Abstract
Background
Visceral leishmaniasis (VL) patients with HIV co-infection should receive antiretroviral treatment (ART). However, the best timing for initiation of ART is not known. Among such individuals, we assessed the influence of ART timing on VL outcomes.
Methods
A retrospective cohort study was conducted in Northwest Ethiopia among VL patients starting ART between 2008 and 2015. VL outcomes were assessed by the twelfth month of starting ART, within 4 weeks of VL diagnosis or thereafter.
Results
Of 213 VL-HIV co-infected patients with ART initiation, 96 (45.1%) had moderate to severe malnutrition, 53 (24.9%) had active TB and 128 (60.1%) had hemoglobin levels under 9 g/dL. Eighty-nine (41.8%) were already on ART before VL diagnosis, 46 (21.6%) started ART within 4 weeks, and 78 (36.6%) thereafter. Definitive cure in those starting ART within 4 weeks 59% (95% CI 43–75%) and those starting thereafter 56% (95% CI 44–68%) was not significantly different. Those starting ART before primary VL had higher 12-months mortality compared to those starting later (RR 0.6; 95% CI 0.4–0.9; p=0.012).
Conclusions
VL-HIV patients are severely ill and with serious additional comorbidities. Outcomes of HIV-VL management are unsatisfactory and early ART initiation was associated with higher mortality. Further research on the optimal timing of ART initiation, and ensuring earlier diagnosis of VL patients, with improved management of comorbidities are needed.
Keywords: Antiretroviral therapy timing, Ethiopia, HIV infection, Tri-dimensional screening, Visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL), also called kala-azar, is a vector borne protozoan disease caused by Leishmania donovani complex.1 The disease is found in over 70 countries and is the second largest global parasitic killer after malaria. Six countries (India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil) account for over 90% of all cases.2,3 The East African region is second to the Indian subcontinent in terms of disease burden with annual incidence reaching 30 000 cases.2,4 VL in Ethiopia is typified by high HIV co-infection with prevalence ranging between 18 and 48%.3,5
The combination of VL and HIV means ‘double trouble’.3,6 In endemic areas, HIV increases the risk of developing VL disease by over 100 times.3 The treatment outcome of VL patients with HIV is generally poor with high proportions of treatment failure, slow responses,7–9 death and relapses.5,10 On the other hand, VL (like HIV) damages the immune system, enhances HIV viral replication and accentuates clinical progression to AIDS.11
The wide use of antiretroviral treatment (ART) in co-infected VL patients brings about clinical and public health benefits as seen in southern Europe.12,13 It is now recommended that all individuals with VL are offered routine HIV testing and those found positive, be started on ART.8,14,15
Médecins Sans Frontiéres (MSF) has been running a leishmaniasis treatment program in Northwest Ethiopia since 1997 and an ART program since 2004.8 Despite the integrated VL and HIV care programs, the VL treatment has remained challenging and generally with poor outcomes. This has raised a number of research questions. Does the timing of ART initiation in HIV positive VL patients have an effect on the outcome of VL treatment as was observed with other opportunistic infections (such as TB)?14–16 WHO recommends early initiation of ART in people with advanced clinical disease (including VL as it is a WHO stage 4 defining illness).17 Early initiation of ART in the context of VL (within 4 weeks of VL diagnosis) means simultaneous use of anti-leishmaniasis drugs and ART. One issue of clinical importance is a possible drug–drug interaction. For example, though miltefosine is expected to be generally safe, the possible interaction with antiretroviral drugs is not completely ruled out.18 The mild and less common risk of nephrotoxicity associated with liposomal amphotericin b (L-AmB) gets accentuated when used with ART warrants consideration, particularly when it is used with another nephrotoxic drug like tenofovir disopoxil fumarate (TDF) as part of ART.19 The pill burden is also another consideration that might impact on tolerability and adherence to prescribed treatments. On the other hand delaying ART initiation until after 4 weeks may have increased unfavorable outcomes as a result of increased viral replication and further deterioration.
The question is, therefore, whether early start of ART simultaneously with VL treatment or before VL diagnosis improves treatment outcome, as compared to waiting with ART initiation till after finishing VL treatment. It is also of paramount importance to identify the risk factors associated with unfavorable treatment outcomes in order to target prioritization of care. A PubMed search revealed no study as yet on the influence of ART timing on VL outcomes. Such information would help improve the management of VL patients with HIV infection. Thus, among individuals with VL and co-infected with HIV, we assessed the timing of ART initiation and its influence on VL treatment outcomes as well as factors associated with unfavorable VL treatment outcomes.
Methods
Study design and setting
A retrospective cohort study was conducted using routine program data.
General setting
In terms of VL-HIV co-infection, Northwest Ethiopia is the most affected region in the country. This is a fertile region with wide cash crop agricultural areas. The region is also known for high number of migrant workers and settlers to the fertile places coming from highlands. These previously unexposed population groups are at higher risk of acquiring VL and HIV infection.5 The major treatment centers for VL and HIV co-infection in this region are the University of Gondar Hospital, the Abdurafi Health Center which is run by MSF, and Humera Hospital (run by MSF until 2009).
Specific setting
The study site was the Abdurafi Health Center, which was located in rural Abdurafi town in Northwest Ethiopia. Since 2003, MSF has established a leishmaniasis treatment program within the health center that provides general primary health care. The MSF program also includes HIV testing and an ART program.
VL management in Abdurafi Health Center
VL was managed according to MSF guidelines.16 VL was suspected when individuals presented with prolonged fever, wasting and enlarged spleen. Diagnostic confirmation was based on a positive rK39 rapid diagnostic test (IT-Leish, BioRad, Hercules, CA, USA). If the rK39 RDT was negative, a second serological test, the direct agglutination test (DAT) (The Royal Tropical Institute, Amsterdam, The Netherlands) was done and a titer result of ≥1:3200 was considered VL. Suspected primary VL patients with rK39 negative and intermediate DAT titer (1:800–1:1600) underwent parasitological diagnosis using tissue aspirate microscopy (spleen or bone marrow). Relapsed VL was diagnosed parasitologically. VL suspected severely ill patients with negative rK39 test result underwent appropriate tissue aspiration immediately, in order not to delay diagnosis.
