Abstract
Background
Data on adult meningitis among patients infected with the human immunodeficiency virus (HIV) is scarce in western sub-Saharan Africa, including Ghana.
Methods
HIV-infected adults with a provisional diagnosis of meningitis were consecutively enrolled, between August 2014 and January 2016. After patient data collection, cerebrospinal fluid (CSF) was obtained and evaluated for microbiological aetiologies, cell counts and biochemistry. Caregiver clinicians provided limited data for inpatients at the end-point of discharge or death.
Results
Complete data sets from 84 patients were analysed (inpatients=63, outpatients=21). Median age was 40 years with 56% (47/84) being females. Only 30% (25/84) of the patients were on antiretroviral therapy (ART). CD4+ T-cell count was available for 81% (68/84) of patients and 61.9% (52/84) had counts below 150 cells/μL [median and interquartile range=56 (13.8–136)]. Microbiological aetiologies were detected in 60.7% (51/84) patients with the following distribution—Toxoplasmosis (25%), Epstein–Barr virus (28.6%), Cytomegalovirus and Cryptococcus (2.4%) each. Co-infection was identified in 20.7% (17/84) of the patients.
Conclusion
Patients presenting with symptoms of meningitis had advanced HIV/AIDS, a quarter of whom had cerebral toxoplasmosis or infection with EBV. A high index of suspicion, laboratory exclusion of cryptococcal meningitis and prompt patient management with anti-toxoplasmosis empiric therapy may thus be required for optimal treatment.
Keywords: ART, Cerebral toxoplasmosis, Clinical presentation, CSF aetiology, Cryptococcus, Outcome at discharge
Introduction
Symptomatic neurological dysfunction is common in individuals infected with HIV and in about 10% of these patients, neurological symptoms are the initial manifestation of acquired immunodeficiency syndrome (AIDS). Neurological dysfunction has been correlated to poor prognosis in late HIV infection. Among the major causes of neurological disorders in HIV/AIDS patients are opportunistic infections of the central nervous system (CNS),1 presenting as meningitis.
The higher prevalence of HIV infections in Africa has had a great impact on CNS aetiologies, which are, unfortunately, sparingly investigated in resource-limited settings in western sub-Saharan Africa, including Ghana. Bacteria, parasites, fungi and viruses are associated with adult meningitis in HIV patients. In eastern and southern Africa, cryptococcosis and TB are commonly reported as aetiologies of adult meningitis among HIV-infected patients, with poor outcomes.2 Retrospective studies in Ghana have associated Mycobacterium tuberculosis-complex meningitis (MTB) as a common cause of death among hospitalized HIV patients.3 However, in settings with inadequate anti-retroviral therapy (ART), cryptococcal meningitis (CM) and meningitis of other aetiologies are commonly associated with opportunistic infections in HIV patients.4 In Nigeria, CM prevalence of 36% was reported in 2010 among HIV-infected patients.5 In Ghana, advanced HIV patients enrolling in an ART programme in 2011 had low (2%) seroprevalence of cryptococcal antigenaemia.6 In a retrospective study in Kumasi, Ghana, in which the HIV status of the patients were unknown, bacteria (71.8%) and Cryptococcus neoformans (11.7%) were the main pathogens confirmed by culture.7 Among HIV seropositive patients from Ghana, PCR identified Toxoplasma gondii in approximately 55% of participants.8
ART is changing the epidemiology of adult meningitis and data are needed to help better manage HIV patients with CNS opportunistic infections. Meningitis associated with other aetiologies, including toxoplasmosis and viruses, have been sparingly reported in western sub-Saharan Africa. The current study investigated the clinical presentation, aetiology and outcome of meningitis among HIV patients presenting at a tertiary-level hospital in Accra, Ghana.
Methods
Setting and patients
A cross-sectional study was conducted at the Fevers’ Unit of the Korle-Bu Teaching Hospital (KBTH), a national tertiary referral hospital in Ghana, between August 2014 and January 2016. The Fevers’ Unit offers both outpatient and inpatient care to several dozen patients on a daily basis. The facility is open to emergencies every day, however, outpatient clinics are held three times a week with patient attendance ranging from 80 to 200 on a clinic day. The inpatient facility has a 24-bed capacity. All HIV patients reporting for routine standard care with a provisional diagnosis of meningitis made by clinicians were enrolled. The majority of these patients were admitted. However, patients who did not present with seizures and/or confusion were managed on an outpatient basis with short interval reviews (3 days), due to the lack of space in the wards. Prophylactic antifungal therapy is not given routinely in this clinic, although patients with other opportunistic infections may be given some antimicrobial agents. All patients 18 years and older with a provisional diagnosis of meningitis were eligible to participate in the study. Patients on prior anti-bacterial or anti-fungal medication 1 week before the clinic visit were excluded from enrolment.
