ABSTRACT
Acute kidney injury (AKI) following transcatheter aortic valve implantation (TAVI) is associated with increased morbidity and mortality. The biomarkers neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and interleukin-18 (IL-18) are predictive of AKI after cardiac surgery, but there is little data regarding these biomarkers after TAVI. We evaluated the associations between NGAL, KIM-1, and IL-18 levels and the incidence and severity of AKI and changes in serum creatinine after TAVI. This was a prospective pilot study of 66 TAVI cases. Urinary biomarkers were measured at baseline and at 2, 4, and 12 hours after TAVI. Demographics, procedural features, and renal function until discharge were compared between patients with and without subsequent AKI. Seventeen patients (25.8%) developed AKI postoperatively (stage 1, n = 14; stage 2, n = 1; stage 3, n = 2). There were no significant differences in unadjusted mean NGAL, KIM-1, and IL-18 levels between patients with and without AKI at 2, 4, and 12 hours following surgery. After adjusting for the Society of Thoracic Surgeons risk of mortality, this study of three urinary biomarkers showed no association with AKI or creatinine after TAVI. Ongoing efforts to predict and modify the risk of AKI after TAVI remain challenging.
KEYWORDS: Acute kidney injury, IL-18, KIM-1, NGAL, renal biomarkers, transcatheter aortic valve implantation
Acute kidney injury (AKI) after transcatheter aortic valve implantation (TAVI) has been described in 10% to 30% of patients undergoing TAVI and is thus one of the most commonly reported complications of this procedure.1–8 AKI after TAVI is associated with both operative and long-term mortality and increases these by a magnitude of 2- to 4-fold.1–5,9–13 Additionally, AKI is associated with an increased risk of early myocardial infarction, life-threatening bleed, need for transfusion and dialysis, as well as prolonged hospital stay.3,13–15 Currently, AKI is detected by measuring serum creatinine (SCr), but this method is not reliable with acute changes in renal function and may not accurately reflect kidney function until a steady state occurs. Several novel biomarkers have shown promising results in predicting AKI after cardiac procedures: neutrophil gelatinase–associated lipocalin (NGAL), which is involved in the innate immune system and allows earlier detection of AKI than SCr; kidney injury molecule-1 (KIM-1), a type-1 transmembrane protein; and interleukin-18 (IL-18), a pro-inflammatory cytokine that plays a role in both the innate and acquired immune response. The goal of this study was to determine whether there are associations between renal biomarker levels of NGAL, IL-18, and KIM-1 and the occurrence of AKI post-TAVI.
MATERIALS AND METHODS
This was a prospective pilot study of consecutive patients with severe symptomatic aortic stenosis who were referred for TAVI at a single institution from June 2012 through May 2013. The protocol and informed consent form were approved by the local institutional review board.
Severe symptomatic aortic stenosis was defined as having an aortic valve area <1.0 cm2, aortic valve Vmax ≥4 m/s, and mean gradient >40 mm Hg on transthoracic echocardiogram with symptoms of exertional dyspnea or decreased exercise tolerance, angina, or syncope. Eligibility for TAVI was determined by a multidisciplinary heart team taking into consideration patient demographics, comorbid conditions as defined by the Society of Thoracic Surgeons (STS), and the STS-predicted risk of mortality (PROM).
Urine levels of NGAL, KIM-1, and IL-18 were measured at baseline and at 2, 4, and 12 hours post-TAVI using the enzyme-linked immunosorbent assay method (Hycult Biotech Inc., Plymouth Meeting, PA). SCr was measured daily until hospital discharge. Patient demographics and in-hospital adverse events were collected using the STS definitions. AKI was defined by the VARC-2 definition16: Stage 1 AKI, increase in SCr to 1.5 to 1.99× baseline or increase of >0.3 mg/dL (>26.4 mmol/L) or urine output <0.5 mL/kg/h for >6 hours but <12 hours; stage 2 AKI, increase in SCr to 2.0 to 2.99× baseline or urine output <0.5 mL/kg/h for >12 hours but <24 hours; stage 3 AKI, increase in SCr to >3× baseline or SCr of >4.0 mg/dL (>354 mmol/L) with an acute increase of ≥0.5 mg/dL (44 mmol/L) or urine output <0.3 mL/kg/h for >24 hours or anuria for >12 hours. Patients receiving renal replacement therapy are considered to meet stage 3 criteria irrespective of other criteria. The maximum relative change in SCr was calculated as the greatest SCr of these postoperative SCr measurements divided by the preoperative level.