The treatment regimens of VL for uncomplicated VL in immunocompetent patients in Abdurafi Health Center largely included sodium stibogluconate (SSG) for 30 days and since March 2013 SSG/paramomycin combination for 17 days through intramuscular injections.20 For severe/complicated and HIV co-infected VL six to eight doses of L-AmB infusion and since 2010 six to eight doses of L-AmB infusion with a combination of oral miltefosine for 28 days were the preferred treatment regimens. The latter two regimens were the preferred first line regimens in HIV co-infection for safety reasons. VL treatment outcome assessment was done by the end of fourth week of treatment. Those who did not get cured (positive parasitological test of cure or persistence of symptoms) were considered as slow responders and continued on a second round of treatment with the same or new treatment regimen until cure was achieved. The definitions and treatment outcomes of VL are shown in Box 1.
Box 1.
Definition of case classification and treatment outcomes for visceral leishmaniasis (VL), in Abdurafi Health Center, Northwest Ethiopia. Adapted from Salih et al.21
| Definition | |
|---|---|
| Primary VL | Patient presenting with VL symptoms with no history of previous VL and currently diagnosed with VL. Diagnosis relies on a positive serological test for VL (rK39 based rapid test and/or DAT direct agglutination test) and/or a positive parasitological test (microscopic detection of Leishmania parasites in splenic aspirate). |
| Relapse | Patient with a history of previous VL and who then presents with symptoms of VL and is parasitologically confirmed. |
| Initial cure | Patient who shows improvement of signs and symptoms at the end of treatment (fever resolution, hemoglobin increase, weight gain and spleen size regression), and a negative parasitological test of cure (TOC) if performed. |
| Initial failure | A positive TOC (parasitological failure) and/or persisting clinical signs/symptoms or failure to continue first-line treatment for safety reasons. |
| Slow responder | Partial clinical response but TOC positive (PVL and VL relapse); or no improvement in clinical symptoms and signs with a decrease in parasite load at the end of first-line VL treatment (defined at 4 weeks). |
| Test of cure (TOC) | Spleen, bone marrow, or Lymph node aspiration performed at the end of treatment to assess the parasitological response to therapy. A TOC is conducted for all VL relapse cases and HIV co-infected cases, and for HIV-negative primary VL cases if clinically indicated. |
| Defaulter | A patient who started VL treatment but interrupted treatment due to the patient leaving the hospital. |
| Lost to follow-up | Patient who was discharged with initial cure, but who did not return for 12 months follow-up visit. |
| Death | Death from any reason during treatment or up to 12 months of follow-up. |
| Definitive cure | Patient with initial cure showing no signs and symptoms of the disease during 12 months of follow-up. Definitive cure is ascertained at 12 months after treatment. |
All VL patients were admitted and treatment was given under observation. The medicine used, dose given, adverse events, interruptions and adherence were recorded on patient files on a daily basis.
HIV diagnosis and treatment
Counseling and HIV testing were offered to all VL patients using two parallel rapid diagnostic tests. Initially with HIV-Determine, Abbott Diagnostics (Abbott Park, IL, USA) and Uni-Gold™ HIV, Trinity Biotech, Bray, Ireland; in recent years KHB (Shanghai Kehua Bio-engineering Co Ltd, Shanghai, China) and STAT-PAK (Chembio Diagnostics Systems, Medford, NY, USA) were used. Positive tests were confirmed by a confirmatory test (Organics Immunocomb Combfirm). In case of discordance, the test was repeated after 6 weeks.
HIV/AIDS management and ART were offered according to national guidelines. VL was considered a WHO stage 4 (AIDS defining) disease and ART was initiated irrespective of CD4 cell count. In 2007, TDF was introduced as one of the first line drugs. Prior to this, zidovudine (AZT) or stavudine (D4T) based regimens were the available first line regimens. Eventually, all patients on D4T were progressively shifted to TDF and AZT based regimens (to avoid long term D4T toxicity) as per WHO guidelines.17
Before starting ART, trained counselors addressed potential issues of adherence to treatment. After starting treatment, adherence to ART regimens was monitored at each visit in sessions with trained adherence counselors using checklists.
Study population and period
Individuals with VL who were HIV co-infected and started on ART at Abdurafi Health Center, from 2008 to 2015, were included.
Data collection, validation and analysis
A dedicated VL database and an HIV follow-up database, were the sources of data related to the study objectives. The two databases were linked with unique identification numbers. Consistency of data was cross-validated with patient files. The data was entered into the Microsoft Excel 2011 Version 14 (Microsoft Corp., Redmond, WA, USA) and analyzed using STATA 12 (StataCorp, College Station, Texas 77845 USA). Descriptive statistics was used to express results. Measures of risk were estimated and adjusted using log binomial regression. The dependent variable was ‘unfavorable treatment outcomes’ defined by any of the following outcomes: died, lost-to-follow- up, slow responders, relapse and transfer out. Initial or definitive cure was considered a favorable outcome (Box 1). Effect of ART timing on VL outcomes were stratified into those who started ART before, within or after 4 weeks of diagnosis of VL. The end of the fourth week was the time for initial treatment outcome assessment with the currently existing VL treatment regimens, except for SSG and paromomycin combination for 17 days. If there is no visible parasite on microscopy of tissue aspiration (test of cure) at this assessment, the patient was declared to have initial cure. But if the test of cure showed persistence of parasites, the treatment will be prolonged. The first 4 weeks were, thus, the period of overlap of treatments. A stepwise backward elimination technique was used initially including variables with an initial p-value cut off of 0.2. A p-value of ≤0.05 (95% CI) was considered significant.