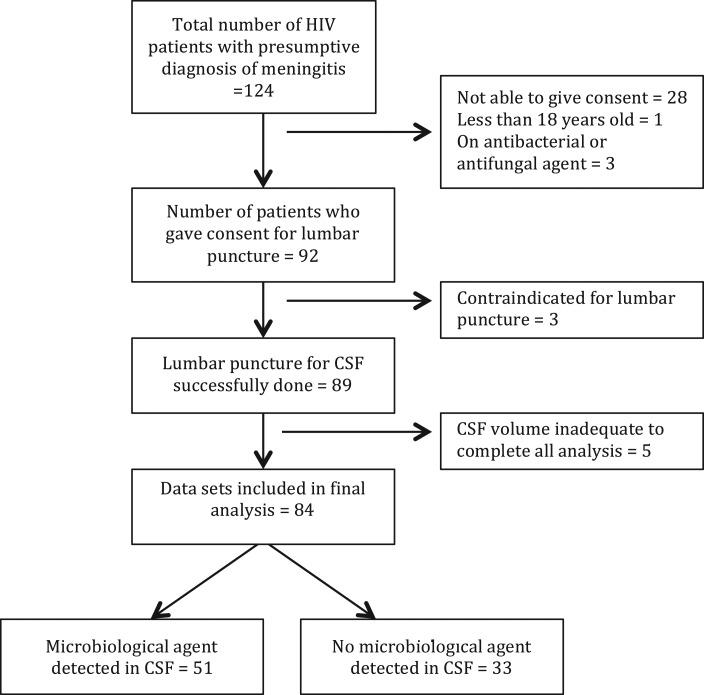
Prior to enrolment and data collection, written informed consent was obtained from each patient. For patients who were too ill to communicate, permission to enrol was obtained from next of kin. Medical officers evaluated eligible patients and consecutive participants were enrolled. Patient information was obtained from administering a questionnaire, as well as chart review of patient folders using an abstraction form. Information extracted included patient demographics, previous history of hospitalization, clinical conditions, ART, and the date of initiation, ART adherence status, last two serial CD4+ T-cell counts, viral load, date of first diagnosis of HIV and type of HIV. Medical officers examined patients and clinical signs, including fever, oral thrush, meningism, tachycardia, Glasgow coma scale (GCS) and, finally, clinical staging of HIV/AIDS by WHO criteria were recorded.9 Other information recorded included social withdrawal and wasting, seizures, stiff neck, headache and nausea. Where there was no contraindication, a lumbar puncture (LP) was performed to obtain cerebrospinal fluid (CSF), prior to the first dose of any antimicrobial therapy and blood was obtained for haematological and viral load assays. Figure 1 shows a consort diagram illustrating patient flow. During the period, 124 patients were screened and 92 enrolled; 89 had a successful lumbar puncture and 84 with complete data sets were included in the analysis.
Figure 1.
Consort diagram showing patients flow. CSF=cerebrospinal fluid, HIV=human immunodeficiency virus.
CSF analysis
CSF samples were taken from each patient for CSF biochemistry and microbiological analysis at the Biochemistry Department of the Central Laboratory, KBTH, and the Medical Microbiology Department, School of Biomedical and Allied Health Sciences (SBAHS), respectively. CSF analyses included white cell count (WCC) and differential, and protein and glucose levels. Initial microbiological analysis included CSF microscopy (Gram and India ink stains), cryptococcal antigen lateral flow assay (CrAgLFA) and culture for bacteria and fungi. For further investigations, CSF aliquots were snap frozen and stored at −80°
Culture for bacteria and fungi
Fresh CSF was centrifuged at 5000 rpm for 5 minutes, and the sediment was used for bacterial and fungal culture. For bacteria, sediment was inoculated onto blood agar (BA) and chocolate agar (CA) plates (Oxoid Ltd., Basingstoke, UK), and incubated at 37°C for 48 hours. CA plates were incubated in 5–10% carbon dioxide overnight. For fungi, sediment was inoculated onto Sabouraud’s dextrose agars (SDA; Oxoid Ltd., Basingstoke, UK). SDA plate was incubated at 35–37°C for 48–72 hours.
No TB culture was performed as part of our microbiological investigations due to resource limitations.
Real time PCR assay for Toxoplasma and viruses
Real-time PCR (qPCR) assays were performed for Toxoplasma gondii (Toxo), Epstein–Barr virus (EBV) and cytomegalovirus (CMV). CSF was centrifuged at 1500 rpm for 10 min and DNA was extracted using the NucliSENS easyMAG automated nucleic acid extraction system (bioMerieux, Inc., Durham, NC) following the manufacturer’s recommendations. For detection of T. gondii, a 133-bp segment of the repeated REP529 element was amplified and detected on the Roche LightCycler (Roche Diagnostics, Indianapolis, IN) using primers and probes as described previously10 with minor modifications. The reaction consisted of 1X LightCycler-FastStart DNA Master Hybridization Probes reaction mixture (Roche) containing FastStart Taq polymerase, primers FRET probes and heat-labile uracil-DNA glycosylase (UNG; Roche). The cycling protocol consisted of a 2-minute incubation at 50°C for UNG activity, followed by an 8-minute incubation at 95°C to activate the DNA polymerase, inactivate the UNG, and melt double-stranded DNA. This was followed by 50 cycles of 5 seconds at 95°C, 10 seconds at 60°C, and 15 seconds at 72°C. Detection of CMV and EBV was then performed on the extracted DNA from each sample using the qPCR assays as described previously.11 Appropriate positive and negative controls for each target and an extraction inhibition control, described previously,12 were included with all qPCR runs.
GeneXpert MTB/RIF assay
CSF from the first 17 consecutive patients in the cohort was screened for MTB using the GeneXpert MTB/RIF (Cepheid, Sunnyvale, Ca, USA).
