host, thus, preventing mosquitoes from picking up the larvae and transmitting LF. In fact, further research showed that a combination of albendazole and ivermectin was even more effective at controlling transmission of LF and so MSD joined GSK in the LF control programme making both albendazole and ivermectin available for LF control in Africa.27,28
In 1999 Pfizer joined the ranks of drug donors and made zithromycin available as a donation in countries where trachoma was endemic and where annual use of zithromycin could control the disease.29
The year 2000 became the pivotal year for schistosomiasis and intestinal helminth control, and proved to be the start of a good news story, which has developed into a massively successful control effort across Africa.
The Bill and Melinda Gates Foundation
It was almost certainly the success of the Egyptian control programme that persuaded the Bill and Melinda Gates Foundation to fund a schistosomiasis control programme in 2000, although while they agreed to fund the proposed implementation of control by the Schistosomiasis Control Initiative (SCI) in African countries, they declined at that time to fund work on developing a vaccine against schistosomiasis.
For schistosomiasis and intestinal helminths (worms) there were three drugs available—praziquantel for schistosomiasis and albendazole, and mebendazole for intestinal worms—but in 2000 no drug company had offered to donate these drugs even for the poorest people who were infected. The breakthrough for people with schistosomiasis in Africa came in 2000 when the Bill and Melinda Gates Foundation agreed to consider funding a project to prove the principle that ‘given the resources governments in sub-Saharan Africa would treat people infected and at risk, and that treatment would have a measurable impact on prevalence and intensity of infection and improve the health of those infected’. Eventually, in 2002, an award of US$34 million was made to establish the Schistosomiasis Control Initiative (SCI) at Imperial College in London. The project proposal had been prepared at Harvard by Alan Fenwick and Michael Reich, with the assistance of two students Scott Gordon and Bea Bezmalinovic, and subsequently this proposal was defended in Geneva with the help of Lorenzo Savioli and Dirk Engels from WHO in front of Gates Foundation staff and Don Hopkins from the Carter Center.
This project was funded with resources to assist six countries in Africa. The six countries were selected from 12 that had prepared proposals by an appointed SCI Technical Review Committee, and they selected Uganda, Tanzania and Zambia in East Africa and Burkina Faso, Mali and Niger in West Africa.30
To get the project accepted at Imperial College was undoubtedly facilitated by the guaranteed award of US$34 million and the support of (now) Sir Roy Anderson head of Department of Infectious Disease Epidemiology. One of the conditions was that the Financial Controller of Imperial College would be on the Advisory Board of the project with the right to veto any proposed plans he deemed against the interests of the College. He asked me how I could allow him the power of veto and I said easy – SCI does not intend to do anything against the interests of the College so the veto will never be used. The College also asked that when the programmes and the budgets for the six countries were proposed that a Memorandum of Understanding be signed with each country.
So in 2003, for the first time since World Bank funded projects in China, The Philippines and Egypt, new schistosomiasis control projects were launched and they were all in sub-Saharan Africa reaching the poorest populations in highly endemic areas. Praziquantel was purchased by tender and albendazole purchased from generic suppliers from India. The children and adults selected for treatment were decided by schistosomiasis prevalence as determined by sampling in selected schools, but every person treated with praziquantel was also offered albendazole for deworming.30
All six countries embraced the programme and in each country the listed schistosomiasis control officers were given the resources with which to offer treatment to populations living in endemic areas of their country, and they all embraced the task of treating their people with enthusiasm. One factor which made the SCI so cost effective was the entry of Shin Poong into the praziquantel manufacturing market. Until Shin Poong started selling praziquantel at below 10 US cents per 600 mg tablet, the cost from European manufacturers had been up to 10 times this price which was too high to tempt countries and donors to purchase the drug in the market place.
Armed with funding, SCI hired scientists as programme managers, the programme grew slowly but surely and almost 40 million treatments were delivered in the first 5 years.
The next steps in the control of schistosomiasis were to find more funding to include more countries, to find donated praziquantel to increase availability and more dewormimg drugs (albendazole and mebendazole). To succeed with these objectives a change in strategy was needed and so the collective name ‘Neglected Tropical Diseases’ (NTDs) was coined and a group of 8 ‘champions’ advocating on both sides of the Atlantic for funding for NTD control.