Ethics considerations
The study fulfilled the exemption criteria set by the Médecins Sans Frontières Ethics Review Board (MSF ERB; Geneva, Switzerland) for a retrospective analysis of routinely collected data and thus did not require MSF ERB review. It was conducted with permission from Médecins Sans Frontières (Medical Director, Operational Centre, Amsterdam). Approval was also received from the Union Ethics Advisory Group (International Union against Tuberculosis and Lung Disease, Paris, France). The issue of informed patient consent did not apply because the study used anonymized routine data.
Results
Characteristics of the study population
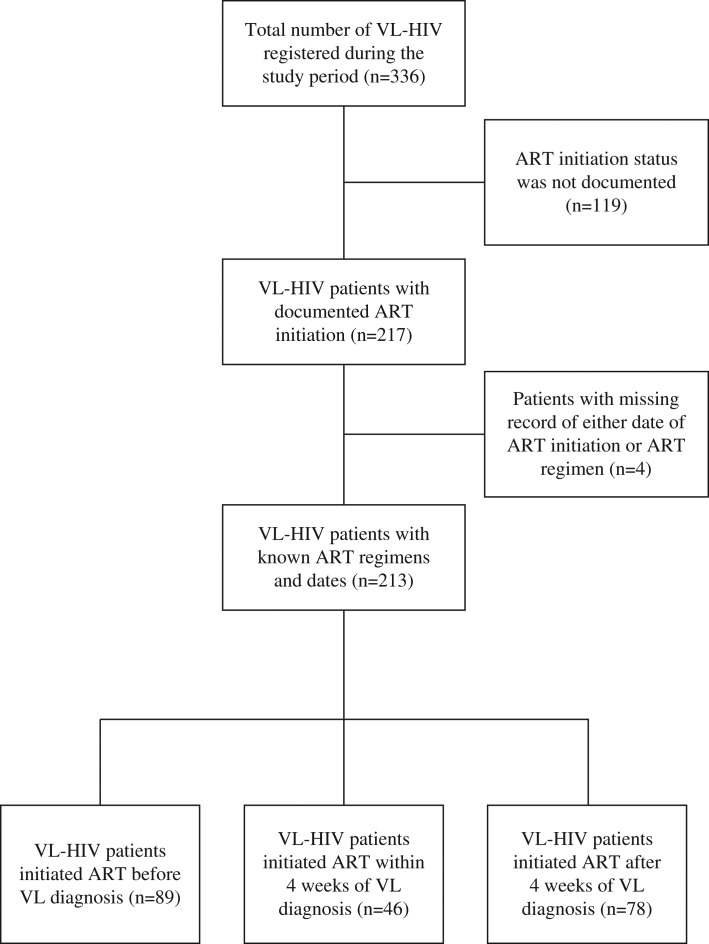
A total of 336 VL-HIV patients were found registered in the study period. Out of these, 217 (64.6%) had a documented ART initiation. One patient did not have date of ART initiation, and three others with no record of ART regimen. Of the remaining 213 patients, 89 (41.8%) patients were already on ART when they were admitted for VL treatment. The remaining 124 (58.2%) presented first with VL and were subsequently diagnosed with HIV and were initiated on ART. Forty-six (21.6%) started ART within 4 weeks of VL diagnosis.
Figure 1 shows the number of patients with VL-HIV at the site with ART uptake. This comprised 162 (76.1%) primary, 49 (23.0%) relapse and 2 (0.9%) post kala-azar dermal leishmaniasis individuals. The majority were male (95%) and migrants (45%) with a median age of 31 years.
Figure 1.
Inclusion of visceral leishmaniasis (VL)/HIV co-infected patients in Antiretroviral (ART) timing study at Abdurafi Health Center, Ethiopia, 2008–2015.
Timing of ART initiation
Table 1 shows the timing of ART initiation and the type of ART and VL regimens. The median length of follow-up after VL diagnosis was 70 weeks (IQR 16–212). Out of 124 VL patients who started ART after VL diagnosis, only 46 (37.1%) started ART within the first 4 weeks of VL diagnosis. Median time to starting ART within 4 weeks was 3.1 weeks (IQR 2.3–3.7) while in those who started thereafter was 7.1 weeks (IQR 4.4–10.9). Those who started ART before VL diagnosis were on ART for a median time of 36 weeks (IQR 9–87) by the time they were diagnosed with the current VL. The majority of VL patients (92%) were started on liposomal amphotericin-B with or without miltefosine.
Table 1.
The timing and type of antiretroviral and antileishmania regimens for patients with visceral leishmaniasis (VL) and HIV, Abdurafi Health Center, Ethiopia, 2008–2015 (n=213)
| Timing and type of regimens | n (%) |
|---|---|
| Antiretroviral treatment (ART) timing (n=213) | |
| ART before VL diagnosis | 89 (41.8) |
| ART initiated within 4 weeks | 46 (21.6) |
| ART initiated after 4 weeks | 78 (36.6) |
| Median time within and after 4 weeks (IQR) | 4.8 (3.5–8.1) |
| Starting antiretroviral combination regimens (n=213) | |
| Zidovudine-lamivudine-nevirapine | 19 (8.9) |
| Zidovudine-lamivudine-efavirenz | 1 (<1) |
| Stavudine-lamivudine-nevirapine | 67 (31.5) |
| Stavudine-lamivudine-efavirenz | 6 (2.8) |
| Tenofovir-lamivudine-nevirapine | 2 (0.9) |
| Tenofovir-lamivudine-efavirenz | 118 (55.4) |
| Initial VL treatment regimena (n=213) | |
| Sodium stibogluconate | 6 (2.8) |
| Liposomal amphotericin B | 108 (50.7) |
| Liposomal amphotericin B with miltefosine | 98 (46.0) |
| Miltefosine | 1 (<1) |
| Second-line VL treatment regimenb (for slow responders); (n=30) | |
| Sodium stibogluconate | 11 (37) |
| Liposomal amphotericin B | 13 (43) |
| Liposomal amphotericin B with miltefosine | 3 (10) |
| Sodium stibogluconate with miltefosine | 3 (10) |
| Pentamidine secondary prophylaxis (PSP); (n=213) | |
| PSP Received within a year of VL | 20 (9.4) |
| PSP Received after over a year of VL | 17 (8.0) |
| Not received PSP | 176 (82.6) |
a Initial treatment means the first attempted treatment regardless of switch for failure or toxicity.