Demographic characteristics and procedural outcomes were compared between patients who developed AKI post-TAVI and those who did not. Group comparisons were performed using the Wilcoxon signed test for continuous variables and using chi-squared or Fisher's exact tests for categorical variables. Unadjusted differences for baseline, 2 hours post-TAVI, 4 hours post-TAVI, and 12 hours post-TAVI levels of NGAL, KIM-1, and IL-18 between groups were tested using univariate logistic regression models with AKI as the dependent variable and each marker (NGAL, KIM-1, or IL-18) as the only independent variable. A generalized estimating equations model accounting for repeated measures and adjusted for STS PROM, baseline NGAL, and baseline NGAL minus 2 hours post-TAVI NGAL, baseline NGAL minus 4 hours post-TAVI NGAL, and baseline NGAL minus 12 hours post-TAVI NGAL was developed to assess maximum relative changes in SCr. The same analysis was repeated for KIM-1 and IL-18. Restricted cubic splines were used to model baseline NGAL, KIM-1, and IL-18 levels.17,18
RESULTS
Of the 66 patients enrolled, 17 patients (25.8%) developed AKI postoperatively: Stage 1 occurred in 14 patients (82.4%), stage 2 in 1 patient (5.9%), and stage 3 in 2 patients (11.4%). Patients who developed AKI were more likely to be female and to have experienced a prior myocardial infarction (Table 1). There was no significant difference in baseline estimated glomerular filtration rate (eGFR) by AKI status. The maximum relative change in SCr ranged from 0.72 to 4.58 with a mean (SD) of 1.19 (0.55), corresponding to a 19% increase in maximum postoperative SCr compared to preoperative levels.
Table 1.
Demographic and clinical characteristics
| Acute kidney injury |
|||
|---|---|---|---|
| Characteristic | Yes (n = 17, 26%) | No (n = 49, 74%) | P value |
| Men | 7 (41%) | 34 (69%) | 0.04 |
| Age (years) | 82.9 ± 6.5 | 82.7 ± 8.9 | 0.76 |
| Height (cm) | 165.4 ± 11.4 | 170.7 ± 9.0 | 0.15 |
| Weight (kg) | 70.3 ± 16.8 | 78.7 ± 21.9 | 0.26 |
| Body mass index (kg/m2) | 26.0 ± 6.5 | 27.1 ± 8.5 | 0.74 |
| Race | 0.22 | ||
| White | 15 (88%) | 47 (96%) | |
| Black | 1 (6%) | 0 (0%) | |
| Asian | 1 (6%) | 2 (4%) | |
| Prior myocardial infarction | 5 (29%) | 4 (8%) | 0.03 |
| Prior stroke | 0 (0%) | 4 (8%) | 0.22 |
| Hypertension | 17 (100%) | 48 (98%) | 0.55 |
| Diabetes | 4 (24%) | 11 (23%) | 0.93 |
| Chronic lung disease | 5 (29%) | 15 (31%) | 0.93 |
| Dyslipidemia | 1 (6%) | 5 (10%) | 0.59 |
| Peripheral artery disease | 4 (24%) | 10 (20.4%) | 0.79 |
| Previous PCI | 6 (35%) | 16 (33%) | 0.84 |
| Prior coronary bypass | 5 (29%) | 18 (37%) | 0.59 |
| Smoking | 1 (6%) | 7 (14%) | 0.36 |
| STS-predicted mortality (%) | 9.6 ± 4.9 | 8.0 ± 4.1 | 0.29 |
| Aortic valve mean gradient (mm Hg) | 39.6 ± 10.9 | 43.0 ± 12.4 | 0.53 |
| Aortic valve area (cm2) | 0.66 ± 0.11 | 0.69 ± 0.23 | 0.96 |
| Preoperative eGFR (mL/min/1.73 m2) | 54.8 ± 29.2 | 70.7 ± 33.8 | 0.09 |
| AKI stage | |||
| Stage 1 | 14 (82%) | N/A | |
| Stage 2 | 1 (6%) | N/A | |
| Stage 3 | 2 (12%) | N/A | |
| Procedural outcomes | |||
| Number of rapid pace runs | 2.9 ± 1.0 | 2.5 ± 1.0 | 0.11 |
| Amount of contrast used (mL) | 131 ± 53 | 145 ± 61 | 0.37 |
| Severe hypotension | 1 (6%) | 2 (4%) | 0.99a |
| Intraoperative arrhythmia | 0 (0%) | 2 (4%) | 0.99a |
| Operative mortality | 2 (12%) | 1 (2%) | 0.16a |
AKI indicates acute kidney injury; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
Fisher's exact test.