Data storage and analysis
Data was stored in Microsoft excel files and later exported to SPSS version 16 (Atlanta, USA) for analysis. Statistical analysis was primarily descriptive with continuous data presented as percentages, median and IQR. Fisher’s exact test was used to compare associations between variables and p-value <0.05 was considered significant. No adjustments were made for multiple comparisons.
Results
Patient characteristics and clinical symptoms
Over the 36-month study period, 92 patients with clinical suspicion of meningitis were enrolled into the study and lumbar puncture for CSF was successfully performed in 89 patients. Five patients were subsequently excluded from final analysis because of insufficient data. The 84 included in the final analysis comprised inpatients 75% (63/84) and outpatients 25% (21/84). Baseline demographic characteristics of the total cohort of 84 patients are shown in Table 1. The median age was 40 years with 56% (48/84) females. The majority of the cohort presented with HIV type-1 87.3% (72/84) with 72% (61/84) at WHO Stage IV. Only about 30% (25/84) of the cohort was on ART at the time of enrolment. GCS was recorded in 61 of cases and 81% (68/84) were normal with a score of 15/15. The median and IQR for CD4 count, and CSF white cell count for the cohort were 56 (13.8–136) cells/μL and 2 (0–10.0) mm3, respectively.
Table 1.
Patient characteristics, clinical presentation and outcome of meningitis associated with HIV-infected patients in Accra, Ghana
| Characteristic | Total cohort (n=84) |
|---|---|
| Age, years† | 40 (34–44) |
| Gender, males‡ | 47 (56) |
| Inpatients‡ | 63 (75) |
| Outpatients‡ | 21 (25) |
| HIV type‡ | |
| HIV-1 | 72 (85.7) |
| HIV-2 | 2 (2.4) |
| Not available | 10 (11.9) |
| Clinical features | |
| cART*‡ | 25 (29.8) |
| WHO clinical staging, IV‡ | 61 (72.6) |
| Glasgow comma score 15/15‡ | 68 (81) |
| Tachycardia‡ | 38 (45.2) |
| Meningismus‡ | 28 (33.3) |
| CD4 cells/μL† | 56 (13.8–136) |
| Seizures‡ | 10 (11.9) |
| Fever‡ | 38 (45.2) |
| Confusion‡ | 22 (26.2) |
| Stiff neck‡ | 19 (22.6) |
| Headache‡ | 65 (77.4) |
| CSF analysis† | |
| Total protein (g/L) | 0.7 (0.35–1.2) |
| Total glucose (mmol/L) | 2.9 (2.5–3.4) |
| Total WBC/mm3 | 2 (0.0–10.0) |
| PMNs (%) | 4.8 (3.4–6.4) |
| Lymphocyte (%) | 22.9 (9.7–38.4) |
| Haematological analysis† | |
| Haemoglobin (g/dL) | 9.8 (8.4–11.2) |
| Platelets | 198 (156–290) |
| Random blood glucose | 6.3 (5.2–8.1) |
| Outcomes at discharge | |
| Survived‡ | 32 (38.1) |
| Died‡ | 30 (35.7) |
| Unknown‡ | 22 (26.2) |
*Currently on anti-retroviral therapy.
†Median and interquartile range.
‡Number, percentage.
PMNs, polymorphonuclear neutrophils.
Patients were primarily managed based on clinical diagnosis and fluconazole (400 mg/day) was administered empirically to a majority of 54.8% (46/84) of these patients. Other empirical therapy administered included sulphadiazine and pyrimethamine, ceftriaxone and anti-tuberculosis drugs. Hospitalized patients spent between 3 and 52 days on admission before discharge or demise. The outcomes after hospital discharge of 22 (26.2%) of the cohort were unknown, but 30 died during hospitalization giving a case fatality of 37.5% for suspected meningitis.
Aetiologies detected in CSF
Table 2 gives a detailed summary of the 51 patients from whom CSF aetiology was detected out of 84 CSF samples obtained, including HIV viral load, CD4+ T cell count and other CSF parameters. In 60.7% (51.84) of patients, evidence of microbiological co-infection was detected, some with multiple microorganisms. The distribution of the organisms in the 51 patients were as follows:
Toxo—25% (21/84 tested);
EBV—45.2% (38/84 tested);
CMV— 7.1% (6/84 tested) and Crypto—6% (5/84 tested).
Table 2.
