Abstract
Human health is intricately intertwined with the composition and function of the trillions of microorganisms that make up the gastrointestinal (GI) microbiome. The GI microbiome is essentially a microbial organ that provides metabolic, immunologic, and protective functions for the host. Habitual diet, changes in macronutrient composition, and consumption of nondigestible dietary fibers have all been shown to impact the human GI microbiome. Intriguingly, the impact of diet on the microbiome may be related not only to what humans eat but also to the timing of food consumption. Emerging preclinical research suggests that gut microbes experience diurnal rhythms, and the health effects of eating patterns, including time-restricted feeding and meal frequency, may be related to the GI microbiome. Herein, the complex connections among circadian rhythms, eating behaviors, the GI microbiome, and health are reviewed, highlighting the need for additional translational research in this area.
Keywords: circadian rhythm, eating frequency, eating patterns, jet lag, microbiome, shift work, time-restricted feeding
INTRODUCTION
The impact of diet on the composition and function of the gastrointestinal (GI) microbial community and human health is an area of rapidly evolving research that is particularly important as the cost and prevalence of chronic diseases rise to staggering figures and standard, efficacious treatments such as dietary and physical activity modifications are infrequently prescribed and even less frequently followed.1 The trillions of microbes that make up the GI microbiome form a microbial organ with a collective gene set 150 times larger than the human genome, providing metabolic, immunologic, and protective functions for the host.2 In addition to contributing to immunologic development and metabolic function, the GI microbiome also influences nervous system development and function.3 Furthermore, the composition and function of the GI microbiome have been shown to be linked to a growing list of metabolic diseases, including obesity, diabetes, and cardiovascular disease.4,5
Diet is an important mediator of the human GI microbiota; habitual intake,6 rapid changes in dietary fat and fiber composition,7 and consumption of dietary fibers8–10 and other nondigestible food components have all been shown to impact both the composition and function of these resident microbes.5,11 Intriguingly, the microbiome may be affected not only by what is eaten but also by when food is consumed. Host–symbiont bidirectional communication occurs via signaling along the gut–microbiota–brain axis by a variety of bacterial metabolites, which have been shown to impact centrally mediated feeding behaviors such as appetite.12–14
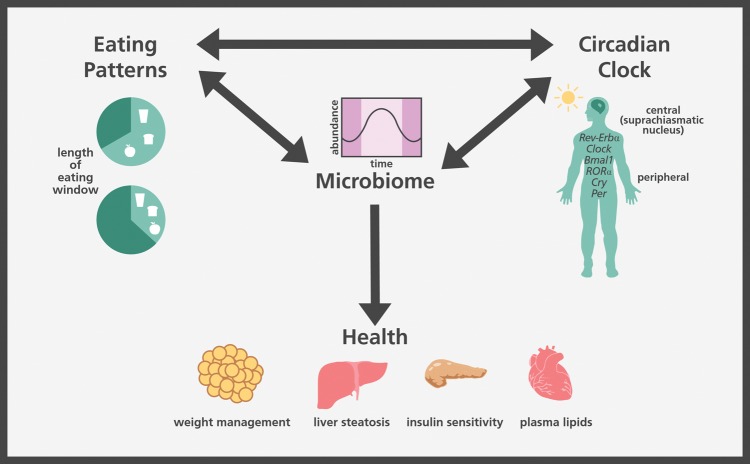
Increasingly, preclinical research has demonstrated that the bacteria in the GI tract vary over the course of a day, with relative abundances of bacterial taxa, proximity of bacteria to the colonic epithelium, and microbial metabolism all exhibiting diurnal rhythms.15,16 Time of eating is considered a potential modulator of circadian rhythms with an effect on both bacterial abundance and function.17 Furthermore, the gut microbiome appears to have a reciprocal relationship with the human body’s circadian clock and eating patterns (Figure 1). Emerging research suggests that some of the observed health effects related to eating patterns, such as time-restricted feeding (TRF) and meal frequency, may also be related to the GI microbiome. Herein, preclinical and clinical research on circadian rhythms, eating behaviors, and the GI microbiome is reviewed.
Figure 1.
Connections between the internal clock, eating patterns, the microbiome, and health.