The advocacy was successful and in 2006 with first the United States Agency for International Development (USAID) and then the UK's Department for International Development (DFID) responding with requests for competitive proposals for funding to implement treatment against NTDs. USAID awarded US$100 million over 5 years to Research Triangle International (RTI) to target initially Burkina Faso, Ghana, Mali, Niger and Tanzania. DFID's funding went to Liverpool School of Tropical Medicine to tackle LF in selected countries and to SCI, Imperial College to implement schistosomiasis and STH control in nine countries (Cote D'Ivoire, Liberia, Malawi, Mozambique, Niger, Tanzania, Uganda, Zambia and Zanzibar). Another donor came forward in 2007 when Legatum through Geneva Global (a philanthropic for profit) donated to launch the Global Network for NTD and control in Burundi and Rwanda.31
Meanwhile the WHO advocacy was successful in persuading the pharmaceutical companies to increase their corporate responsibility, and from 2007 through 2012 were exciting times. In 2007 Merck KGaA entered the donation field by committing 200 million tablets of praziquantel over 10 years for schistosomiasis control and in 2012 they increased their commitment to reach 250 million tablets annually by 2017. In 2010, GSK increased their donation of albendazole by 400 million tablets a year so that children in Africa could receive deworming tablets once or even twice a year. Johnson and Johnson increased their donation of mebendazole for deworming school aged children.
Schistosomiasis Consortium for Operational Research and Elimination (SCORE)
The Bill and Melinda Gates Foundation recognised the need for increased research into the epidemiology of schistosomiasis and the strategy for delivery of praziquantel (in terms of annual treatment, targeting children etc). They then funded a Schistosomiasis Consortium for Operational Research and Elimination (SCORE) directed by Dan Colley based in the University of Georgia at Athens Georgia (UGA).32,33 This programme has funded several implementation strategies in parallel sites in Cote D'Ivoire, Kenya, Mozambique, Niger and Tanzania, and SCORE has instigated an elimination programme in Zanzibar and funded excellent research on new and more sensitive diagnostics (circulating cathodic antigen, CCA and circulating anodic antigen, CAA). The results from the latter work have raised many new questions as to how we are going to define elimination of schistosomiasis, because the previously highly regarded Kato Katz stool examination method has been shown to be too insensitive to detect very low infections as we head for elimination. The CCA test has been extensively tested in a number of countries and compared with the Kato Katz stool examination method. The results suggest that in individuals with a heavy infection there is little difference between the methods. However, as soon as we reach a level of intensity at which the Kato Katz test fails to detect eggs, the CCA test differential is increased – an extreme example being in Burundi and Rwanda where Kato Katz results were less than 3% and the CCA results were nearer 40%. The first question which arises is the interpretation of ‘trace’ lines, which are not negative but we are unsure what they mean. Do trace lines mean that individuals have a light infection, a single sex infection or what? Then the question arises does this level of infection need treating, and if untreated will that individual contribute to transmission? It may be that these CCA tests are too sensitive to help governments decide when and where to treat as prevalence and particularly intensity of infections are reduced to very low levels.34,35
Expansion of control programmes
Despite there still being just two bilateral donor countries supporting SCI and RTI to implement schistosomiasis control, more and more countries in Africa have established control programmes and expanded their coverage. The additional resources have come from a variety of sources.
WHO has successfully mobilised resources to support mapping of prevalence across most countries in Africa, and activated Ministers of Health to adopt a control programme offering donated drugs as a strong incentive. But the fact of the donated drugs meant that more careful accountability was required and more evidence needed to determine who should benefit and be offered the donated tablets. Additional resources were needed to meet the costs of mapping, advocacy, logistics, health education, training, delivery, and monitoring and evaluation. The first source of these extra funds came from Legatum, a philanthropic organisation based in Dubai, and Geneva Global a for-profit philanthropic company based in USA. The Legatum/Geneva Global support amounted to US$9 million for a 5 year programme to support NTD control in both Rwanda and Burundi from 2007. When the Burundi and Rwanda programmes were successfully concluded in 2012, Legatum established ‘The ENDFUND’, which raises funding for NTD control from High Net Worth (HNW) individuals all over the world. The ENDFUND then directs the money raised to non-governmental organisations (NGOs) to support implementation in countries short of support from other donors.