The trends in postoperative SCr indicated that 1 patient who developed AKI had a measured rise in creatinine within 24 hours, 8 patients with AKI had a measured rise in creatinine from 24 to 48 hours, 6 patients had a measured rise in creatinine from 48 to 72 hours, and 2 patients had a measured rise in creatinine >72 hours post-TAVI. There were no significant unadjusted differences in mean NGAL or IL-18 levels between patients with AKI and those without AKI seen at 2, 4, and 12 hours postprocedure (Figure 1). Patients with AKI had a marginally greater KIM-1 at 2 hours post-TAVI (P = 0.05).
Figure 1.
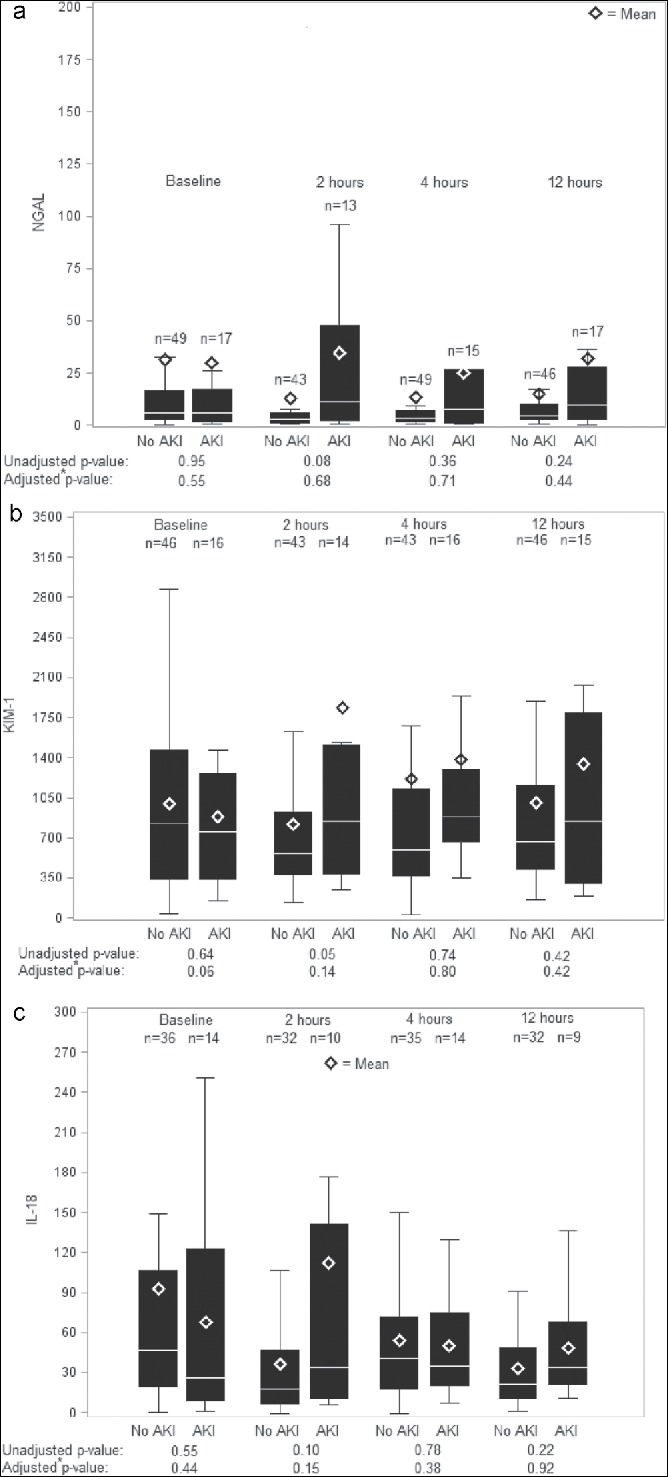
Box plots of (a) urinary neutrophil gelatinase–associated lipocalin (NGAL), (b) interleukin-18 (IL-18), and (c) kidney injury molecule-1 (KIM-1) by acute kidney injury (AKI) status. Shown are unadjusted boxplots of 0-, 2-, 4-, and 12-hour biomarker measurements comparing patients who did not develop AKI to those who did develop AKI. The adjusted P values were obtained from a linear generalized estimating equations model with maximum relative change in serum creatinine as the dependent variable and the following dependent variables: baseline, 0 hour − 2 hour, 0 hour − 4 hour, and 0 hour − 12 hour biomarker measurements and Society of Thoracic Surgeons predicted risk of mortality.