Clinical presentation, diagnostic parameters and outcomes of patients with microbiological aetiologies in cerebrospinal fluid
| Codified ID | Age/years | Sex | Patient category (IN/OUT) | HIV type (1/2) | HIV viral load/copies | Aetiology | mCD4/cells/μL | cART | WHO staging | GCS | CSF protein/g/L | CSF glucose/mmol/L | CSF WBC/mm3 | Initial therapy administered based on clinical diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CM077 | 39 | F | OUT | 1 | 1.01×10^6 | CMV | 61 | Yes | IV | 15/15 | 0.77 | 2.7 | 1 | – | Unknown |
| CMO92 | 39 | F | IN | 1 | 1.07×10^6 | CMV | 1 | No | III | 15/15 | 1.77 | 2.3 | 1 | Oral fluconazole | Died |
| CM086 | 37 | F | IN | 1 | 3.38×10^3 | Crypto | 1 | Yes | IV | 15/15 | 1.03 | 1.9 | 27 | Oral fluconazole | Survived |
| CM085 | 44 | M | IN | 1 | ND | Crypto | 27 | No | III | 15/15 | 0.49 | 2.3 | 0 | IV fluconazole | Died |
| #CM014 | 31 | F | OUT | 1 | 1.94×10^3 | EBV | 132 | No | III | 15/15 | 0.94 | 4.3 | 16 | – | Unknown |
| CM018 | 38 | F | OUT | 1 | 3.78×10^4 | EBV | 106 | No | III | 15/15 | 0.31 | 3.5 | 2 | – | Unknown |
| CM032 | 35 | F | IN | 1&2 | 3.80×10^5 | EBV | 133 | No | IV | 15/15 | 2.8 | 1.6 | 22 | Oral fluconazole | Survived |
| CM035 | 48 | F | IN | 1 | 8.66×10^2 | EBV | ND | Yes | IV | 15/15 | 2.5 | 1.1 | 108 | IV ceftriaxone | Survived |
| CM037 | 44 | F | IN | 1 | 1.44×10^5 | EBV | 290 | Yes | IV | 15/15 | 0.42 | 3.3 | 1 | Oral fluconazole | Survived |
| CM044 | 44 | F | IN | 1 | 2.59×10^3 | EBV | 169 | No | IV | 15/15 | 5.84 | 2.8 | 250 | oral fluconazole | Died |
| CM050 | 34 | F | IN | 1 | ND | EBV | 20 | No | IV | 14/15 | UN | 2.7 | 0 | Oral fluconazole | Died |
| CM053 | 38 | F | OUT | ND | 7.49×10^2 | EBV | 118 | Yes | IV | 15/15 | 0.055 | 2.9 | 17 | Oral fluconazole | Unknown |
| CM054 | 45 | F | IN | ND | 1.78×10^6 | EBV | 483 | No | IV | 15/15 | 0.28 | 2.1 | 0 | Oral fluconazole | Survived |
| CM064 | 44 | F | OUT | 1 | 2.08×10^5 | EBV | 583 | No | IV | 15/15 | UN | UN | ND | – | Unknown |
| CM079 | 33 | F | IN | 1 | 1.03×10^5 | EBV | 108 | No | IV | 15/15 | 0.59 | 3.3 | 0 | Oral fluconazole | Died |
| CM089 | 57 | F | IN | 1 | 2,55×10^5 | EBV | 251 | No | IV | 15/15 | 7.43 | 5.9 | 32 | Oral fluconazole | Survived |
| CM095 | 65 | F | IN | 1 | 1.92×10^5 | EBV | 25 | No | III | 15/15 | 3.56 | 5.2 | 2 | IV ceftriaxone | Died |
| CM098 | 62 | F | IN | 1 | ND | EBV | ND | Yes | IV | 3/15 | 0.79 | 1 | 2 | Oral fluconazole | Died |
| CM099 | 37 | F | IN | 1 | 5.89×10^5 | EBV | ND | No | IV | 15/15 | 2.1 | 1.9 | 240 | Oral fluconazole | Died |
| #CM006 | 35 | M | IN | 1 | 1.61×10^5 | EBV | 175 | No | IV | 14/15 | 2.5 | 4 | 14 | – | Died |
| CM022 | 41 | M | IN | 1 | 3.84×10^4 | EBV | 403 | No | IV | 15/15 | 0.61 | 2.9 | 31 | – | Survived |
| CM027 | 36 | M | IN | 1 | 3.55×10^6 | EBV | ND | No | IV | 14/15 | 0.49 | 4.2 | 0 | Septrin | Died |
| CM033 | 40 | M | IN | 1 | 1.06×10^5 | EBV | 156 | No | IV | 15/15 | 0.24 | 4 | 23 | Oral fluconazole | Survived |
| CM046 | 34 | M | OUT | 1 | 4.30×10^4 | EBV | 80 | No | IV | 15/15 | 0.21 | 2.7 | 5 | Oral fluconazole | Unknown |
| CM048 | 47 | M | IN | 1 | 8.23×10^4 | EBV | 430 | No | IV | 15/15 | 1.4 | 2.6 | 59 | Oral fluconazole | Survived |
| CM093 | 16 | M | IN | 1 | 1.16×10^6 | EBV | 118 | Yes | ND | 15/15 | 3.8 | ND | 3 | Oral fluconazole | Survived |
| CM104 | 37 | M | IN | 1 | ND | EBV | 73 | Yes | III | 15/15 | 4.65 | 2.5 | 10 | Oral fluconazole | Survived |
| #CM001 | 40 | F | OUT | 1 | 1.00×10^6 | Toxo | 51 | No | IV | 15/15 | 0.52 | 4.1 | 56 | – | Unknown |
| #CM012 | 30 | F | IN | ND | 3.61×10^5 | Toxo | ND | No | IV | 15/15 | 3.12 | 2.1 | 0 | – | Survived |