CIRCADIAN RHYTHMS
Most of the life on Earth experiences a daily 24-hour light/dark cycle created by Earth’s rotation in relation to the sun and, as a result, engages in a 24-hour cycle of feeding and fasting.18 Circadian rhythms are cycles of gene expression, metabolism, and behaviors created by the internal clock that govern a multitude of metabolic functions such as hepatic lipid metabolism, cardiovascular function, obesity regulation, and glucose homeostasis.19,20 Circadian rhythms are regulated in humans in 2 ways: (1) by light via the suprachiasmatic nucleus in the brain and (2) by clock proteins that are present in nearly every cell and provide a transcriptional rhythm based on a 24-hour day.21 The suprachiasmatic nucleus also regulates the circadian release of digestive peptides, including vasoactive intestinal polypeptide and gastrin-releasing peptide.21 Almost all human cells have circadian regulatory genes, including Clock, Bmal1, RORα, Cry, Per, and Rev-erbα.21 In mice, 45% of transcripts have 24-hour oscillations.21 The homeostatic sleep/wake cycles of the central nervous system, combined with pituitary gland activity, exert substantial influences on the endocrine system.21
Health implications of misaligned circadian rhythms
The natural condition of most living organisms on Earth is to spend one phase of a 24-hour day (either light or dark) in an active and feeding state and the other in a resting and fasting state.18 Humans naturally spend the light phase in the active and feeding state and the dark phase in the resting and fasting state. However, with the development of artificial light, humans have deviated from the original pattern of eating only during the light portion of the day. Furthermore, individuals who work night shifts experience an almost complete reversal of food intake, with intake occurring primarily during the night and rest and fasting occurring during daylight hours. Dietary intake that is misaligned with the natural rhythms of the circadian clock has been shown to negatively impact human health. Specifically, disruptions to the normal sleep/wake cycle in relation to the night/day cycle of Earth, as seen in shift work, are associated with a 40%–60% increased risk for obesity and metabolic syndrome.22,23
A misaligned circadian rhythm is one in which the normal schedule of feeding and fasting is disrupted. Circadian rhythms can be misaligned through environmental conditions, such as shift work or jet lag, or through genetic manipulation of relevant genes as in the case of the creation of knockout models in preclinical research. The importance of cellular clock mechanisms to health has been demonstrated in Bmal1 knockout mice and Rev-erbα and Rev-erbβ double-knockout mice, which display dysmetabolism in glucose and lipid homeostasis, respectively.21 Restricting the food access of wild-type mice to only the light phase (when mice are normally not active or eating) resulted in a 23% increase in weight gain and an 8% higher body fat percentage in mice that had their environmental conditions manipulated compared with mice that had access to food during their normal active phase.24 Thaiss et al.25 reported that circadian-disrupted mice fed a high-fat diet had 17% greater body weight than nondisrupted mice on a similar diet. Antibiotic-treated mice were resistant to these detrimental metabolic changes, suggesting a connection between misaligned rhythms and the microbiome.25
Addition of dim light to an animal’s habitual dark phase has also been shown to disrupt circadian rhythms and thus metabolism. Mice housed in dim light conditions have been shown to increase energy consumption during the light phase to 55% of total intake, compared with 36% in mice kept in standard light/dark phase conditions. Body mass and insulin resistance were also greater in mice exposed to both dim light and constant light situations when compared with mice exposed to standard light/dark conditions, despite the use of isocaloric diets and matched physical activity levels.26 Chronic sleep fragmentation, a model of obstructive sleep apnea, also affects both the murine microbiome and health.27 Poroyko et al.27 demonstrated that mice exposed to chronic sleep fragmentation by tactile stimulation every 2 minutes during the sleep phase had increased food intake and decreased colonic barrier function and that transplantation of the Firmicutes-enriched microbiome of these animals into germ-free animals resulted in enhanced inflammatory response and insulin resistance in the recipient animals. Recent work has also examined sleep restriction in both mice and a small group of humans.28 The authors found that, after mice and humans were restriced to 4 hours of sleep per night for 5 nights, there were minor changes in the murine microbiota but no changes in the human microbiota (n = 11). They concluded that, although weight and behavioral alertness were impacted in humans, the microbiota was resistant to sleep restriction–induced change. However, because of the small sample size of human participants and the acute nature of the study, the study may have not been adequately powered to detect changes in the highly individualistic human microbiota.29
Results from preclinical circadian rhythm misalignment studies are further supported by clinical studies that reveal associations between specific single nucleotide polymorphisms in the Clock gene and risk for metabolic syndrome.30 There are 3 known polymorphisms of this gene, which are relatively equally distributed throughout the population, and haplotype is significantly associated (P = 0.02) with presence of metabolic syndrome.30 The functional role of these single nucleotide polymorphisms has not yet been elucidated.21 Furthermore, it has been demonstrated in shift workers, both observationally and in a laboratory setting, that working at times other than daylight hours negatively impacts health outcomes, including a 66% elevated risk of obesity and a 57% elevated risk of metabolic syndrome.23 Shift workers also have been shown to have 12%–16% reduced energy expenditure,31 and they are more likely to experience dyslipidemia, including elevated blood triglycerides and reduced high-density lipoprotein cholesterol.22 In 1 experiment, human participants experiencing circadian misalignment through a 12-hour reversal from their habitual schedule had 17% lower leptin concentrations compared with their values under circadian alignment. Furthermore, insulin concentrations, glucose concentrations, and mean arterial pressure were 22%, 6%, and 3% higher, respectively, under misalignment than under alignment.32 A summary of clinical findings of misaligned circadian rhythms can be found in Table 1.21,23,25,28,31,32
Table 1.