b Second-line regimens are the anti-leishmaniasis regimens used to extend the treatment of slow responding patients after initial VL regimen failed.
Outcomes of VL treatment in relation to timing of ART initiations
Table 2 shows the baseline socio-demographics and clinical features of VL patients in relation to timing of ART initiation. Of all patients, 45.1% (n=96/213) had moderate to severe malnutrition, 24.9% (n=53) had active TB and 60.1% (n=128) had hemoglobin levels under 9 gms/dl (moderate to severe anemia). Only 62 (29.1%) had CD4 count record 90 days around the time of VL diagnosis (baseline) and of those 41 (66%) had <200 cells/mm3.
Table 2.
Baseline socio-demographic and clinical characteristics of visceral leishmaniasis (VL) patient by timing of antiretroviral treatment (ART) initiation at Abdurafi Health Center, Ethiopia, 2008–2015
| ART before current VL episode, n (%) | ART within 4 weeks, n (%) | ART after 4 weeks, n (%) | RRR (95% CI)b | p-value | |
|---|---|---|---|---|---|
| Total | 89 | 46 | 78 | ||
| Age | |||||
| Mean, years (SD) | 35 (8) | 32 (7) | 31 (8) | 33c (32–4) | NS |
| Gender | |||||
| Male | 84 (94) | 45 (98) | 74 (95) | 1 | |
| Female | 5 (6) | 1 (3) | 4 (5) | 0.9 (.5–1.9) | NS |
| Residence status in West Armachiho | |||||
| Resident | 64 (72) | 20 (44) | 32 (41) | 1 | |
| Migrant worker | 25 (28) | 26 (57) | 46 (59) | 1.9 (1.4–2.6) | 0.000 |
| Category of leishmaniasis | |||||
| Primary VL | 59 (66) | 37 (80) | 66 (85) | 1 | |
| Relapse VL | 29 (33) | 8 (17) | 12 (15) | 0.6 (0.4–0 .9) | 0.000 |
| PKDL | 1 (1) | 1 (<1) | 0 | NA | NA |
| Malnutrition status (BMI) | |||||
| Normal (>18), (n=39) | 14 (16) | 11 (24) | 14 (18) | 1 | |
| Mild (16.0–17.9), (n=78) | 30 (34) | 14 (30) | 34 (44) | 1.1 (0.7–1.6) | NS |
| Moderate (14.0–15.9), (n=77) | 35 (39) | 17 (37) | 25 (32) | 0.8 (0.5–1.3) | NS |
| Severe (<14.0), (n=19) | 10 (11) | 4 (9) | 5 (6) | 0.7 (0.4–1.3) | NS |
| Active TBa | |||||
| Yes | 23 (26) | 12 (26) | 18 (23) | 1.1 (0.8–1.5) | NS |
| Edema or ascitesa | |||||
| Yes | 4 (4) | 5 (11) | 3 (4) | 0.9 (0.5–1.8) | NS |
| Jaundicea | |||||
| Yes | 1 (1) | 1 (2) | 4 (5) | 2.2 (0.8–6.5) | NS |
| Unrecorded | 10 (11) | 5 (11) | 8 (10) | NA | |
| Palpable spleena | |||||
| Yes | 78 (88) | 39 (85) | 69 (89) | 0.9 (0.6–1.5) | NS |
| Unrecorded | 2 (2) | 1 (3) | 0 | NA | |
| CD4 count (cell/ml) | |||||
| <200 | 9 (10) | 10 (22) | 22 (28) | 1 | |
| >200 | 5 (6) | 8 (17) | 8 (10) | 0.8 (0.4–1.5) | NS |
| Unrecorded | 75 (84) | 28 (61) | 48 (62) | NA | |
| Hemoglobin (g/dL) | |||||
| >9 | 26 (29) | 20 (44) | 35 (45) | 1 | |
| 5–9 | 53 (60) | 24 (52) | 38 (49) | 0.7 (0.5–1.0) | NS |
| <5 | 7 (8) | 1 (3) | 5 (6) | 0.7 (0.4–1.4) | NS |
| Unrecorded | 3 (3) | 1 (3) | 0 | NA | |
| Pentamidine secondary prophylaxis (PSP) PSP | |||||
| PSP received before or in 1 year (n=20) | 11 (12) | 3 (7) | 6 (8) | 0.8 (0.4–1.3) | NS |
| Leishmaniasis treatment regimen | |||||
| SSG (n=6) | 2 (2) | 2 (4) | 2 (3) | 1 | NS |
| L-AmB (n=108) | 38 (43) | 26 (57) | 44 (56) | 1.1 (0.4–2.7) | NS |
| L-AmB with MF (n=98) | 48 (54) | 18 (39) | 32 (42) | 0.8 (0.3–2.1) | NS |
| dMF (n=1) | 1 (1) | 0 | 0 | NA | |
| Starting antiretroviral regimens | |||||
| AZT-3TC-NVP (n=19) | 12 (14) | 3 (6) | 4 (5) | ||
| AZT-3TC-EFV (n=1) | 0 | 0 | 1 (1) | NA | |
| D4T-3TC-NVP (n=67) | 35 (39) | 15 (33) | 17 (22) | 1.3 (0.7–2.4) | NS |
| D4T-3TC-EFV (n=6) | 3 (3) | 0 | 3 (4) | 1.8 (0.6–5.3) | NS |
| TDF-3TC-NVP (n=2) | 2 (2) | 0 | 0 (0) | NA | |
| TDF-3TC-EFV (n=118) | 37 (42) | 28 (61) | 53 (68) | 2.1 (1.2–3.9) | 0.014 |
3TC: lamivudine; AZT: zidovudine; BMI: body mass index; D4T: stavudine; EFV: efavirenz; L-AmB: liposomal amphotericin B; MF: miltefosine; NA: not applicable; NS: not significant; NVP: nevirapine; PKDL: post-kala azar dermal leishmaniasis (excluded for RR calculation); RRR: relative risk ratio; SSG: sodium stibogluconate; TDF: tenofovir disoprosil fumarate.