| CM039 | 43 | F | IN | 1 | 8.87×10^4 | Toxo | 9 | No | IV | 15/15 | 0.72 | 1.2 | 6 | Pyrimethamine | Survived |
| CM083 | 38 | F | IN | 1 | ND | Toxo | 38 | Yes | IV | 15/15 | 0.98 | 2.9 | 1 | Oral fluconazole | Survived |
| CM097 | 38 | F | IN | 1 | 2.78×10^6 | Toxo | ND | No | IV | 10/15 | 0.63 | 1.8 | 21 | IV ceftriaxone | Died |
| CM020 | 50 | M | OUT | 1 | 4.25×10^4 | Toxo | 5 | No | IV | 15/15 | 0.81 | 3.1 | 0 | – | Unknown |
| CM073 | 40 | M | IN | 1 | ND | Toxo | 16 | No | IV | 15/15 | 1.11 | 3.1 | 2 | IV fluconazole | Survived |
| #CM004 | 24 | F | IN | 1 | 7.90×10^5 | Toxo/EBV | 66 | No | IV | 15/15 | 9.86 | <1.1 | 3 | – | Died |
| CM047 | 43 | F | IN | 1 | 4.66×10^4 | Toxo/EBV | ND | No | IV | 15/15 | 0.52 | 2.7 | 21 | – | Survived |
| CM059 | 51 | F | IN | 1 | ND | Toxo/EBV | 27 | No | IV | 15/15 | 0.7 | 2.8 | 20 | Oral fluconazole | Survived |
| CM060 | 27 | F | OUT | 1 | 2.23×10^5 | Toxo/EBV | 42 | No | IV | 15/15 | 0.24 | 2.9 | 1 | – | Unknown |
| CM066 | 32 | F | IN | 1 | 1.73×10^5 | Toxo/EBV | 69 | No | IV | 14/15 | 1.8 | 2.8 | 8 | IV fluconazole | Survived |
| CM068 | 49 | F | IN | ND | 9.29×10^4 | Toxo/EBV | 21 | Yes | ND | 15/15 | 0.59 | 2.7 | 0 | IV ceftriaxone | Survived |
| #CM005 | 45 | M | IN | 1 | 9.40×10^4 | Toxo/EBV | ND | No | IV | 12/15 | 1 | 3.2 | 1 | – | Died |
| #CM007 | 47 | M | IN | 1 | 2.40×10^5 | Toxo/EBV | ND | No | IV | 15/15 | 0.69 | 3 | 1 | Sulphadiazine | Survived |
| CM029 | 42 | M | IN | 1 | 2.82×10^5 | Toxo/EBV | 6 | No | IV | 11/15 | 0.73 | 5 | 2 | Oral fluconazole | Survived |
| CM045 | 38 | M | OUT | 1 | 4.21×10^3 | Toxo/EBV | 175 | Yes | IV | 15/15 | 1.12 | 3.3 | 6 | – | Unknown |
| CM051 | 26 | M | IN | 1 | 2.35×10^6 | Toxo/EBV | ND | No | IV | 12/15 | 0.14 | 3.7 | 0 | Oral fluconazole | Died |
| CM075 | 29 | M | IN | 1 | 3.93×10^5 | Toxo/EBV | 73 | No | IV | 14/15 | 0.78 | 8.8 | 2 | – | Died |
| CM103 | 34 | F | IN | 1 | ND | Toxo/CMV | 247 | Yes | IV | 15/15 | 2.19 | 2.7 | 10 | Oral fluconazole | Died |
| CM021 | 54 | M | IN | 1 | 5.71×10^2 | EBV/CMV | 76 | No | IV | 15/15 | 2.3 | 1.7 | 28 | Oral fluconazole | Died |
| CM088 | 42 | M | IN | ND | ND | EBV/Crypto | 3 | Yes | IV | 15/15 | 1.25 | 3.2 | 6 | Oral fluconazole | Died |
| CM076 | 42 | F | IN | 1 | 6.23×10^6 | CMV/Crypto | 65 | No | IV | 15/15 | 0.37 | 1.6 | 3 | IV ceftriaxone | Survived |
| CM011† | 42 | M | IN | 1 | 3.09×10^5 | Toxo/EBV/Crypto | ND | No | IV | 15/15 | IF | 3.4 | 5 | Anti-TB | Died |
IN/OUT, inpatient or outpatient; CMV, cytomegalovirus; Crypto, Cryptococcus, EBV, Epstein–Barr virus; GCS, Glasgow Coma Scale; WBC, white cell count
mCD4, most recent CD4 count before recruitment into study; cART, patient on antiretroviral therapy prior to enrolment; ^, exponential; ND, not done; IF, insufficient CSF; IV, intravenous.
†CSF GeneXpertTB assay for Mycobacterium tuberculosis was performed, but was negative.
–, Indicates no data.
CSF co-infection distribution was as follows:
Toxo/EBV—15.5% (13/84);
Toxo/CMV—2.4% (2/84);
EBV/Crypto, CMV/Crypto, and Toxo/EBV/CMV were approximately 1% each.
Interestingly, culture, CrAgLFA assay and India ink tests detected Cryptococcus with equal sensitivities with positive results in all samples tested. While bacterial cultures were negative in all specimens, Gram stain of three CSF deposits demonstrated Gram-positive diplococci, suggestive of a bacterial aetiology. Other co-infection, predominately Cryptococcus was also detected in these three patients with bacterial aetiology. In 31 of 84 (36.9%) patients no co-infection was identified. CSF GeneXpertTB assay, performed on the first 17 consecutive patients did not identify MTB. Other co-infections were identified in eight of this subset cohort (Table 2).