The adjusted associations between baseline NGAL, KIM-1, and IL-18 and maximum relative change in creatinine were not significant (P = 0.71, P = 0.37, and P = 0.69, respectively; Figure 2). Likewise, the adjusted association between NGAL, KIM-1, and IL-18 differences at 2, 4, and 12 hours from baseline measurement and maximum change in SCr was not significant (Figure 3).
Figure 2.
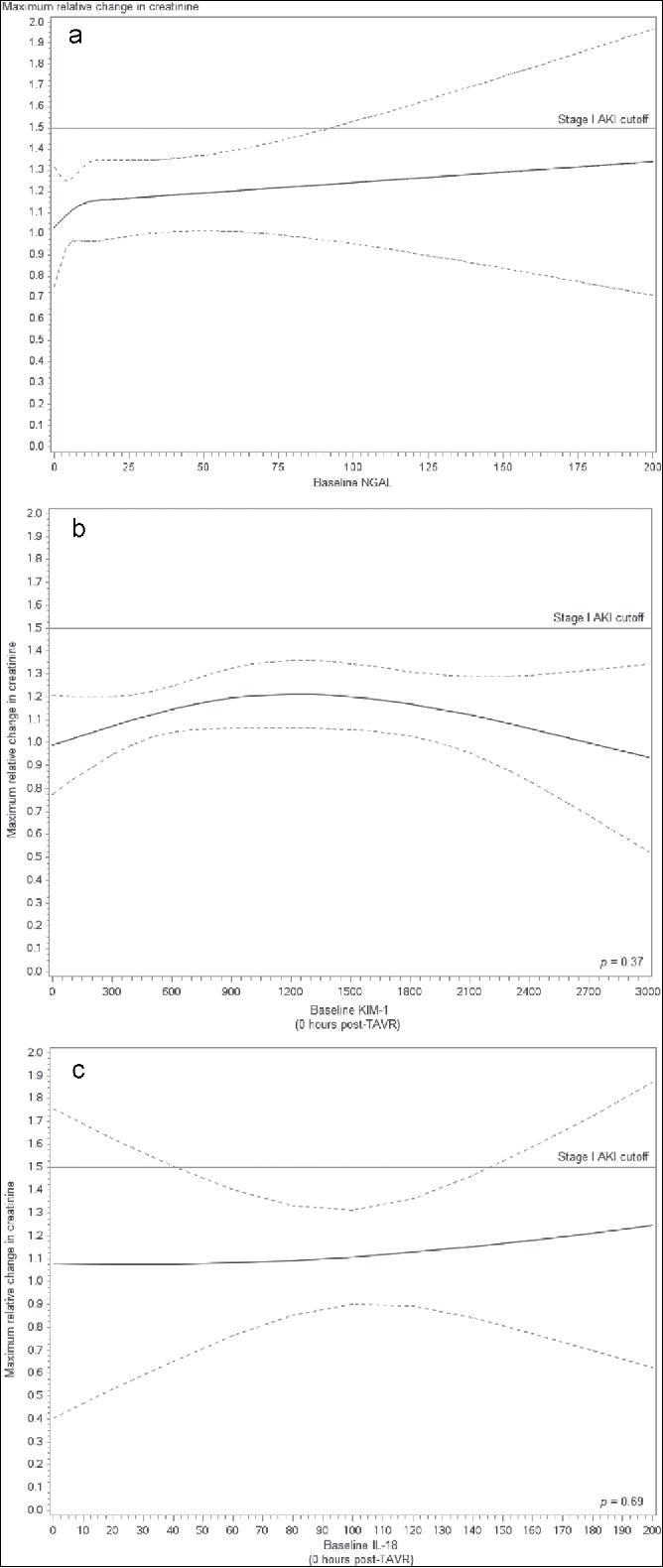
Adjusted association (P = 0.714) between baseline neutrophil gelatinase–associated lipocalin (NGAL) and maximum change in creatinine. A generalized estimating equations model accounting for patients, repeated measures and adjusted for Society of Thoracic Surgeons predicted risk of mortality, baseline, 0 hour − 2 hour, 0 hour − 4 hour, and 0 hour − 12 hour post–transcatheter aortic valve implantation (TAVI) NGAL was developed to assess maximum changes in creatinine. Restricted cubic splines were used to model baseline NGAL.