a Comparison is done with non missing values.
b Derived from multiple logistic regression model and it is estimated for each delay in ART start from ‘before current VL episode’ to ‘within 4 weeks’ and to ‘after 4 weeks’ categories.
c Mean (95% CI) stated instead of RR.
d Excluded from comparison.
There were no significant differences in characteristics between those who started ART within 4 weeks and those who started ART after 4 weeks. However, patients who had started ART before VL diagnosis differed in their clinical and socio-demographic characteristics. There was a lower proportion of the migrant population, and a higher proportion of relapsed VL patients than the other two groups. Additionally, TDF-based ART regimen was used less frequently in these groups (Table 2).
Of the 213 patients 37 (17.4%) received pentamidine secondary prophylaxis and of these 20 (9.4 %) received it before or within one-year of VL diagnosis. There was no association between the receptions of pentamidine secondary prophylaxis and the timing of ART initiation (OR 0.8; 95% CI 0.4–1.3; p=0.295).
Tables 3 and 4 show the initial and the 12 month outcomes of VL patients in relation to timing of ART initiation. Among primary VL patients, initial cure was 78% (n=29/37; 95% CI 65–92%) in those starting ART within 4 weeks and 80% (n=53/66; 95% CI 71–90%) among those starting thereafter. At 12 months, definitive cure was 59% (n=22/37; 95% CI 43–75%) among those starting ART within 4 weeks and 56% (n=37/66; 95% CI 44–68%) among those started ART thereafter. This was not significantly different (RR 1.1; p=0.738).
Table 3.
Initial outcomes of visceral leishmaniasis (VL) treatment (primary and relapse) in relation to timing of antiretroviral initiation, Abdurafi Health Center, Ethiopia, 2008–2015
| Time of ART initiation | p-valuea | RRb (95%CI) | |||
|---|---|---|---|---|---|
| Before current VL episode n (%) | Within 4 weeks n (%) | After 4 weeks n (%) | |||
| Primary VL (n=162) | |||||
| Total | 59 | 37 | 66 | ||
| Initial cure | 35/59 (59) | 29/37 (78) | 53/66 (80) | ||
| Unfavorable outcome | 24/59 (41) | 8/37 (22) | 13/66 (20) | 0.010 | 0.7 (0.5–0.9) |
| Slow responders | 9/59 (15) | 1/37 (3) | 8/66 (12) | NS | 0.9 (0.5–1.5) |
| Lost to follow-up | 1/59 (2) | 1/37 (3) | 0 | NS | 0.5 (0.1–2.9) |
| Transferred out | 3/59 (5) | 1/37 (3) | 3/66 (5) | NS | 0.9 (0.4–2.2) |
| Died | 11/59 (19) | 5/37 (13) | 2/66 (3) | 0.006 | 0.4 (0.2–0.8) |
| Relapse VL (n=49) | |||||
| Total | 29 | 8 | 12 | ||
| Cured | 16/29 (55) | 5/8 (63) | 10/12 (83) | ||
| Unfavorable outcome | 13/29 (45) | 3/8 (37) | 2/12 (17) | NS | 0.7 (0.4–1.1) |
| Slow responders | 7/29 (24) | 3/8 (37) | 2/12 (17) | NS | 0.9 (0.4–1.9) |
| Lost to follow-up | 1/29 (3) | 0 | 0 | NA | NA |
| Transferred out | 3/29 (10) | 0 | 0 | NA | NA |
| Died | 2/29 (7) | 0 | 0 | NA | NA |
| Post kala-azar dermal leishmaniasis (n=2) | |||||
| Total | 1 | 1 | 0 | ||
| Cured | 1 | 1 | 0 | ||
ART: antiretroviral treatment; NA: not applicable; NS: not significant.
a The p-value and RR are derived from generalized linear model for respective unfavorable outcome and all other outcomes.
b Relative risk, is estimated for each delay in ART start from ‘before current VL episode’ to ‘within 4 weeks’ and to ‘after 4 weeks’ categories.
Table 4.