Effective altruism embraces schistosomiasis and deworming treatments
In 2011, more resources were directed to SCI from unexpected sources. Two organisations were established to evaluate smaller charities with a view to recommending to their philanthropic members the most cost effective and deserving of charitable giving. After extensive research, including a site visit to Malawi, Givewell (www.givewell.org) based in the USA selected SCI and Against Malaria Foundation as their two top selections as cost effective charities and the same two have remained high on their recommended list every year since. The same two charities were selected by Giving What We Can (www.givingwhatwecan.org), an Oxford University, UK, based group, and the result of these recommendations has been an incremental amount of donations from the public all over the world plus some HNW individuals. The support to schistosomiasis and deworming by these evaluators has raised the profile of these diseases in countries where previously they were unknown. The donations from a few HNW individuals have been substantial (over US$1 million from each individual); some of these donations were unrestricted donations and some tied to specific country programmes.
The current situation
By 2016, most countries in Africa were receiving some support for their schistosomiasis control programmes together with deworming and often as required support for LF, trachoma and Onchocerciasis. Some countries are on the way to elimination of one or more of these NTDs, although schistosomiasis is usually some way behind the others because of the donation of praziquantel only reaching the committed 250 million tablets by 2017. In 2015 the reported coverage was over 66.5 million individuals (53.2 million school-aged children and 13.3 million adults) who received preventive chemotherapy (PC) for schistosomiasis, and 711 million (150 million preschool-aged children, 417 million school-aged children and 144 million women of childbearing age) for STH. In relation to progress made in 2015 towards achieving the NTD road-map targets, the coverage of school-aged children with PC was 42.2% for schistosomiasis and 63.3% for STH; coverage of preschool-aged children with PC for STH was 48.2%.36
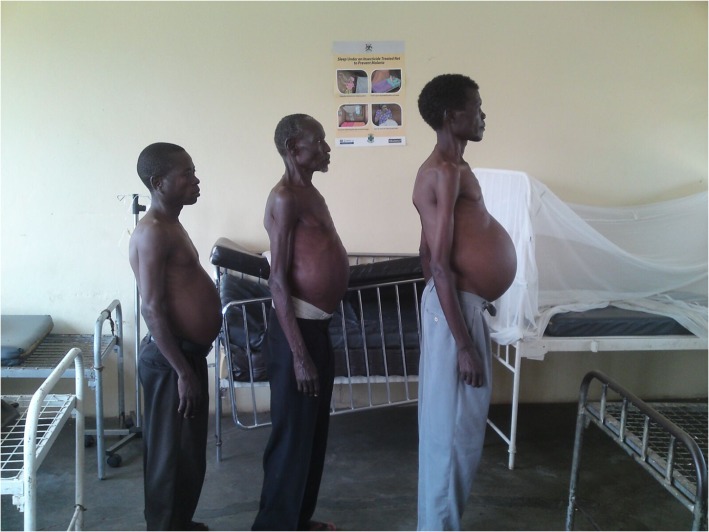
It is very difficult to predict when and where and if schistosomiasis can be eliminated on a national level. There are many places where several years of annual treatment will be enough to lower prevalence and intensity below a ‘break point’ and lead to elimination of transmission by 2020. In these areas then regular treatment has reduced the impact of schistosomiasis, and it can be labelled as ‘not a public health problem’. However we have already discovered some very ‘hot spots’ in countries with lake-shore transmission, such as Malawi, Senegal, Uganda, Tanzania and Kenya, and in these areas frequent regular treatments over a number of years do not seem to have reduced prevalence, intensity or morbidity due to schistosomiasis (Figure 2). In many other countries, because of the previous shortage of praziquantel, national coverage against schistosomiasis and indeed worms has still to be achieved, and so elimination is still a long way away. Most people believe that improved water and sanitation will also be an essential improvement before schistosomiasis and intestinal worms can be eliminated, and these are expensive contributions to improvement in socio-economic development, improved health and control of parasitic infections.37
Figure 2.