Figure 3.
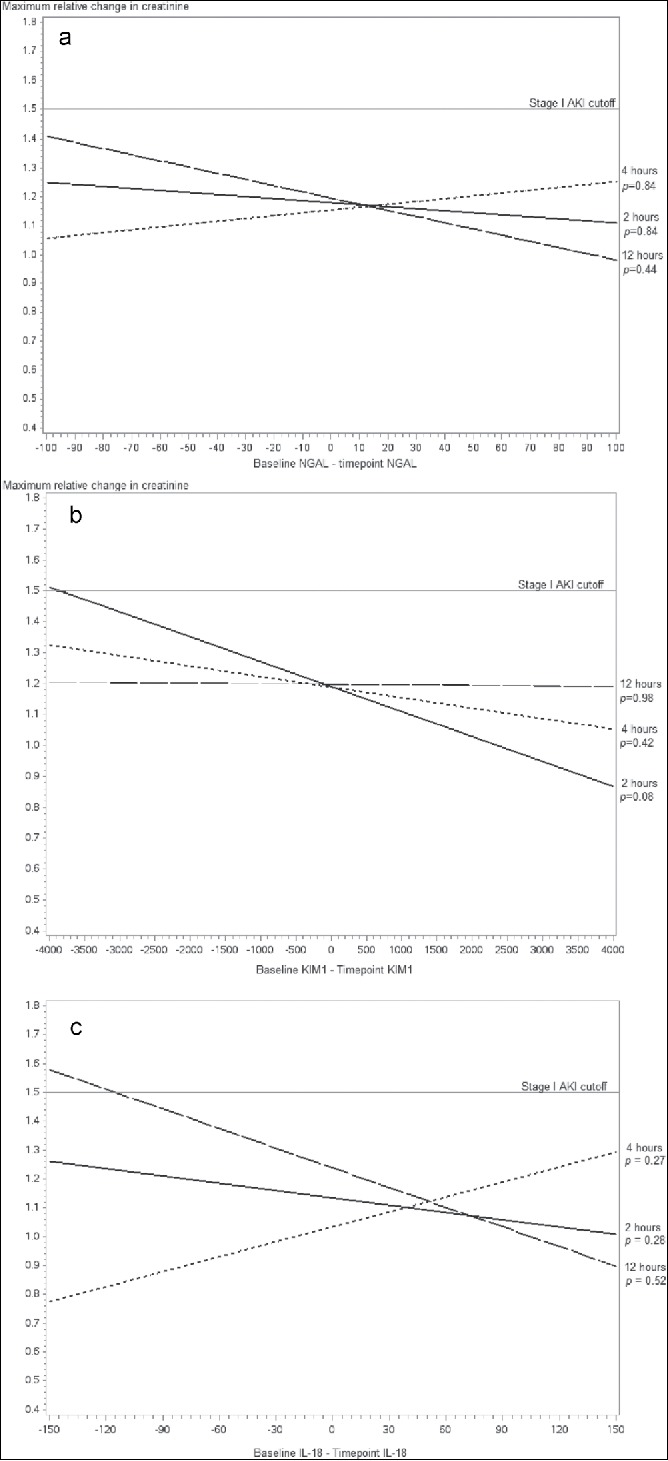
Adjusted associations between neutrophil gelatinase–associated lipocalin (NGAL) differences at (a) 2 hours (P = 0.844), (b) 4 hours (P = 0.845), and (c) 12 hours (P = 0.440) from baseline measurement and maximum change in creatinine.