Twelve month outcomes of visceral leishmaniasis (VL) patients (primary and relapse) in relation to timing of antiretroviral initiation, Abdurafi Health Center, Ethiopia, 2008–2015
| Time of ART initiation | p-valuea | RRb (95%CI) | |||
|---|---|---|---|---|---|
| Before current VL episode n (%) | Within 4 weeks n (%) | After 4 weeks n (%) | |||
| Primary VL (n=162) | |||||
| Total | 59 | 37 | 66 | ||
| Definitive cure | 30/59 (51) | 22/37 (59) | 37/66 (56) | NA | NA |
| Unfavorable outcome | 29/59 (49) | 15/37 (41) | 29/66 (44) | NS | 0.9 (0.8–1.1) |
| Transferred out | 2/59 (3) | 3/37 (8) | 3/66 (5) | NS | 1.1 (0.5–2.4) |
| Lost to follow-up | 4/59 (7) | 5/37 (14) | 7/66 (11) | NS | 1.2 (0.7–2.1) |
| Died | 17/59 (29) | 6/37 (16) | 7/66 (11) | 0.012 | 0.6 (0.4–0.9) |
| Relapsedc | 6/59 (10) | 1/37 (3) | 12/66 (18) | NSc | 1.5 (0.9–2.4) |
| Relapse VL (n=49) | |||||
| Total | 29 | 8 | 12 | ||
| Definitive cure | 9/29 (32) | 3/8 (38) | 8/12 (66) | NA | NA |
| Unfavorable outcome | 20/29 (68) | 5/8 (62) | 4/12 (34) | NS | 0.7 (0.5–1.0) |
| Transferred out | 3/29 (10) | 0/8 (0) | 0/12 (0) | NA | NA |
| Lost to follow-up | 0/29 (0) | 1/8 (12) | 2/12 (17) | NS | 4.1 (0.8–21.5) |
| Died | 5/29 (17) | 2/8 (25) | 0/12 (0) | NS | 0.5 (0.2–1.6) |
| Relapses | 12/29 (41) | 2/8 (25) | 2/12 (17) | NS | 0.6 (0.3–1.2) |
ART: antiretroviral treatment; NA: not applicable; NS: not significant.
a The p-value and RR are derived from generalized linear model for respective unfavorable outcome and all other outcomes.
b Relative risk, is estimated for each delay in ART start from ‘before current VL episode’ to ‘within 4 weeks’ and to ‘after 4 weeks’ categories.
c When compared between within 4-weeks and after 4-weeks of primary VL diagnosis RR 0.1; 95% CI 0–1.1; p=0.023.
However, patients who were ART initiated after primary VL diagnosis had less frequent unfavorable initial outcomes than patients who had started ART before the current primary VL episode (RR 0.7; 95% CI: 0.5–0.9; p=0.010). Although there was no significant difference in overall unfavorable outcomes at 12 months between the groups, patients who were initiated after VL diagnosis had significantly lower mortality than patients who were already on ART before current VL diagnosis (RR 0.6; 95% CI 0.4–0.9; p=0.012).
In patients with triple infection including VL, HIV and TB (n=53), definitive cure at 12 months was lower, 42% (CI 28–55%) compared with 56% (95% CI 48–64%) in those with dual infection (VL+HIV, n=159). However, this was not statistically significant (RR 1.3; 95% CI 1.0–1.8; p=0.068). On the other hand, mortality at 12 months was higher in those with triple infection 15 (28%) compared with double infections 22 (14%); (RR=2.0; 95% CI 1.1–3.6; p=0.016).
Among the primary VL patients who started ART within 4 weeks, 1/37 (3%; 95% CI 0–8%) relapsed compared to 12/66 (18%; 95% CI 9–28%), among those who started ART after the fourth week, indicating early initiation of ART was protective of relapse (RR 0.1; p=0.023). However, when those who were on ART before VL diagnosis (n=59) were also compared, there was no difference (RR 1.5; 95% CI 0.9–2.5%; p=0.152).
For VL relapses, outcomes were worse but there were no significant differences associated with the timing of ART initiation (Table 3 and 4).
Factors associated with unfavorable VL treatment outcomes
There were no significant factors associated with unfavorable outcomes in both the univariate and multivariate analysis of the two groups who were initiated within 4 weeks of VL diagnosis (n=46) and thereafter (n=78).
However, in a stepwise backward elimination of risk factors for unfavorable initial treatment outcomes for all co-infected patients BMI, active TB, severity of illness, enlargement of spleen, admission hemoglobin, and VL treatment regimen qualified for the final model. While admission hemoglobin <9 gm/dL (OR 2.1; 95% CI 1.3–3.2; p=0.001) and lower BMI (OR 1.4; 95% CI 1.0–1.9; p=0.024) increased the odds of unfavorable initial outcome, treatment with L-AmB containing regimen was protective (OR 0.2; 95% CI 0.1–0.4; p=0.000).
A similar stepwise backward elimination of risk factors for unfavorable outcomes by 12 months for all co-infected patients, BMI, jaundice, severity of illness, enlargement of spleen, presence of edema/ascites, and VL treatment regimen qualified for the final model. Lowered BMI was the independent risk factor for unfavorable outcome (OR 1.3; 95% CI 1.0–1.8; p=0.052).
Discussion
This is the first study examining the relationship between timing of ART start and treatment outcomes for VL. It shows that close to half of all patients at baseline were severely ill and timing of ART after VL diagnosis did not influence VL treatment outcomes. When we included patients on ART before the time of primary VL diagnosis, their mortality was significantly higher at the end of VL treatment and by twelve months follow-up periods. Unfavorable outcomes were also more common among VL patients already on ART before the VL disease both by end of initial VL treatment and by 12 months of follow-up especially among the primary VL patients. These findings contradicted what was known about ART benefits because one would expect that patients already on ART have better immunity and therefore better survival.4,8 While this is normally not expected; the development of VL while on ART raises the question if it was working. VL is a stage four AIDS defining illness17 and probably shows that the ART has failed. These patients are at higher risk for other opportunistic conditions, further immunosuppression and death. Unfortunately, we had limited CD4 count records and HIV viral load monitoring in place to substantiate this.
The important differences we observed in socio-demographic and clinical characteristics between ART time groups make interpretations difficult. For example, the higher proportion of loss to follow-up among the migrant population probably hides most mortalities in the later ART initiated groups since they are mostly migrant workers. The lesser proportion of TDF-based (presumably less toxic) ART regimens in those already on ART before VL diagnosis could also explain the higher mortality.
