Abstract
This study aimed to distinguish between the single and interactive effects of phosphorus (P), calcium (Ca), and phytase on products of phytate degradation, including the disappearance of myo-inositol (MI), P, Ca, and amino acids (AA) in different segments of the digestive tract in broiler chickens. Additionally, all dephosphorylation steps from myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate) (InsP6) to MI were investigated in the digesta of the terminal ileum. Unsexed Ross 308 broiler chickens were allocated to 56 pens with 19 birds per pen, and assigned to one of 8 dietary treatments. The dietary treatments included diets without (P−, 4.1 g/kg DM) or with (P+, 6.9 g/kg DM) monosodium phosphate supplementation, without (Ca−, 6.2 g/kg DM) or with (Ca+, 10.3 g/kg DM) additional fine limestone supplementation, and without or with 1,500 FTU phytase/kg feed in a factorial design. Adding Ca or P had no effect on InsP6 disappearance in the crop when phytase was added. InsP6 disappearance up to the terminal ileum (P−Ca− 56%) was decreased in P+Ca− (40%), and even more so in P+Ca+ (21%), when no phytase was added. Adding phytase removed all effects of P and Ca (77 to 87%); however, P+Ca+ increased the concentrations of lower InsP esters and reduced free MI in the ileum, even in the presence of phytase. These results indicate that mineral supplements, especially P and Ca combined, reduce the efficacy of endogenous microbial or epithelial phosphatases. Supplementation with phytase increased, while supplementation with Ca decreased the concentration of MI in all segments of the digestive tract and in blood plasma, demonstrating the ability of broilers to fully degrade phytate and absorb released MI. While AA disappearance was not affected by P or Ca, or an interaction among P, Ca, and phytase, it increased with the addition of phytase by 2 to 6%. This demonstrates the potential of the phytase used to increase AA digestibility, likely independent of P and Ca supply.
Keywords: phosphorus, calcium, phytase, myo-inositol, amino acid
INTRODUCTION
Phosphorus (P) is an important element in poultry nutrition, which must be adequately supplied in the diet. However, for non-ruminant animals, it is only partially available from plant seeds, where P is predominantly bound as phytic acid [myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6] and in salt form, called phytate (Eeckhout and de Paepe, 1994; Rodehutscord et al., 2016). InsP6-P can be utilized after the stepwise cleavage of the P from the phytate molecule by phytases (myo-inositol hexaphosphate phosphohydrolases) and other phosphatases. Because plant seeds and byproducts are major components of poultry diets, and P is of specific economic and environmental relevance, the improvement of the digestibility of plant P in poultry is of great interest.
In this context, the determination of potential inhibiting factors on InsP6 degradation is of high relevance. Previous studies have found negative effects of adding P and calcium (Ca) to poultry diets on InsP6 disappearance in the digestive tract (Tamim et al., 2004; Shastak et al., 2014). In contrast, the addition of microbial phytases to feed results in the increased availability of P from plant ingredients, and a reduction in the complex formation between phytate and susceptible minerals (Lei and Porres, 2007). However, supplements of phytase and mineral P have been shown to exert interactive effects on InsP6 disappearance and P digestibility in broilers. In a broiler study by Zeller et al. (2015b), supplementing low P and Ca diets with monocalcium phosphate in the presence of a practical level of a microbial 6-phytase led to decreased InsP6 and P disappearance up to the terminal ileum. However, a very high dose of phytase (12,500 FTU/kg) diminished the negative effects of monocalcium phosphate. Owing to the simultaneous supplementation of P and Ca, the authors were not able to distinguish the single effects of P and Ca supplementation.
The detection and measurement of lower InsP esters and their positional isomers in the digestive tract of poultry was first carried out by Zeller et al. (2015a). To optimize P utilization in the animal, it is of great relevance to link the intermediate products of phytate degradation to phytases of different origins. Moreover, specific InsP isomers can have distinct biological functions (Irvine and Schell, 2001; Żyła et al., 2004). However, only the isomers of InsP3–5 have been measured to date, and thus no information exists on the concentrations of isomers of InsP1–2 in the ileum of broilers. In addition, recent studies assume a direct effect of the myo-inositol (MI) released after the complete dephosphorylation of InsP6 on growth performance. A study by Walk et al. (2014) that used a very high level of phytase supplementation concluded that the improvements in feed conversion ratio probably were not only caused by reduced nutrient-phytate interactions, but also by the provision of MI.
Several studies were carried out to investigate the effect of a phytase supplementation on prececal amino acid (AA) digestibility in broilers. Proteins and AA can build complexes with phytate, resulting in reduced digestibility. Moreover, it is suggested that increasing dietary phytate concentrations may lead to increased endogenous AA losses (Cowieson et al., 2004). The results regarding this, however, are conflicting. In some studies, phytase increased AA digestibility in broilers (Ravindran et al., 1999; Rutherfurd et al., 2002; Amerah et al., 2014), whereas in other studies, no effect of phytase was observed (Sebastian et al., 1997; Peter and Baker, 2001; Rodehutscord et al., 2004). As Ca and P were shown to exert effects on phytate degradation, and as phytate can be complexed with proteins and AA, it is possible that the Ca and P concentrations used in the aforementioned studies contributed to the contradictory results.