DISCUSSION
Given the well-described higher rate of mortality and morbidity in patients who develop AKI after TAVI, there is a clinical need for earlier detection of AKI as well as development of therapeutic options for prevention to improve patient outcomes. Even more important, an enhanced ability to predict AKI prior to TAVI would be optimal. Risk factors associated with the development of AKI after TAVI include baseline renal function, diabetes mellitus, blood transfusion, hypertension, and peripheral vascular disease.5,9–12 This pilot study demonstrated the occurrence of AKI in approximately 25% of the patients in a high-risk aortic stenosis cohort. The vast majority of these were stage 1 AKI. Though baseline eGFR did not differ between the group that developed AKI and the group that did not, there was a numerically lower eGFR in the AKI group, perhaps reflecting the possibility that the small numbers of patients limited our ability to detect differences.
NGAL is involved in the innate immune system and has been reported to predict AKI after cardiopulmonary bypass (CPB) in a pediatric population19,20 as well as in adults21–26 and after coronary angiography and percutaneous coronary intervention.27,28 Similarly, KIM-1, a type-1 transmembrane protein, has been shown to be predictive of AKI following CPB,29,30 coronary artery bypass grafting,31 and valve surgery.31 IL-18 is a pro-inflammatory cytokine that plays a role in both the innate and acquired immune response32 and has also been shown to be predictive of AKI following CPB,29,33 coronary artery bypass grafting,33 and valve surgery,33,34 as well as following thoracic aortic aneurysm repair.34 These data lead to the choice of these biomarkers for evaluation. However, in our study, there were no significant differences in the trend of urinary NGAL, KIM-1, or IL-18 in patients who developed AKI post-TAVI versus those who did not, in both unadjusted and adjusted analyses. Baseline levels of the biomarkers also did not differ based on whether AKI developed or not. Additionally, there was no association between the urinary biomarker levels and maximal change in SCr post-TAVI.
Only one other study has evaluated urinary NGAL levels after TAVI.35 That study evaluated levels of NGAL 4 hours after TAVI in 34 patients, 6 (17.7%) of whom developed AKI (all of them stage 1), but demonstrated no significant difference in the single NGAL measurement between patients who developed AKI and those who did not. Our results are very similar to their results even with approximately twice the number of patients evaluated and with biomarker evaluation at multiple time points instead of just 4 hours postprocedure.
Therefore, the question becomes, why can we not demonstrate a difference in renal biomarkers after TAVI, though this has been demonstrated after cardiac surgery and in a few studies after percutaneous coronary intervention? First, perhaps there is something unique about the population with aortic stenosis that makes detection of differences in renal biomarkers more challenging. Elevated NGAL levels have been described in coronary artery disease, heart failure, and stroke, with evidence of up-regulation in the setting of failing myocardium and in atherosclerotic plaque.36–38 Perhaps the magnitude of elevated baseline NGAL levels in patients with aortic stenosis makes it challenging to detect an additional increase in value after TAVI, with a physiologic mechanism that differs from that after coronary intervention. Finally, as a recent metaanalysis showed,39 there is variability across published studies in the performance of biomarkers of AKI; several studies have reported nonsignificant associations of biomarker levels, including postoperative urinary NGAL,30,34,40 KIM-1,40 and IL-18,30,41 with the development of AKI, despite having larger sample sizes than in the study presented here.
Renal biomarkers other than the ones studied here may also be considered for predicting AKI after TAVI.39 One study has evaluated cystatin C in the prediction of AKI post-TAVI.42 Additionally, combinations of renal biomarkers may be considered. Urinary liver-type fatty acid–binding protein has been evaluated as a predictor of AKI after cardiac surgery, with increased performance in detecting AKI (as measured by receiver operator characteristic analysis) when combined with urinary NGAL.43 Thus, perhaps the most accurate prediction of AKI in the TAVI population will come from a combination of multiple biomarkers.
The limitations of this study are that it is a small study with significant selection bias related to the screening criteria of the population undergoing TAVI. TAVI was used as an alternative to surgical aortic valve replacement in patients who were classified by the multidisciplinary heart team to be high risk or inoperable for surgical aortic valve replacement. As a result, the selected TAVI population might have an unequal distribution of comorbidities. This was evident in the STS PROM in the 8.0% to 9.0% range in the study patients.
In conclusion, urinary levels of the renal biomarkers NGAL, KIM-1, and IL-18 were not found to be significantly different in patients who developed AKI post-TAVI compared to those who did not. Additionally, these biomarkers were not associated with maximum changes in SCr levels postoperatively. The lack of significant association may be due to the small study size versus no real differences in the TAVI population. Further research on renal biomarkers in TAVI is required to optimize outcomes in this high-risk population.