Clinical outcomes
A total of 47.6% (30/64) hospitalized patients died on admission, while 52.4% (32/64) survived and were discharged home, after spending an average of 16.9 days in hospital. For those who died in hospital, the median duration of hospitalization was 15 (3–61) days. Records on post-discharge outcomes were not available. Patients with toxoplasma identified by PCR had a mortality rate of 25% (6/16), for which mortality data was available and three of five patients with Cryptococcus (60%). EBV co-infection alone was associated with 44% deaths (8/18) for which data was available. For the 33 patients without a microbiological aetiology in CSF, a majority 63.6% (21/33) were inpatients that were hospitalized between 3 and 41 days (median=15 days). Their CSF white cell counts and CD4 counts were generally low, 1 (0–312 mm3) and 27 (1–760 cells/μL; median and IQ range), respectively. A third (11 of 33) of these patients died in hospital.
Discussion
CSF aetiologies
Symptoms of opportunistic infections in HIV/AIDS patients may be non-specific, providing little to suggest possible infecting microorganisms. Knowledge of the epidemiological incidence of infecting agents in a given community is thus invaluable to decisions regarding empiric therapy. In the current study, real-time PCR detected CSF co-infections that would have otherwise remained undetected by routine diagnostics available in resource-limited settings, such as in Ghana. Cerebral toxoplasmosis and cryptococcosis may be responsible for unrecognized infections in Ghana, similar to findings from other countries within sub-Saharan Africa and Asia.13 The high prevalence of CSF co-infection including CMV and EBV in our cohort also suggest severe immunosuppression. Multiple factors including low ART coverage and late presentation of patients to hospitals may account for this. ART coverage in sub-Sahara Africa is between 10 and 25%.14 In Ghana, only accredited facilities provide ART services to HIV patients mostly through National HIV Control Programmes. Less than 30% of our cohort was on ART at the time of enrolment, and among patients with a CSF aetiology, just about 25% of them were on ART. Scaling up of ART services in Ghana may be required, but should be supported by a comprehensive research. Though achievable, the 90-90-90 ambitious treatment target to help end the AIDs epidemic15 appears to be far from reality, especially in low-income-middle-countries (LIMCs).
Toxoplasma was identified in more than 25% of our patients, some patients co-infected with EBV, CMV and Cryptococcus. Cerebral toxoplasmosis may be responsible for hospitalization and CNS infections among HIV patients seeking healthcare at hospitals in Accra,8 although lack of imaging prevented the diagnosis of typical mass lesions associated with this organism. In the sub-Saharan African region, the few studies conducted on T. gondii in HIV patients focused only on seroprevalence. A previous study in Ghana reported a relatively high (54.7%) active T. gondii antibodies or antigen among HIV seropositive treatment naive individuals, but low (3%) prevalence among blood donors.8 In a 2-year retrospective study in Abidjan, enrolling 447 HIV patients, cerebral toxoplasmosis (17.9%) and cryptococcal (8%) was observed.16 In a similar 6-year retrospective study in Kenya, enrolling 708 HIV patients, the prevalence of cerebral toxoplasmosis was (12.7%) and Cryptococcus (22%).17 Data available from the few sub-Saharan studies show that the leading cause of adult meningitis varies according to geographical region. Around the West African regions, cerebral toxoplasmosis tops aetiologies associated with CNS opportunistic infection, while Cryptococcus is often found in East and South African regions.6,8,16–18 The high amount of Toxoplasma in Ghana may suggest that empiric treatment be considered for those unable to obtain CT scans due to cost constraints.
The relatively high incidence of viral co-infections by EBV and CMV in our cohort is also worth noting, although most did not have high levels of virus. The literature reports limited data on CSF infections with EBV among HIV/AIDS patients in Africa, with no records from Ghana. Only a handful of studies provided information on EBV seroprevalence in symptomatic HIV/AIDS patients in Ghana, with seroprevalence ranging between 14.6 and 87.2%.19,20 In the current study, EBV was identified in over 45% (38/84) of patients, often co-infected with organisms of higher virulence such as Toxoplasma and Cryptococcus. Studies elsewhere found similar EBV co-infection in the CSF of HIV patients,18,21 and in Malawi, it was associated with an increased risk of death,22 which was also found in the present study and may be indicative of a poor overall performance status. Indeed, EBV has a ubiquitous biology and persists latently in B lymphocytes long after colonization/infection. While EBV is associated with CNS lymphoma in a minority of cases, additional investigation by imaging would be required to elucidate the role of EBV positive PCR findings in CSF samples.23 The current study utilized real-time PCR with established in-house protocols at the clinical centre at the National Institutes of Health in the US, using a high cycle threshold cut-off point for EBV detection. However, no neuroimaging was done as part of this study to evaluate for possible EBV-related pathologies such as lymphoma. CMV prevalence in our cohort was less than 6% and was often co-infected with EBV, Toxoplasma and Cryptococcus. In Uganda, less than 1% CMV prevalence was detected in a subset of patients from whom a further diagnosis was conducted.18 In China, clinical data of CMV among HIV/AIDS patients was relatively high (22%),24 contrary to what is found in African regions.
Cryptococcus infection among our cohort was relatively low, but the mortality rate among these patients was high, typical of that found globally.4,25 All assay methods used, culture, CrAgLFA and India ink staining on CSF samples showed 100% sensitivity. This suggests that, in settings where culture or the CrAgLFA test is not available, India ink microscopy may be reliable. Training and expertise of personnel performing India ink microscopy is, however, critical for accurate and prompt diagnostics. Contrary to the low cryptococcal prevalence in the current study, data from the southern and central African regions have consistently identified Cryptococcus as the leading cause of adult meningitis among HIV/AIDS patients, with prevalence ranging between 27 and 63%.24,25 Why Cryptococcus causes a disproportionately low incidence of meningitis in West Africa remains poorly understood, but could be related to either environmental or genetic factors.