Another possible explanation was the longer time on simultaneous anti-leishmaniais and ART drugs in those who started ART earlier than VL diagnosis leading to increased risk of toxicity.
On the other hand, definitive cure assessed at 12 months (59%) was also unsatisfactory compared with that among immunocompetent patients as seen in other studies (>90%).22 In addition to the ‘double trouble’ of having to cope with HIV and VL co-infection, several also had active TB—in effect, these patients were in ‘triple trouble’.
The findings are important as they highlight two fundamental operational challenges. First, patients are reaching the treatment facility too late by which time they have advanced disease. Immunological recovery is poor despite ART after profound immunodeficiency and outcome remains poor. Second, VL treatment with the ‘best’ available drugs for now (L-AmB, with or without miltefosine) is ineffective12,23 in achieving an acceptable threshold (in our opinion 90% or over) of definitive cure in Ethiopia. The latter is an ‘urgent call’ for a new armamentarium of drugs needed to manage VL in Africa. Achieving the newly declared Sustainable Development Goal (SDG) target24 of eliminating VL as a neglected tropical disease by the year 2030, will only become a reality if we can address these two challenges. Advocacy and funding, coupled with innovative Research and Development is needed to change the current paradigm. The study strengths include selection of one of the largest NGO supported VL management sites in Ethiopia which was well-resourced in logistics and supervision; availability of dedicated and comprehensive databases for HIV and VL; and data validation using patient cards. In addition, the subject per se is of national and global public health importance and may influence policy and practice.
The main study limitation is the limited cohort of VL patients who were found HIV positive and then placed on ART. On one side, this affects statistical power of the comparisons but on the other side, it is a likely reflection of field-level operational challenges associated with starting VL patients on ART. This thinking is supported by the fact that we ended up with a limited number of patients despite inclusion of 7 years of data from one of the largest VL centers in Ethiopia. A second limitation is that losses to follow-up and transfer outs might include unascertained deaths. The use of co-trimoxazole prophylaxis was not captured in this study.
These limitations notwithstanding, the study has a number of policy and practice implications. First, why are patients severely ill on VL diagnosis? Patients with these two infections have rapid deterioration in their immunity and are further predisposed to a number of other opportunistic conditions. Hurdles in access to diagnosis and treatment may be the other factors to blame. Diagnosis of VL and HIV in co-infected patients is a challenge as the existing first line serological tests have only moderate performance.25 This complicates the diagnostic algorithms, which may result in delayed diagnosis. The centralized treatment sites and a migratory nature of the population may also add to the problem of access. For example, transport may not be available for patients within large, arid and farmland areas implying that ill patients have to walk to get to the treatment center. Furthermore, even when transport is available, patients may be unable to afford the related costs. Improving the current state of affairs will need access to robust ‘point-of-care’ diagnostic tests and multiplying the numbers of accessible treatment centers to improve geographic access. The latter seems logical but will need further reflection on the practicalities. In the meantime, advocacy and increased community awareness raising activities that are already underway (including by MSF) needs to be enhanced.
Second and surprisingly, we did not find any significant differences in VL outcomes in relation to time (within 4 weeks and after 4 weeks) of ART initiation and overall VL outcomes were unsatisfactory. This was contrary to the general expectation based on the impact of ART on VL outcomes reported previously.12,23 The fact that mortality at initial and 12 months outcome was higher in patients who had started ART before VL indicate ART and VL related drug–drug interactions may have played a greater role. However, this conclusion is not warranted, because we also observed significant differences in regard to the ART constituents, primary versus relapse VL composition and socio-demographic characteristics between the groups. The severe baseline clinical status of patients may be to blame as this might have negated any added benefit of early ART. In addition, drug–drug interactions, poor tolerance of multiple drugs in malnourished individuals and immune reconstitution syndrome linked to TB and HIV may also have been culprits in both comparison groups.26,27 The way forward is to catch patients early. In this regard, tri-dimensional screening seems logical. From a program perspective, this implies that individuals presenting with fever and weight loss (with or without a palpable spleen) and living in VL endemic areas should be screened for HIV, TB and VL. This is all the more justified, since those with triple infection (including TB) had lower proportions of definitive cure than those with dual infections. The potential role of introducing GeneXpert and Urine LAM tests in improving early TB diagnosis is an important operational consideration.28 While the prevention of TB through use of isoniazid preventive treatment could be of potential importance, implementation can be complicated and this too, needs further reflection.28
Finally, although limited by small numbers, about one in three individuals with primary VL who started ART after 4 weeks ended up in relapse, which was higher than those starting ART earlier. It may indicate ART related immune benefit in preventing relapse.13,23
Conclusions
In conclusion, we have highlighted the dire predicament currently faced by VL patients in Ethiopia. ART initiation within 4 weeks of VL diagnosis was protective of relapse among primary VL patients by 12 month follow-up, as compared to later ART initiation. However, initiation of ART before primary VL diagnosis was associated with higher mortalities at end of VL treatment and by 12 month follow-up period, but may have been confounded ART regimen and/or comorbidities. More research on the optimal timing of ART initiation in HIV/VL co-infected patients is needed.
Acknowledgments
Authors’ contributions: EMA, ED, RZ, and KR were involved with conception and design of the protocol, which was critically reviewed by MSdF. EMA, MSdF and CA were involved with acquisition of data, and EMA, ED, and RZ did the data analysis and all authors were involved with interpretation. The first draft manuscript was written by EMA, ED and RZ and then critically reviewed by KR and then by all co-authors. All authors have given approval for the final version to be published and are accountable. KR is the guarantor of this paper.
Acknowledgements: This research was conducted through the Structured Operational Research and Training Initiative (SORT-IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at WHO (WHO/TDR). Abdurafi Health Center staff working in care of HIV and VL care assisted during data validation for this study. Zulisile Zulu, Hsin-yi Lee, and Philip Owiti have contributed during the development of the first draft protocol.