Summary of human studies of misaligned circadian rhythms
| Relationship | Reference |
|---|---|
| SNPs of Clock gene ↑ or ↓ risk of metabolic syndrome | Zarrinpar et al. (2016)21 |
| Shift work ↑ risk of obesity and metabolic syndrome | Wang et al. (2014)23 |
| Shift work ↓ daily energy expenditure | McHill et al. (2014)31 |
| Shift work | Scheer et al. (2009)32 |
| ↓ leptin | |
| ↑ insulin | |
| ↑ glucose | |
| ↑ mean arterial pressure | |
| Sleep restriction ↑ BMI ↓ alertness No change in microbiota | Zhang et al. (2017)28 |
| Jet lag ↑ relative abundance of Firmicutes ↑ weight gain and blood glucose in mice receiving transplants from jetlagged humans | Thaiss et al. (2014)25 |
Abbreviations: BMI, body mass index; SNP, single-nucleotide polymorphism.
Impact of misaligned circadian rhythms and diet on the microbiota
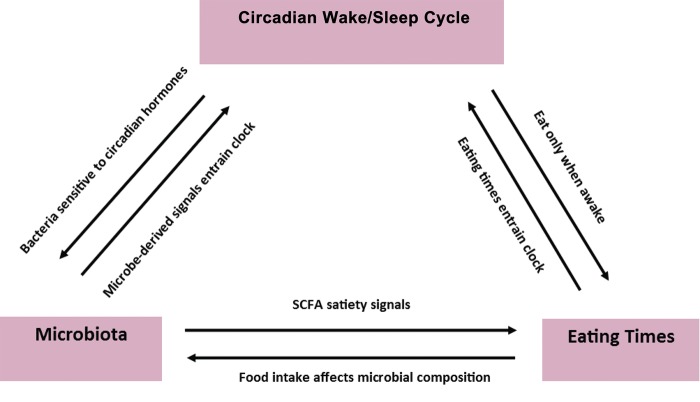
There is increasing evidence that the microbiota, diet, circadian rhythms, and internal clock mechanisms are connected (Figure 2). Interactions among these factors can be explored through the provision of continuous intravenous nutritional support, which eliminates effects of feeding time or even intestinal presence of food on the GI microbiome. Interestingly, mice given continuous parenteral nutrition have been shown to have substantial changes in microbial community structure, but the microbiota did not completely lose diurnal variation.33 Specifically, although beta diversity indices, a measure of dissimilarity or distance between samples, revealed clustering by treatment group when enteral and parenteral nutrition were compared, samples also clustered by time of day within each treatment group. Additionally, relative abundances of different phyla varied between treatments, with Verrucomicrobia dramatically blooming at the expense of Firmicutes in the parenteral group. For Bacteroidetes, a cyclical abundance pattern was observed over the course of the day, increasing during the light phase and decreasing during the dark phase; this occurred independent of the study treatment provided.
Figure 2.
Integration of circadian homeostasis with eating patterns and the microbiota. Abbreviation: SCFA, short-chain fatty acids.
Research has also been conducted to investigate interactions among environmental circadian rhythm disruption, diet, and the GI microbiome in murine models.34 Continuous circadian rhythm disruption was induced by reversing the 12-hour light/dark cycle every week for 12 weeks in mice receiving chow or a high-fat/high-sugar diet. Mice in the misalignment group experienced intestinal hyperpermeability and disrupted circadian gene expression in intestinal cells. Cycle shifting resulted in significant weight gain in mice consuming chow (P = 0.04), but there were no significant changes in GI microbial composition of these animals (P = 0.81). There were, however, significant differences in microbial community composition between high-fat shifted and high-fat nonshifted groups (P = 0.04). The high-fat shifted mice had decreased alpha diversity, a measure of the number of different microbial species (richness) within a sample and their relative abundances (evenness), and a higher ratio of Firmicutes/Bacteriodetes compared with the other high-fat diet fed mice who were not exposed to a light/dark cycle shift. This specific microbial ratio has been associated with obesity or ill health in both rodent models and humans.34 These results suggest that a combination of a high-fat diet and circadian disruption may be what drives microbial dysbiosis in mice.