We did not identify any bacterial aetiology by culture of CSF deposits. TB-GeneXpert did not identify any MTB meningitis in the few CSF samples we analysed. However, in the majority of the assays from which no MTB was detected, cerebral toxoplasmosis or viral aetiologies were detected. Meningitis, generally of bacterial aetiology, mounts a high inflammatory response, resulting in CSF white cell counts greater than 5 mm3. Although the current study did not grow any bacteria on culture, some patients had CSF white cell counts less than the accepted diagnostic criteria for meningitis (5 mm3). It is also commonly observed that patients with advanced HIV do not mount a large inflammatory response, and some patients in our cohort may fall into this category, despite their neurological presentations. Based on clinical diagnosis, a handful of the patients in our cohort were given anti-TB drugs. Systematic reviews26 and individual studies27 showed varying sensitivities of MTB detection in CSF; ranging between 51 and 100%. We were constrained with available CSF volumes to perform analysis. However, experts recommend large CSF volumes (ideally 8–10 mL) for GeneExpert testing.28 Sputum microscopy and post-mortem records, however, show that MTB meningitis causes significant morbidity, as well as mortality among HIV/AIDs patients in Ghana.3,29,30 In our setting, patients with low CD4 counts (≤100 cells/μL) are presumptively treated for TB, Toxoplasma, Cryptococcus and bacterial meningitis. Clinicians typically have a high index for TB and patients generally not doing well were given anti-TB drugs as standard of care.3
Clinical presentation and mortality at discharge
Only about a quarter of the patients were on ART, and the most common presenting symptoms included headache, fever, altered mental status and stiff neck. The majority of patients examined were alert and orientated, clinical stage IV with a GCS of 15/15 being common. Although real-time PCR unveiled aetiologies that would have remained undetected with routine diagnosis, these results were unavailable to guide patient management. Even with the additional diagnostic analysis, CSF aetiologies were not detected in approximately 40% of our patient population. Additionally, based on clinical diagnosis alone, fluconazole was administered to over 50% of the patients in our cohort, resulting in poor treatment outcomes even in those with cryptococcal meningitis as described previously.31 Importantly, none of the patients who died with PCR evidence of toxoplasmosis were on therapy for this organism, suggesting that the addition of these agents to empiric regimens for meningitis could improve outcome in regions of West Africa, including Ghana. In addition to education, we recommend co-trimoxazole and fluconazole prophylaxis to patients in Ghana. Furthermore, a more sensitive and available laboratory diagnostics are critically needed to guide the management of HIV patients presenting with meningitis and obviate the need for extensive empiric therapy.
Limitations
This study had several limitations worth mentioning. We enrolled patients with a provisional diagnosis of meningitis. However, HIV patients with advanced disease may not demonstrate any symptomatology of meningitis. Additionally, written informed consent was a critical requirement for participation in our study. We were unable to meet this requirement for a majority of the patients, as many died before enrolment. Occasionally, a complete medical history could not be obtained for patients with an altered mental state and investigations such as CD4 count and HIV viral load was incomplete for most of the patients due to reagent shortages and prohibitive costs of testing in the private sector. Testing for tuberculosis could only be performed in some patients due to lack of reagents for the GeneXpert MTB/RIF assay. Our GeneXpert investigation is, indeed, skewed as relatively few samples were analysed. In addition, chest X-rays, CT scans and post-mortem information on patients who died were unavailable and survivors were not followed-up for possible sequelae. Documentation for immune reconstitution inflammatory syndrome was scanty and outcomes after hospital discharge were not readily available. In spite of the above limitations, our study revealed that cerebral toxoplasmosis is an important unrecognized cause of adult meningitis among HIV infected patients.
Conclusions
In conclusion, HIV-infected patients presenting with symptoms of meningitis were critically ill, requiring hospitalization. Cerebral toxoplasmosis is an important causes of CNS opportunistic infection in our cohort followed by Cryptococcus with important co-morbidities associated with EBV and CMV co-infection. In the absence of point-of-care diagnostics to improve patient management outcomes, empiric therapy for toxoplasmosis may improve outcomes in this patient population.
Acknowledgments
Author’s contributions: JAO and ML conceived the study; JAO, ML, MJN, EK and PRW designed the study; BKA, IJKB, VG, RD, RFA collected the specimens; BKA, MMO, AA, MC, GAF performed microbiological and molecular testing; JAO, MJN, ML, PRW, GAF collated, analysed and interpreted data; JAO drafted the manuscript and undertook revisions; PRW and ML made significant contributions to the preparation and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements: The authors are grateful to Adwoa Obo-Akwa and Ruth A. Yalley for providing technical support. They are thankful to all the staff at the Fevers’ Unit, Korle-Bu Teaching Hospital. Cristina Carvalheiro did toxoplasma screening.
Funding: The Office of Research, Innovation and Development, University of Ghana supported this work [Grant number: URF/7/ILG-043/2013-2014, to JAO]. Partial funding was also received from the Admer project. The Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health also supported this work. The postgraduate committee of the Noguchi Memorial Institute for Medical Research supported BKA, a student at the Medical Microbiology Dept., University of Ghana.
Competing interests: None declared.