Patients with advanced hepatosplenamegaly due to schistosomiasis in Uganda.
Recent initiatives
Two recent initiatives have improved the visibility of schistosomiasis and have brought together researchers and funders to improve the chances of elimination people to work towards better.
The consortium to develop a paediatric formulation of praziquantel (www.pediatricpraziquantelconsortium.org)
This consortium (Box 1) has been brought together in response to advocacy concerning the bitter taste and large tablet that describes the current 600 mg tablet of praziquantel. Funded initially by Merck KGaA the consortium now has funds from GHIT for Phase II, which are currently underway in Cote D'Ivoire, and Phase III trials will start after the completion of the Phase II testing.
Box 1. Members of the consortium to develop a paediatric formulation of praziquantel.
Merck KGaA (Darmstadt, Germany) leads the program and provides expertise and support relating to praziquantel (PZQ), including internal resources from different areas needed for clinical development – drug product manufacturing; preclinical; clinical and regulatory. Also responsible for the development and manufacturing of the L-PZQ Active Pharmaceutical Ingredient (API).
Astellas Pharma Inc. (Japan) has developed the new pediatric PZQ formulations, and provides expert advice on clinical development in children, and pharmacokinetic modeling.
Swiss Tropical & Public Health Institute is a not-for-profit institute internationally renowned for its research, services, teaching and training in global health. It contributes with extensive experience in helminths biological and pharmacological research; epidemiology; and clinical research in endemic regions.
Lygature, a Dutch not-for-profit foundation, acts as the independent coordinator of the Consortium, providing governance in terms of progress, finance and collaboration. Since 2006, Lygature has supported close to a hundred public-private partnerships in the field of life sciences and health, including poverty-related diseases.
Farmanguinhos, the federal governmental pharmaceutical laboratory of the Fiocruz Foundation in Brazil, brings unique expertise to addressing the production and distribution of new pediatric formulations in endemic countries.
Simcyp, a UK-based research company, provides pharmacokinetics modeling capabilities and expertise.
The Schistosomiasis Control Initiative at Imperial College London, has been working for 15 years to provide treatments against schistosomiasis and three soil-transmitted helminths to the rural poor in Sub-Saharan Africa and Yemen. SCI will work with the Pediatric Praziquantel Consortium to facilitate preparation and implementation of the Access and Delivery plan in schistosomiasis endemic countries, to ensure optimal use for the new pediatric praziquantel formulation.
The Global Schistosomiasis Alliance (www.eliminateschisto.org)
The Global Schistosomiasis Alliance (GSA) was launched in 2014 both at the World Health Assembly at the Merck lunch and at the partners meeting in Addis Ababa. The secretariat is headed by Dr Johannes Waltz and secretary is Anouk Gouvras. Professor David Rollinson has recently taken over as Director of GSA from Founding Director Dr Lorenzo Savioli. The aim of the alliance is to promote the best use of donated praziquantel to treat those in need but also to advocate for schistosomiasis control and promote operational research.
2017 through 2020 and 2030
The way forward is clear as far as simple PC is concerned.
The drugs to treat 100 million school aged children annually are available from Merck KGaA. Funding to assist the countries to deliver these drugs are mostly available, and so its up to the countries to implement their programmes.
A paediatric formulation of praziquantel to treat preschool-aged children is being developed and will be available by 2020. However, it is not clear whether countries appreciate where these drugs should be used, and the access plan available does not yet show provision for purchasing the paediatric formulation of praziquantel nor how the distribution will take place and be paid for.
Praziquantel is not yet available for all the adults who need treatment, because the Merck donation was requested by WHO for school-aged children only. Within the grant from DFID, SCI has the funds to purchase some 27 million tablets a year, and some drugs may be made available from World Vision, but WHO has predicted a large gap of 300 million tablets in supply in order for adults to receive treatment.