A “jet lag” model has also been used to explore the effects of shorter (eg, ≤12 h) circadian cycle disruptions. In 1 study, mice exposed to an 8-hour shift in the light/dark cycle every 3 days for 4 weeks experienced a loss of rhythmicity in oscillating GI bacterial taxa.25 Microbiome community composition was impacted after 4 weeks of this jet lag model intervention, and dysbiosis was even more pronounced by 16 weeks. Fecal transplant of jet-lagged mouse microbiome into germ-free mice resulted in weight gain and glucose intolerance in the recipient animals.25
Interestingly, there is evidence of bacteria containing clock genes35 and regulating the behavior of their host in a circadian manner.36 For example, Enterobacter aerogenes is purported to contain an endogenous circadian clock that synchronizes with the human host through melatonin secreted into the GI tract.37 In mice, reprogramming of the hepatic circadian clock that occurred following a high-fat diet intervention has been attributed to microbiota-driven induction and activation of the transcription factor PPARγ.38 Both liver and intestinal circadian genes are affected by unconjugated bile acids, known products of microbial metabolism.39 The absence of a microbiota, both in germ-free and antibiotic-induced murine models, has been shown to alter intestinal epithelial cell transcription of nuclear receptors and clock components such as Rev-erbα, RORα, Bmal1, Cry1, Per1, and Per2.40 Additionally, these microbiota-deficient mice have decreased insulin concentrations and elevated blood glucose, triglyceride, and free fatty-acid concentrations as a result of intestinal corticosterone overproduction.40 Circadian regulation within ileal and colonic epithelial cells has been found to be completely disrupted in animals without a microbiota.40 The researchers theorized that microbe-associated molecular patterns are released from the microbiota in a continuous fashion, but Toll-like receptors translate this information into rhythmic signals of gene expression.40 Conversely, Leone et al.33 asserted that circadian shifts in bacterial composition result in corresponding shifts in concentration of bacterial metabolites, such as butyrate, which peaks during fasting, and hydrogen sulfide, which peaks during feeding. Fecal butyrate has been shown to cycle in mice on a standard, but not high-fat, diet, whereas hydrogen sulfide has been shown to exhibit cyclical behavior in the ceca of mice on a high-fat, but not normal, diet. It has been previously demonstrated in vitro that these metabolites can directly impact the cycling of hepatic clock genes Per2 and Bmal1.33 Taken together, the negative consequences observed following circadian disruption may be related to inflammatory processes caused by alterations in intestinal barrier function, increased abundances of proinflammatory bacteria, and etiologies of circadian disruption–related diseases.17
There is emerging evidence that the circadian clock impacts eating time among humans.25 It has also been established that habitual diet and dietary alterations affect the GI microbial composition.5 Given these relationships, it is purported that changes to the circadian clock and/or feeding time may impact the human gut microbiome. However, to date, there is only preliminary data on time shift–induced microbiota dysbiosis in humans. Jet lag induced by flying ahead 8 time zones resulted in changes in microbial abundances, including a higher relative abundance of Firmicutes, in 2 individuals.25 Observed microbial changes in these jet-lagged individuals resolved within 2 weeks after landing.25 Fecal transplant from jet-lagged human participants into germ-free mice resulted in weight gain that was 37% greater and peak blood glucose concentrations that were 35% higher during an oral glucose challenge than the levels in mice that received fecal material from the same individuals taken before jet lag occurred.25 Although the sample size was small in this study, the similarities to results from animal trials are promising.
TIME-RESTRICTED FEEDING
Time-restricted feeding is defined as the consumption of as much food as desired by a person or animal during a specific window of time, followed by denial of food access during a subsequent period.41 A common theory for the observed benefits of TRF is that it mimics natural eating patterns based on circadian rhythms, the way humans ate before artificial lighting and high-energy foods became available 24 hours a day.21,26 TRF among humans results in food being consumed during the light phase when the body is in the active state and not consumed during the dark phase when the body is ready to rest and repair. Thus the body receives the energy it needs when it is metabolically expecting and prepared for it. Evidence suggests that there are protective effects on weight, blood lipids, and glucose homeostasis associated with eating only within a specific window of the day (eg, TRF).42,43
Health implications of time-restricted feeding
Much of the research on TRF has been conducted in animal models, commonly through the use of diet-induced-obesity murine models involving a high-fat diet intervention. When mice are given ad libitum access to normal chow, intake occurs almost entirely during the dark phase, and the mice consume an adequate amount of energy for their needs and maintain normal body weight.44,45 However, when mice are given ad libitum access to high-fat chow, the tendency to eat only during the dark phase disappears.15,45,46 Contrary to their natural rhythm, the mice eat around the clock, which results in obesity and metabolic dysfunction. Introducing TRF in the context of a high-fat diet has been shown to reverse many detrimental metabolic consequences.41,46 Mice provided 8-hour access to a high-fat diet, for example, were protected against obesity, hyperinsulinemia, hepatic steatosis, and inflammation, despite consuming an equivalent number of calories from identical diets as the animals in the 24-hour-access treatment arm.46 Time-restricted feeding has also been linked to 26%–62% lower fat mass, 60% lower postprandial glucose concentrations, and a 93% reduction in insulin resistance in mice undergoing a 6-month TRF intervention compared with ad libitum–fed controls.41
In humans, TRF has been shown to result in modest weight reductions (1%–3%) when food intake is limited to a window of 10–12 hours; small to moderate (5%–31%) improvements in plasma lipid measures (low-density lipoprotein [LDL], high-density lipoprotein, triglyceride, and total cholesterol) when food intake is limited to windows of 7–8 hours and 10–12 hours; and improved insulin sensitivity and fasting blood glucose concentrations across interventions with food intake limited to windows ranging from 4 hours to 10–12 hours.42 Recently, a study in healthy, overweight adults demonstrated that decreasing the food intake window from approximately 14 hours per day to 10–11 hours for a 16-week period resulted in an average weight loss of 3.27 kg.43 Clinical studies of TRF are summarized in Table 2.25,42,43
Table 2.