Ethics and confidentiality: The Institutional Review and Protocol Review Board of the University of Ghana Medical School, reviewed and approved the study [reference number: MS-Et/M.11-P3.2/2013-2014]. Written informed consent was obtained from all participants/next of kin before enrolment into the study and also for specimen collection and subsequent processing. Participants’ records and laboratory data were codified and kept under lock and key.
References
- 1. Boissé L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin 2008;26:799–819. [DOI] [PubMed] [Google Scholar]
- 2. Jarvis JN, Meintjes G, Williams A et al. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lartey M, Asante-Quashie A, Essel A et al. Causes of death in hospitalized HIV patients in the early anti-retroviral therapy era. Ghana Med J 2015;49:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park BJ, Wannemuehler KA, Marston BJ et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009;23:525–30. [DOI] [PubMed] [Google Scholar]
- 5. Gomerep SS, Idoko JA, Ladep NG et al. Frequency of cryptococcal meningitis in HIV-1 infected patients in north central Nigeria. Niger J Med 2010;19:395–9. [DOI] [PubMed] [Google Scholar]
- 6. Mamoojee Y, Shakoor S, Gorton RL et al. Short communication: low seroprevalence of cryptococcal antigenaemia in patients with advanced HIV infection enrolling in an antiretroviral programme in Ghana. Trop Med Int Health 2011;16:53–66. [DOI] [PubMed] [Google Scholar]
- 7. Owusu M, Nguah SB, Boaitey YA et al. Aetiological agents of cerebrospinal meningitis: a retrospective study from a teaching hospital in Ghana. Ann Clin Microbiol Antimicrob 2012;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayi I, Kwofie KD, Blay EA et al. Clonal types of Toxoplasma gondii among immune compromised and immune competent individuals in Accra, Ghana. Parasitol Int 2016;65:238–44. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: WHO, 2007. http://apps.who.int/iris/handle/10665/43699 [accessed 28 March 2017].
- 10. Cassaing S, Bessières MH, Berry A et al. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J Clin Microbiol 2006;44:720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klion AD, Mejia R, Cowen EW et al. Chronic active Epstein–Barr virus infection: a novel cause of lymphocytic variant hypereosinophilic syndrome. Blood 2013;121:2364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JI, Fahle G, Kemp MA et al. Human herpesvirus 6-A, 6-B, and 7 in vitreous fluid samples. J Med Virol 2010;82:996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wall EC, Cartwright K, Scarborough M et al. High mortality amongst adolescents and adults with bacterial meningitis in sub-Saharan Africa: an analysis of 715 cases from Malawi. PLoS One 2013;8:e69783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. HIV/AIDS. Antiretroviral therapy coverage in sub-Saharan Africa. Available at: http://www.who.int/hiv/data/art_coverage/en/ [accessed 5 March 2017].
- 15. Lima VD, St-Jean M, Rozada I et al. Progress towards the United Nations 90-90-90 and 95-95-95 targets: the experience in British Columbia, Canada. J Int AIDS Soc 2017;20:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kra O, Aba YT, Yao KH et al. Clinical, biological, therapeutic and evolving profile of patients with HIV infection hospitalized at Infectious and tropical diseases unit in Abidjan (Ivory Coast). Bull Soc Pathol Exot 2013;106:37–42. [DOI] [PubMed] [Google Scholar]
- 17. Jowi JO, Mativo PM, Musoke SS. Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi hospital. East Afr Med J 2007;84:67–76. [DOI] [PubMed] [Google Scholar]
- 18. Rajasingham R, Rhein J, Klammer K et al. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg 2015;92:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adjei AA, Armah HB, Gbagbo F et al. Seroprevalence of HHV-8, CMV, and EBV among the general population in Ghana, West Africa. BMC Infect Dis 2008;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Compston LI, Li C, Sarkodie F et al. Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J Med Virol 2009;81:1860–8. [DOI] [PubMed] [Google Scholar]
- 21. Siddiqi OK, Ghebremichael M, Dang X et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis 2014;58:1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly MJ, Benjamin LA, Cartwright K et al. Epstein–Barr virus coinfection in cerebrospinal fluid is associated with increased mortality in Malawian adults with bacterial meningitis. J Infect Dis 2012;205:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimura H, Ito Y, Suzuki R, et al. Measuring Epstein–Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol 2008;18:305–19. [DOI] [PubMed] [Google Scholar]
- 24. Xiao J, Gao G, Li Y et al. Spectrums of opportunistic infections and malignancies in HIV-infected patients in tertiary care hospital, China. PLoS One 2013;8:e75915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson PR. The relentless march of cryptococcal meningitis. Lancet Infect Dis 2017;17:790–1. [DOI] [PubMed] [Google Scholar]
- 26. Denkinger CM, Schumacher SG, Boehme CC et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014;44:435–46. [DOI] [PubMed] [Google Scholar]
- 27. Patel VB, Theron G, Lenders L et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 2013;10:e1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bahr NC, Marais S, Caws M et al. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clin Infect Dis 2016;62:1133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bjerrum S, Kenu E, Lartey M et al. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 2015;15:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarfo FS, Sarfo MA, Norman B et al. Risk of deaths, AIDS-defining and non-AIDS defining events among Ghanaians on long-term combination antiretroviral therapy. PLoS One 2014;9:e111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bicanic T, Meintjes G, Wood R et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007;45:76–80. [DOI] [PubMed] [Google Scholar]