CCA and CAA diagnostic techniques have been used to map some countries that were thought to be close to elimination. The results have been interesting and have also caused confusion and concern. They have shown that many more people are positive by the urine dipstick test using CAA than are found by the Kato Katz (40% as against 3% in Rwanda [Dr Michelle Clements personal communication] and Burundi38). A percentage of the CCA positives are however ‘trace’ lines and the meaning of these lines is not clear – do they reflect very low infections (one or two worm pairs) or single sex infections? In either case do they represent a true positive that contributes to transmission and do they need further treatment?
Water supplies and sanitation are recognised as being needed and vital in the elimination of schistosomiasis, and athough programmes to make improve water and sanitation available as part of development programmes few if any are linked to NTDs.
Acknowledgments
AuthorAuthors's contribution: AF has undertaken all the duties of authorship and is guarantor of the paper.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1. Manson-Bahr P. The malaria story. Proc R Soc Med 1961;54:91–100. [PMC free article] [PubMed] [Google Scholar]
- 2. Manson-Bahr P. Sir Patrick Manson, father of tropical medicine, and founder of the School of Tropical Medicine (October 3, 1844-April 9, 1922) [article in French]. Bull Soc Pathol Exot Filiales 1957;50:516–28. [PubMed] [Google Scholar]
- 3. Christopherson JB. Intravenous injections of antimonium tartaratum in bilharziosis. Br Med J 1918;2:652–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christopherson JB. The cure of bilharzia disease by intravenous injections of antimony tartrate: the prophylactic action of the drug. Br Med J 1919;2:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greany WH. Schistosomiasis in the Gezira irrigated area of the Anglo-Egyptian Sudan. II. Clinical study of Schistosomiasis mansoni. Ann Trop Med Parasitol 1952;46:298–310. [DOI] [PubMed] [Google Scholar]
- 6. Greany WH. Schistosomiasis in the Gezira irrigated area of the Anglo-Egyption Sudan. I. Public health and field aspects. Ann Trop Med Parasitol 1952;46:250–67. [DOI] [PubMed] [Google Scholar]
- 7. Bell DR, Farooq M, Samaan SA et al. Transmission of urinary schistosomiasis after the introduction of snail control: a study of children at the Egypt-49 project by means of an egg-counting technique. Ann Trop Med Parasitol 1967;61:422–8. [DOI] [PubMed] [Google Scholar]
- 8. Abdel-Aziz F, Habib M, Mohamed MK et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 2000;32:111–5. [DOI] [PubMed] [Google Scholar]
- 9. Frank C, Mohamed MK, Strickland GT et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 2000;355:887–91. [DOI] [PubMed] [Google Scholar]
- 10. Abdel-Salam E, Peters PA, Abdel Meguid AE et al. Discrepancies in outcome of a control program for schistosomiasis haematobia in Fayoum governorate, Egypt. Am J Trop Med Hyg 1986;35:786–90. [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Wahab MF, Esmat G, Ramzy I et al. The epidemiology of schistosomiasis in Egypt: Fayoum Governorate. Am J Trop Med Hyg 2000;62:55–64. [DOI] [PubMed] [Google Scholar]
- 12. Amin MA, Fenwick A. Aerial application of N-trityl morpholine to irrigation canals in Sudan. Ann Trop Med Parasitol 1975;69:257–64. [DOI] [PubMed] [Google Scholar]
- 13. Amin MA, Fenwick A. The development of an annual regimen for blanket snail control on the Gezira Irrigated Area of the Sudan. Ann Trop Med Parasitol 1977;71:205–12. [DOI] [PubMed] [Google Scholar]
- 14. Amin MA, Fenwick A, Osgerby JM et al. A large-scale snail control trial with trifenmorph in the Gezira irrigation scheme, Sudan. Bull World Health Organ 1976;54:573–85. [PMC free article] [PubMed] [Google Scholar]
- 15. Fenwick A, Savioli L, Engels D et al. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol 2003;19:509–15. [DOI] [PubMed] [Google Scholar]