Summary of human studies of time-restricted feeding
| Relationship | Reference |
|---|---|
| 4-h feeding window ↑ insulin sensitivity | Rothschild et al. (2014)42 |
| 7–8-h feeding window | Rothschild et al. (2014)42 |
| ↑ insulin sensitivity | |
| ↑ HDL cholesterol | |
| ↓ LDL cholesterol | |
| ↓ triglycerides | |
| ↓ total cholesterol | |
| 10–12-h feeding window | Rothschild et al. (2014)42 |
| ↓ weight | |
| ↑ insulin sensitivity | |
| ↑ HDL cholesterol | |
| ↓ LDL cholesterol | |
| ↓ triglycerides | |
| ↓ total cholesterol | |
| 10–11-h window vs 14-h window ↓ weight | Gill et al. (2015)43 |
| Human microbiome displays cyclical behavior, likely as a result of feeding times | Thaiss et al. (2014)25 |
Impact of time-restricted feeding on the microbiota
In addition to impacting health, restricting the time of food access has also been shown to affect the GI microbial community structure in mice. In healthy mice fed a standard chow diet ad libitum, 17% of bacterial operational taxonomic units (OTUs) showed cyclical behavior, with 20%–83% of bacterial sequences at a single point in time belonging to cyclical OTUs.15 Cyclical behavior among bacteria may be a result of microbial adaptation to the availability of food in the intestine at different points in the day.25 Firmicutes were found to peak during the normal murine feeding phase (eg, dark), and Bacteroidetes peaked during the normal fasting phase (eg, light).15 Interestingly, a reduced Bacteroidetes/Firmicutes ratio is associated with increased body weight and obesity in rodent models and humans. Fecal transplants in gnotobiotic mice suggest that these perturbations increase energy harvest and weight gain.4 Another major phylum that displayed cyclical behavior is Verrucomicrobia, which followed the same pattern as Bacteroidetes of peaking in relative abundance during the fasting phase. Interestingly, this phylum contains the species Akkermansia muciniphila, which has been associated with positive health outcomes, such as improved glucose homeostasis and decreased inflammation.47–49
Preclinical research has demonstrated that feeding pattern alterations also disrupt the cyclical nature of OTUs. Mice fed a high-fat diet ad libitum alter their daytime fast/nighttime feed behaviors.15,45,46 These mice also lose much of their normal OTU cycling.15 Although TRF of a high-fat diet may be beneficial metabolically, it did not completely restore OTU cycling.15 This is of note because others have reported that microbiome alterations can persist for longer than dietary interventions and even longer than the metabolic consequences of dysbiosis.50 TRF did, however, decrease the relative abundances of several presumed obesogenic microbes, such as Lactobacillus and Lactococcus species, and increase the abundances of presumed obesity-protective bacteria, such as Oscillibacter and other Ruminococcaceae species.15
Mice lacking a circadian clock through knockout of the Per1 and Per2 genes that were given ad libitum food access ate irregularly and had lower alpha diversity.25 These circadian clock–absent mice also showed a distinct microbial community and lacked microbial cycling. When placed on a TRF regime, in either the light or dark phase, the cycling of the microbiota was restored. Several bacteria, including Bacteroides and Lactobacillus reuteri, showed cycling in these Per1/Per2–deficient animals under TRF, but the time of peaks and troughs was reversed in animals under TRF in the dark phase compared with TRF in the light phase. This further confirms the impact of feeding times on GI microbial composition within murine models.
To the best of the authors’ knowledge, there are no published studies on the effect of TRF on the human GI microbiota. Given the preclinical findings related to relationships between the microbiota and TRF, it is probable that TRF interventions in humans would impact the microbiota; however, additional TRF research is needed to make a determination.
Health implications of eating frequency
Some observational evidence links differences in eating frequency with varying health effects in humans (Table 3).51–56 Results from a study of nearly 2000 adults revealed an inverse relationship between adiposity and number of eating occasions after controlling for energy intake per kilogram of ideal body weight.51 Furthermore, analyses of the 1988–1992 National Health and Nutrition Examination Survey III cohort followed through 2006 revealed a lower hazard ratio (0.68) for cardiovascular disease–related mortality for those eating >6 meals per day compared with eating 4 times per day.52 This association was stronger for those consuming >2500 calories per day. Another large epidemiologic study (n = 29 206) reported that men who skipped breakfast had a 21% higher risk of diabetes and men who ate 1–2 meals per day had a 25% higher risk of diabetes compared with those who consumed 3 meals per day.53
Table 3.