References
- 1.Barbanti M, Latib A, Sgroi C, et al.. Acute kidney injury after transcatheter aortic valve implantation with self-expanding CoreValve prosthesis: results from a large multicentre Italian research project. EuroIntervention. 2014;10:133–140. https://doi.org/ 10.4244/EIJV10I1A20. [DOI] [PubMed] [Google Scholar]
- 2.Barbash IM, Ben-Dor I, Dvir D, et al.. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. https://doi.org/ 10.1016/j.ahj.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Gargiulo G, Sannino A, Capodanno D, et al.. Impact of postoperative acute kidney injury on clinical outcomes after transcatheter aortic valve implantation: a meta-analysis of 5,971 patients. Catheter Cardiovasc Interv. 2015;86:518–527. https://doi.org/ 10.1002/ccd.25867. [DOI] [PubMed] [Google Scholar]
- 4.Genereux P, Kodali SK, Green P, et al.. Incidence and effect of acute kidney injury after transcatheter aortic valve replacement using the new valve academic research consortium criteria. Am J Cardiol. 2013;111:100–105. https://doi.org/ 10.1016/j.amjcard.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khawaja, et al.. The effects of VARC-defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2012;8:563–570. https://doi.org/ 10.4244/EIJV8I5A87. [DOI] [PubMed] [Google Scholar]
- 6.Munoz-Garcia AJ, Munoz-Garcia E, Jimenez-Navarro MF, et al.. Clinical impact of acute kidney injury on short- and long-term outcomes after transcatheter aortic valve implantation with the CoreValve prosthesis. J Cardiol. 2015;66:46–49. https://doi.org/ 10.1016/j.jjcc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Nuis RJ, Van Mieghem NM, Tzikas A, et al.. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;77:881–889. https://doi.org/ 10.1002/ccd.22874. [DOI] [PubMed] [Google Scholar]
- 8.Saia F, Ciuca C, Taglieri N, et al.. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol. 2013;168:1034–1040. https://doi.org/ 10.1016/j.ijcard.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Aregger F, Wenaweser P, Hellige GJ, et al.. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–2179. https://doi.org/ 10.1093/ndt/gfp036. [DOI] [PubMed] [Google Scholar]
- 10.Bagur R, Webb JG, Nietlispach F, et al.. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. https://doi.org/ 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatani K, Abdel-Wahab M, Wubken-Kleinfeld N, et al.. Acute kidney injury after transcatheter aortic valve implantation: impact of contrast agents, predictive factors, and prognostic importance in 203 patients with long-term follow-up. J Cardiol. 2015;66:514–519. https://doi.org/ 10.1016/j.jjcc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Elhmidi Y, Bleiziffer S, Piazza N, et al.. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J. 2011;161:735–739. https://doi.org/ 10.1016/j.ahj.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Gebauer K, Diller GP, Kaleschke G, et al.. The risk of acute kidney injury and its impact on 30-day and long-term mortality after transcatheter aortic valve implantation. Int J Neprhol. 2012;2012:483748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong WY, Yong G, Irish A. Incidence, risk factors and prognosis of acute kidney injury after transcatheter aortic valve implantation. J Nephrol. 2012;17:445–451. https://doi.org/ 10.1111/j.1440-1797.2012.01593.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinning JM, Ghanem A, Steinhauser H, et al.. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141–1149. https://doi.org/ 10.1016/j.jcin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Kappetein AP, Head SJ, Genereux P, et al.. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. https://doi.org/ 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Filardo G, Hamilton C, Hamman B, Ng HK, Grayburn P. Categorizing BMI may lead to biased results in studies investigating in-hospital mortality after isolated CABG. J Clin Epidemiol. 2007;60:1132–1139. https://doi.org/ 10.1016/j.jclinepi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE., Jr. Regression Modeling Strategies: With Application to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 19.Bennett M, Dent CL, Ma Q, et al.. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. https://doi.org/ 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra J, Dent C, Tarabishi R, et al.. Neutrophil gelatinase–associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. https://doi.org/ 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 21.Cai L, Borowiec J, Xu S, Han W, Venge P. Assays of urine levels of HNL/NGAL in patients undergoing cardiac surgery and the impact of antibody configuration on their clinical performances. Clin Chim Acta. 2009;403(1-2):121–125. https://doi.org/ 10.1016/j.cca.