- 16. Fenwick A, Jourdan P. Schistosomiasis elimination by 2020 or 2030. Int J Parasitol 2016;46:385–8. [DOI] [PubMed] [Google Scholar]
- 17. Goll PH, Lemma A, Duncan J, Mazengia B.. Control of schistosomiasis in Adwa, Ethiopia, using the plant molluscicide endod (Phytolacca dodecandra). Tropenmed Parasitol 1983;34:177–83. [PubMed] [Google Scholar]
- 18. Clark TE, Appleton CC, Kvalsvig JD. Schistosomiasis and the use of indigenous plant molluscicides: a rural South African perspective. Acta Trop 1997;66:93–107. [DOI] [PubMed] [Google Scholar]
- 19. Webbe G, Jordan P. Recent advances in knowledge of schistosomiasis in East Africa. Trans R Soc Trop Med Hyg 1966;60:279–312. [DOI] [PubMed] [Google Scholar]
- 20. Jordan P, Woodstock L., Unrau GO, Cook JA. Control of Schistosoma mansoni transmission by provision of domestic water supplies. A preliminary report of a study in St Lucia. Bull World Health Organ 1975;52:9–20. [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan P, Woodstock L, Cook JA.. Preliminary parasitological results of a pilot mollusciciding campaign to control transmission of Schistosoma mansoni in St Lucia. Bull World Health Organ 1976;54:295–302. [PMC free article] [PubMed] [Google Scholar]
- 22. el Gaddal AA. The Blue Nile Health Project: a comprehensive approach to the prevention and control of water-associated diseases in irrigated schemes of the Sudan. J Trop Med Hyg 1985;88:47–56. [PubMed] [Google Scholar]
- 23. El-Khoby T, Galal N, Fenwick A et al. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg 2000;62:88–99. [DOI] [PubMed] [Google Scholar]
- 24. Chu KY. Trials of ecological and chemical measures for the control of Schistosoma haematobium transmission in a Volta Lake village. Bull World Health Organ 1978;56:313–22. [PMC free article] [PubMed] [Google Scholar]
- 25. Benton B, Dadzie Y, Boatin B. The onchocerciasis control program in West Africa (OCP): essential characteristics [article in French]. Sante 1998;8:26. [PubMed] [Google Scholar]
- 26. Collins K. Profitable gifts: a history of the Merck Mectizan donation program and its implications for international health. Perspect Biol Med 2004;47:100–9. [DOI] [PubMed] [Google Scholar]
- 27. Ottesen EA, Ismail MM, Horton J.. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today 1999;15:382–6. [DOI] [PubMed] [Google Scholar]
- 28. Maher D, Ottesen EA. The Global Lymphatic Filariasis Initiative. Trop Doct 2000;30:178–9. [DOI] [PubMed] [Google Scholar]
- 29. Cook JA. Eliminating blinding trachoma. N Engl J Med 2008;358:1777–9. [DOI] [PubMed] [Google Scholar]
- 30. Fenwick A. New initiatives against Africa's worms. Trans R Soc Trop Med Hyg 2006;100:200–7. [DOI] [PubMed] [Google Scholar]
- 31. Fenwick A, Webster JP, Bosque-Oliva E et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002-2008. Parasitology 2009;136:1719–30. [DOI] [PubMed] [Google Scholar]
- 32. Colley DG, Secor WE. A schistosomiasis research agenda. PLoS Negl Trop Dis 2007;1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colley DG, Binder S, Campbell C et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg 2013;88:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Dam GJ, Bogitsh BJ, van Zeyl RJ et al. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol 1996;82:557–64. [PubMed] [Google Scholar]
- 35. Stothard JR, Sousa-Figueiredo JC, Standley C et al. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Trop 2009;111:64–70. [DOI] [PubMed] [Google Scholar]
- 36. WHO Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec 2016;91:585–600. [PubMed] [Google Scholar]
- 37. Freeman MC, Ogden S, Jacobson J et al. Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis 2013;7:e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ortu G, Ndayishimiye O, Clements M et al. Countrywide reassessment of Schistosoma mansoni infection in Burundi using a urine-circulating cathodic antigen rapid test: informing the national control program. Am J Trop Med Hyg 2017;96:664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]