Summary of human studies of eating frequency
| Relationship | Reference | |
|---|---|---|
| ↑ eating frequency ↓ adiposity | Metzner et al. (1977)51 | |
| >6 meals/d vs 4 meals/d ↓ CVD mortality | Chen et al. (2016)52 | |
| No breakfast ↑ type 2 diabetes mellitus risk | Mekary et al. (2012)53 | |
| 1–2 meals/d vs 3 meals/d ↑ type 2 diabetes mellitus risk | Mekary et al. (2012)53 | |
| 17 meals/d vs 3 meals/d | Jenkins et al. (1989)54 | |
| ↓ total cholesterol | ||
| ↓ LDL cholesterol | ||
| ↓ apolipoprotein B | ||
| ↓ insulin | ||
| 1 meal/d vs 3 meals/d ↑ weight loss | Kant (2014)56 | |
| Vast majority of weight maintenance research no relationship | Raynor et al. (2015)55 | |
Abbreviations: CVD, cardiovascular disease; LDL, low-density lipoprotein.
Intervention trials focused on the impact of eating occasions on metabolic health are lacking. One small crossover study (n = 7) investigated serum markers of metabolic health following 2 isocaloric interventions: (1) a snacking pattern consisting of 17 small meals eaten 1 hour apart and (2) a 3-meal pattern.54 The researchers reported that participants in the snacking pattern group had reductions of 8%–15% in total cholesterol, LDL, apolipoprotein B, and serum insulin concentrations compared with participants in the 3-meal pattern group. A potential confounding aspect of this study was that the eating time window differed between the 2 treatments—participants in the 3-meal pattern group, ate during an 11-hour period, whereas those in the hourly snacking pattern ate during a 17-hour period. As discussed earlier, restricting food intake to a smaller time interval is associated with improvements in metabolic health. Intriguingly, those in the snacking pattern group had improved metabolic health despite the longer eating duration.
Raynor et al.55 conducted a systematic review of human and animal studies on eating frequency and weight status and concluded that the relationship between eating frequency and weight is unclear. More than 60% of the studies reviewed found no effect of eating frequency on consumption/intake, body weight, or body mass index. Inconsistent findings related to eating frequency and body weight may be due to reporting bias. It has been suggested that underreporting of food consumption and eating occasions in adults who are overweight or obese may result in an erroneous connection between fewer meals and higher weight.56,57 A review by Kant of 4 prospective cohort studies and 12 controlled trials of eating frequency and body weight found mixed results among the cohort studies with 1 reporting a benefit, 2 reporting a detriment, and 1 showing no effect; the majority of the randomized controlled trials found no relationship between eating frequency and weight loss. Only 1 study reported a significant difference (loss of 0.6 kg; P = 0.01) in participants consuming 1 meal per day over 8 weeks compared with participants consuming 3 meals per day over 8 weeks (gain of 0.8 kg).56
The connection between the number of eating occasions and health is not fully understood. Although beneficial clinical results from greater eating frequency have been reported in the areas of adiposity, insulin, blood lipids, and risk of diabetes and cardiovascular-related death, effects on weight status are less clear. Plausible mechanisms underlying the inverse relationships between more frequent eating occasions and lower glucose, insulin, total cholesterol, and LDL cholesterol concentrations include a lower glycemic load as food is spread throughout the day, slower stomach emptying from smaller meals leading to a lower need for insulin, decreased insulin leading to decreased stimulation of enzymes for cholesterol synthesis, and increased LDL receptors as a result of the lower circulating cholesterol.58
Impact of eating frequency on the microbiota
Although there is considerable research on eating frequency and health, the impact of eating frequency on the GI microbiome has only recently been explored. In horses, the cecal microbiota is impacted by feeding frequency, with higher frequency being associated with increased relative abundance of the genus YRC22 and decreased relative abundances of Prevotella, Lactobacillus, Streptococcus, Coprococcus, and Phascolarctobacterium.59 Additional research is necessary to determine whether changes in eating patterns affect taxa associated with glucose response, lipid metabolism, and adiposity in humans.
Independent of eating frequency, certain bacterial taxa are associated with improved glucose, lipids, and adiposity. For example, A. muciniphila, a mucin-degrading intestinal bacterium, has been associated with improved glucose homeostasis47 and inversely correlated with inflammation in animal models.48 In humans, an increased abundance of Akkermansia was found to inversely relate to fasting glucose, waist-to-hip ratio, and subcutaneous adipocyte diameter.49
LIMITATIONS
This review is limited by the novelty of the field it explores. Recent advances in microbiome investigation technologies have only lately made this research possible. Additionally, performing circadian rhythm or food-timing interventions on humans can be challenging. Self-reported behaviors are subject to bias and error, and in-house experiments are costly and difficult to execute. Furthermore, human studies involving eating pattern alterations (eg, TRF) must be carefully monitored to assess and control energy intake because humans generally decrease energy intake when given a reduced eating window, whereas animals will not.