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Cruz DN, Soni S, Ronco C. NGAL and cardiac surgery–associated acute kidney injury. Am J Kidney Dis. 2009;53:565–566. https://doi.org/ 10.1053/j.ajkd.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Delcroix G, Gillain N, Moonen M, et al.. NGAL usefulness in the intensive care unit three hours after cardiac surgery. J Nephrol. 2013;2013:865164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebetrau C, Dorr O, Baumgarten H, et al.. Neutrophil gelatinase–associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand J Clin Lab Invest. 2013;73:392–399. https://doi.org/ 10.3109/00365513.2013.787149. [DOI] [PubMed] [Google Scholar]
- 25.Sargentini V, Mariani P, D'Alessandro M, et al.. Assessment of NGAL as an early biomarker of acute kidney injury in adult cardiac surgery patients. J Biol Regul Homeost Agents. 2012;26:485–493. [PubMed] [Google Scholar]
- 26.Wagener G, Jan M, Kim M, et al.. Association between increases in urinary neutrophil gelatinase–associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. https://doi.org/ 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26:287–292. https://doi.org/ 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 28.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil gelatinase–associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant. 2007;22:295–296. https://doi.org/ 10.1093/ndt/gfl408. [DOI] [PubMed] [Google Scholar]
- 29.Liang XL, Liu SX, Chen YH, et al.. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case–control study. Biomarkers. 2010;15:332–339. https://doi.org/ 10.3109/13547501003706558. [DOI] [PubMed] [Google Scholar]
- 30.Liangos O, Tighiouart H, Perianayagam MC, et al.. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–431. https://doi.org/ 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyner JL, Vaidya VS, Bennett MR, et al.. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165. https://doi.org/ 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw. 2000;11:483–486. [PubMed] [Google Scholar]
- 33.Parikh CR, Thiessen-Philbrook H, Garg AX, et al.. Performance of kidney injury molecule-1 and liver fatty acid–binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–1088. https://doi.org/ 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejaz AA, Kambhampati G, Ejaz NI, et al.. Post-operative serum uric acid and acute kidney injury. J Nephrol. 2012;25:497–505. https://doi.org/ 10.5301/jn.5000173. [DOI] [PubMed] [Google Scholar]
- 35.Vermi AC, Costopoulos C, Latib A, et al.. Urinary neutrophil gelatinase–associated lipocalin as a predictor of acute kidney injury after transcatheter aortic valve implantation. Hellenic J Cardiol. 2014;55:77–79. [PubMed] [Google Scholar]
- 36.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase–associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med. 2012;50:1533–1545. https://doi.org/ 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palazzuoli A, Ruocco G, Pellegrini M, et al.. Comparison of neutrophil gelatinase–associated lipocalin versus B-type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol. 2015;116:104–111. https://doi.org/ 10.1016/j.amjcard.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 38.Ronco C, Cruz D, Noland BW. Neutrophil gelatinase–associated lipocalin curve and neutrophil gelatinase–associated lipocalin extended-range assay: a new biomarker approach in the early diagnosis of acute kidney injury and cardio-renal syndrome. Semin Nephrol. 2012;32:121–128. https://doi.org/ 10.1016/j.semnephrol.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Ho J, Tangri N, Komenda P, et al.. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66:993–1005. https://doi.org/ 10.1053/j.ajkd.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Paarmann H, Charitos EI, Beilharz A, et al.. Duration of cardiopulmonary bypass is an important confounder when using biomarkers for early diagnosis of acute kidney injury in cardiac surgical patients. Appl Cardiopulm Pathophysiol. 2013;17:284–297. [Google Scholar]
- 41.Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A. Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care Med. 2008;12(4):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansson M, Nozohoor S, Bjursten H, Kimblad PO, Sjogren J. Acute kidney injury assessed by cystatin C after transcatheter aortic valve implantation and late renal dysfunction. J Cardiothorac Vasc Anesth. 2014;28:960–965. https://doi.org/ 10.1053/j.jvca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Che M, Xue S, et al.. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers. 2013;18:95–101. https://doi.org/ 10.3109/1354750X.2012.740687. [DOI] [PubMed] [Google Scholar]