Funding: The program was funded by the United Kingdom's Department for International Development (DFID), The Union, MSF and Médecins sans Frontières-Operational Center Amsterdam. Médecins Sans Frontières- Operational Center Amsterdam supported open access publication. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Ethical approval: Approval was received from the Union Ethics Advisory Group (International Union against Tuberculosis and Lung Disease, Paris, France). The study protocol was also submitted to the MSF ethical review process. MSF medical editors critically reviewed the protocol as part of this process. The protocol did not have issues that required a full ethical review by the board because it was determined by the medical editors to fulfill the MSF six-point criteria for exception of a full ethical review.
References
- 1. van Griensven J, Diro E. Visceral leishmaniasis. Infect Dis Clin North Am 2012;26:309–22. doi:10.1016/j.idc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 2. Alvar J, Vélez ID, Bern C et al. . Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012;7 doi:10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarvis JN, Lockwood DN. Clinical aspects of visceral leishmaniasis in HIV infection. Curr Opin Infect Dis 2013;26:1–9. doi:10.1097/QCO.0b013e32835c2198. [DOI] [PubMed] [Google Scholar]
- 4. Alvar J, Aparicio P, Aseffa A et al. . The relationship between leishmaniasis and AIDS: The second 10 years. Clin Microbiol Rev 2008;21:334–59. doi:10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diro E, Lynen L, Ritmeijer K et al. . Visceral leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis 2014;8 doi:10.1371/journal.pntd.0002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvar J, Cañavate C, Gutiérrez-Solar B et al. . Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev 1997;10:298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diro E, Lynen L, Mohammed R et al. . High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis 2014;8 doi:10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horst R, Collin SM, Ritmeijer K et al. . Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis 2008;46:1702–9. doi:10.1086/587899. [DOI] [PubMed] [Google Scholar]
- 9. Diro E, van Griensven J, Mohammed R et al. . Atypical manifestations of visceral leishmaniasis in patients with HIV in north Ethiopia: A gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect Dis 2015;15:122–9. doi:10.1016/S1473-3099(14)70833-3. [DOI] [PubMed] [Google Scholar]
- 10. Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in hiv-infected patients: A systematic review. PLoS Negl Trop Dis 2011;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berhe N, Wolday D, Hailu A et al. . HIV viral load and response to antileishmanial chemotherapy in co-infected patients. AIDS 1999;13:1921–5. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Velez R. The impact of highly active antiretroviral therapy (HAART) on visceral leishmaniasis in Spanish patients who are co-infected with HIV. Ann Trop Med Parasitol 2003;97(Suppl 1):143–7. [DOI] [PubMed] [Google Scholar]
- 13. Marques N, Cabral S, Sa R et al. . Visceral leishmaniasis and HIV infection in the HAART era. Acta Med Port 2007;20:291–8. [PubMed] [Google Scholar]
- 14. Blanc F-X, Sok T, Laureillard D et al. . Earlier versus Later Start of Antiretroviral Therapy in HIV-Infected Adults with Tuberculosis. N Engl J Med 2011;365:1471–81. doi:10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Havlir D V, Kendall MA, Ive P et al. . Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011;365:1482–91. doi:10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdool Karim SS, Naidoo K, Grobler A et al. . Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362:697–706. doi:10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf [accessed 19 November 2016].
- 18. Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 2012;67:2576–97. doi:10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 19. Wade RL, Chaudhari P, Natoli JL et al. . Nephrotoxicity and other adverse events among inpatients receiving liposomal amphotericin B or amphotericin B lipid complex. Diagn Microbiol Infect Dis 2013;76:361–7. doi:10.1016/j.diagmicrobio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 20. FMOH , ed. Guidelines for Diagnosis, Treatment and Prevention of Leishmaniasis in Ethiopia. 2nd ed. Addis Ababa: Federal Ministry of Health Ethiopia; 2013.
- 21. Salih NAW, van Griensven J, Chappuis F et al. . Liposomal amphotericin B for complicated visceral leishmaniasis (kala-azar) in eastern Sudan: How effective is treatment for this neglected disease? Trop Med Int Health 2014;19:146–52. doi:10.1111/tmi.12238. [DOI] [PubMed] [Google Scholar]
- 22. Ritmeijer K, Dejenie A, Assefa Y et al. . A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis 2006;43:357–64. doi:10.1086/505217. [DOI] [PubMed] [Google Scholar]
- 23. Fernandez Cotarelo MJ, Abellan MJ, Guerra Vales JM et al. . Effect of highly active antiretroviral therapy on the incidence and clinical manifestations of visceral leishmaniasis in human immunodeficiency virus-infected patients. Clin Infect Dis 2003;37:973–7. [DOI] [PubMed] [Google Scholar]
- 24. United Nations Sustainable Development GOALS - 17 Goals to transform our world. Sustainable development goals - United Nations. http://www.un.org/sustainabledevelopment/sustainable-development-goals/ [accessed 19 November 2016].
- 25. ter Horst R, Tefera T, Assefa G et al. . Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg 2009;80:929-–34.. doi:80/6/929. [PubMed] [Google Scholar]
- 26. Lin J-N, Lai C-H, Chen Y-H et al. . Immune reconstitution inflammatory syndrome presenting as chylothorax in a patient with HIV and Mycobacterium tuberculosis coinfection: a case report. BMC Infect Dis 2010;10:321 doi:10.1186/1471-2334-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agarwal U, Kumar A, Behera D et al. . Tuberculosis associated immune reconstitution inflammatory syndrome in patients infected with HIV: meningitis a potentially life threatening manifestation. AIDS Res Ther 2012;9:17 doi:10.1186/1742-6405-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harries AD, Zachariah R, Corbett EL et al. . The HIV-associated tuberculosis epidemic-when will we act? Lancet 2010;375:1906–19. doi:10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]