Limitations of rodent models in microbiota research include the anatomy of the GI tract as well as coprophagy. Rodents are cecal fermenters, meaning most of their bacterial fermentation takes place in the cecum. In humans, most bacterial fermentation occurs in the large intestine. Additionally, the distribution of goblet (mucin-producing) cells is consistent throughout the human colon but decreases along the length of the mouse colon, which could affect the distribution of mucin-degrading bacteria.60 Furthermore, rodents practice coprophagy, consuming their fecal material as well as fecal material of other animals. Lastly, in rodent studies, in addition to fecal samples, the bacterial content of the cecum is frequently examined, whereas in human studies fecal samples are used to characterize the microbiota.
The use of undefined animal diets limits comparisons among rodent studies as well as translation to clinical populations. Chow varies in composition and sources of nutrients due to price and availability of ingredients, whereas refined diets, such as the American Institute of Nutrition diet, have defined nutrient composition. The high-fat diets used in animal research are infrequently representative of the proportion and composition of fat in human diets. In high-fat chow, up to 60% of total energy comes from fat, with 24% of total energy coming from saturated fat. In contrast, NHANES data from 2009 to 2012 show that men and women aged >20 years get an average of 33% of their total energy from fat and 10.6% of their total energy from saturated fat.61
Despite these limitations, the murine model is still a powerful tool in microbiota research. Mice have a different core microbiome than humans, but many of the species comprising the microbiome are similar and present in relatively similar abundances. Interventions also tend to show similar shifts in the microbiota of both mice and humans for many conditions, although it may take longer for diet to affect shifts in humans than in mice.60 Colonization of germ-free mice with fecal transplants from humans overcomes some of these challenges, but the cross-talk between host and symbiont is not identical. Overall, the mouse is a valuable model that allows isolation of variables that would be impossible in humans. Helpful conclusions from these studies can be drawn, although the drawbacks to the translation of results should be kept in mind.
CONCLUSION
Research on disrupted circadian rhythms suggests a reciprocal relationship between the microbiome and the internal clock. Animal evidence supporting the detrimental health effects of disrupting the normal circadian rhythm is robust.21,24,25 Human evidence is preliminary but promising. In both models, the microbiome has been suggested as a potential mediator between circadian misalignment and negative health consequences. Further work is needed with interventions rather than observational studies to establish a causal link between misaligned rhythms and the microbiome, investigating not only microbial composition but also the functional capacity of the microbiome and/or metabolomics.
Results from TRF studies indicate that restricting the time of food access may be protective against weight gain, insulin resistance, and dyslipidemia.41,46 These results also indicate there is a connection between the microbiome and metabolic health. Results demonstrate an absence of diet-induced obesity in germ-free mice and a lack of microbial cycling in mice without an internal clock. To date, evidence from human studies has not been robust because of issues such as small sample sizes and methodologic limitations inherent to human research, including the inability to conduct germ-free experimentation.
There is extensive research on the connection between eating frequency and health but almost no published research on the connection between eating frequency and the microbiome. Additional adequately powered, well-controlled, randomized trials investigating the impact of eating frequencies on the GI microbiome as a primary outcome are necessary to translate preclinical research findings to human populations.
In general, additional well-designed randomized controlled trials of eating behaviors, including trials that focus on circadian rhythm alignment, TRF, and eating frequency, with their effect on the microbiome as a primary outcome, will vastly increase knowledge and strengthen the evidence for using “when to eat” as a novel intervention to prevent or treat disease through GI microbiota manipulation.
Acknowledgments
The authors wish to acknowledge Charlee Walker for assistance with figure design.
Author contributions. J.L.K., H.D.H. designed the review. J.L.K., S.V.T. performed the review. All authors wrote and edited the paper. H.D.H. had primary responsibility for final content. All authors read and approved the final manuscript.
Funding/support. This work was supported by a Research Excellence Fellowship from the Division of Nutritional Sciences at the University of Illinois (J.L.K.), a Jonathan Baldwin Turner Fellowship within the College of Agriculture, Consumer, and Environmental Science at the University of Illinois (S.V.T.), and USDA National Institute of Food and Agriculture, Hatch project ILLU-698-902 (J.L.K., S.V.T., H.D.H.).
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337–343. [DOI] [PubMed] [Google Scholar]
- 2. Qin J, Li R, Raes J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mu C, Yang Y, Zhu W. Gut microbiota: the brain peacekeeper. Front Microbiol. 2016;7:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Vos WM, De Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012;70(suppl. 1):S45–S56. [DOI] [PubMed] [Google Scholar]
- 5. Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu GD, Chen J, Hoffmann C et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334;105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. David LA, Maurice CF, Carmody RN et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC, Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:2025–2032. [DOI] [PubMed] [Google Scholar]
- 9. Holscher HD, Caporaso JG, Hooda S, Brulc JM, Fahey GC, Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr. 2015;101:55–64. [DOI] [PubMed] [Google Scholar]
- 10. Holscher HD. Dietary fiber, prebiotics, and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota–gut–brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–244. [DOI] [PubMed] [Google Scholar]
- 13. Perry RJ, Peng L, Barry NA et al. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frost G, Sleeth ML, Sahuri-Arisoylu M et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. Article 3611:2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thaiss CA, Levy M, Korem T et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510. [DOI] [PubMed] [Google Scholar]
- 17. Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A. Circadian rhythm and the gut microbiome. Int Rev Neurobiol. 2016;131:193–205. [DOI] [PubMed] [Google Scholar]
- 18. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. [DOI] [PubMed] [Google Scholar]
- 19. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang W, Ramsey KM, Marcheva B et al. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27 485 people. Occup Environ Med. 2001;58:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Zhang L, Zhang Y et al. Meta-analysis on night shift work and risk of metabolic syndrome. Obes Rev. 2014;15:709–720. [DOI] [PubMed] [Google Scholar]
- 24. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thaiss CA, Zeevi D, Levy M et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. [DOI] [PubMed] [Google Scholar]
- 26. Fonken LK, Workman JL, Walton JC et al. Light at night increases body mass by shifting time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poroyko VA, Carreras A, Khalyfa A et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang SL, Bai L, Goel N et al. Human and rat gut microbiome composition is maintained following sleep restriction. Proc Natl Acad Sci U S A. 2017;114:E1564–E1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 30. Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes. 2008;32:658–662. [DOI] [PubMed] [Google Scholar]
- 31. McHill AW, Melanson EL, Higgins J et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014;111:17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leone V, Gibbons SM, Martinez K et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voigt RM, Forsyth CB, Green SJ et al. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9:e97500 doi:10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kondo T. A cyanobacterial circadian clock based on the Kai oscillator. Cold Spring Harb Symp Quant Biol. 2007;72:47–55. [DOI] [PubMed] [Google Scholar]
- 36. Heath-Heckman EAC, Peyer SM, Whistler CA, Apicella MA, Goldman WE, McFall-Ngai MJ. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis. MBio. 2013;4:e00167–13–e00167-13. doi:10.1128/mBio.00167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paulose JK, Wright JM, Patel AG, Cassone VM. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One. 2016;11:e0146643 doi:10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murakami M, Tognini P, Liu Y et al. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016;17:292–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Govindarajan K, MacSharry J, Casey PG et al. Unconjugated bile acids influence expression of circadian genes: a potential mechanism for microbe–host crosstalk. PLoS One. 2016;11:e0167319 doi:10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–827. [DOI] [PubMed] [Google Scholar]
- 41. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. [DOI] [PubMed] [Google Scholar]
- 43. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. [DOI] [PubMed] [Google Scholar]
- 45. Kohsaka A, Laposky AD, Ramsey KM et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 46. Hatori M, Vollmers C, Zarrinpar A et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin N-R, Lee J-C, Lee H-Y et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. [DOI] [PubMed] [Google Scholar]
- 48. Schneeberger M, Everard A, Gómez-Valadés AG et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dao MC, Everard A, Aron-Wisnewsky J et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. [DOI] [PubMed] [Google Scholar]
- 50. Thaiss CA, Itav S, Rothschild D et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–551. [DOI] [PubMed] [Google Scholar]
- 51. Metzner HL, Lamphiear DE, Wheeler NC, Larkin FA. The relationship between frequency of eating and adiposity in adult men and women in the Tecumseh Community Health Study. Am J Clin Nutr. 1977;30:712–715. [DOI] [PubMed] [Google Scholar]
- 52. Chen H-J, Wang Y, Cheskin LJ. Relationship between frequency of eating and cardiovascular disease mortality in U.S. adults: the NHANES III follow-up study. Ann Epidemiol. 2016;26:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jenkins D, Wolever T, Vuksan V et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–934. [DOI] [PubMed] [Google Scholar]
- 55. Raynor HA, Goff MR, Poole SA, Chen G. Eating frequency, food intake, and weight: a systematic review of human and animal experimental studies. Front Nutr. 2015;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kant AK. Evidence for efficacy and effectiveness of changes in eating frequency for body weight management. Adv Nutr. 2014;5:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr. 1997;77(suppl 1):S57–S70. [DOI] [PubMed] [Google Scholar]
- 58. Palmer MA, Capra S, Baines SK. Association between eating frequency, weight, and health. Nutr Rev. 2009;67:379–390. [DOI] [PubMed] [Google Scholar]
- 59. Venable EB, Fenton KA, Braner VM et al. Effects of feeding management on the equine cecal microbiota. J Equine Vet Sci. 2017;49:113–121. [Google Scholar]
- 60. Nguyen TLA, Vieira-Silva S, Liston A et al. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Hyattsville, MD; 2016. [PubMed] [Google Scholar]