Therefore, the first objective of the present study was to distinguish between the single and interactive effects of P and Ca, with and without phytase supplementation, on InsP6, P, Ca, and AA disappearance up to the terminal ileum. The second objective was to characterize the degradation steps from InsP6 in the digesta of the terminal ileum via all lower InsP esters and their positional isomers down to MI, and the effects of P, Ca, and phytase on the degradation pattern. The addition of P was hypothesized to have greater effects on InsP6 and P disappearance than the addition of Ca owing to end product inhibition. Additionally, the MI concentration in the digestive tract was hypothesized to be higher in the presence of phytase.
MATERIALS AND METHODS
Birds and Housing
The trial was performed at the Agricultural Experiment Station of the University of Hohenheim and approved by the Regierungspräsidium Tübingen, Germany (Project no. HOH 33/14 TE) in accordance with the German Animal Welfare Legislation. A total of 1,064 unsexed Ross 308 broiler hatchlings was supplied by a commercial hatchery (Brüterei Süd GmbH & Co. KG, Regenstauf, Germany) and allocated to 56 floor pens (115 × 230 cm ground area, 260 cm height) on deep litter bedding, each comprising 19 hatchlings. The animals were fed a commercially available starter diet [containing 220 g CP/kg, 9.5 g Ca/kg, 6.5 g P/kg, 12.2 MJ ME/kg, 1 mg coccidiostat Diclazuril/kg, 2,250 VU endo-1,3 (4)-β-glucanase/kg, 1,650 VU endo-1,4-β-xylanase/kg, and 750 phytase units (FTU) 6-phytase/kg] until d 15. At the beginning of the experimental phase on d 15, 7 pens were randomly allocated to each of the 8 treatments in a completely randomized block design. For this period, the animals were allocated on perforated floors. Feed and tap water were provided for ad libitum consumption from placement to the end of the trial. The light program was as follows: 24L:0D from hatch to d 3, 22L:2D from d 4 to 7, 16L:8D from d 8 to 10, 12L:12D from d 11 to 20, and 16L:8D from d 21 to the end of the experiment. The temperature in the barn was set at 34°C on the first d, and then gradually decreased every 3 to 5 d to achieve a temperature of 24°C on the last 3 d of the experiment. The well-being of the animals was checked twice daily, and the occurrence and weight of dead animals, as well as the feed consumption up to that point, were recorded.
Diets and Treatments
Diets were calculated to contain adequate levels of all nutrients, with the exception of Ca and P, according to the recommendations of the Gesellschaft für Ernährungsphysiologie (1999), and were mainly based on solvent-extracted soybean meal and corn (Table 1). The calculated concentration of CP and ME were 250 g/kg DM and 13.5 MJ/kg DM, respectively. Five g/kg titanium dioxide (TiO2) was included in the diets as an indigestible marker. The experiment was designed as a 2 × 2 × 2-factorial arrangement of treatments (2 P levels, 2 Ca levels, and 2 phytase levels), and included diets without (P−, 4.1 g/kg DM) or with (P+, 6.9 g/kg DM) monosodium phosphate supplementation, without (Ca−, 6.2 g/kg DM) or with (Ca+, 10.3 g/kg DM) additional fine limestone supplementation, and without (Phy−) or with (Phy+) 1,500 FTU phytase/kg feed (modified E. coli-derived 6-phytase; Quantum BlueTM, AB Vista, Marlborough, UK). The experimental diets were produced by first mixing all ingredients with the exception of variable ingredients. This mix was divided into 4 parts. Each part was then supplemented with an individual mixture of monosodium phosphate and limestone or sand or both, and mixed again. Each of the resulting mixtures was then divided into 2 parts and supplemented, or not, with the microbial phytase product. Subsequent to phytase supplementation, the diets were remixed and pelleted using a 3-mm pelleting matrix without using steam conditioning. The pelleting temperature stayed below 65°C, which was confirmed by the temperature measurement of the pellets immediately after release from the press. Representative samples of each diet were taken and ground through a 0.5 mm sieve, or pulverized by a vibrating cup mill (PULVERISETTE 9, Fritsch GmbH, Idar-Oberstein, Germany). The calculated and analyzed concentrations of P and Ca differed by less than 10%. The analyzed phytase activity ranged between 1,150 and 1,230 FTU/kg (Table 1), hence similar for all treatments but lower than formulated. The experimental diets contained similar concentrations of InsP6, InsP5, and MI. Other InsPs were not detected in the diets.
Table 1.
Ingredient and analyzed composition of the experimental diets.
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | |||||
|---|---|---|---|---|---|---|---|---|
| Ingredient, g/kg as fed | ||||||||
| Corn | 541 | 541 | 541 | 541 | ||||
| Extracted soybean meal | 400 | 400 | 400 | 400 | ||||
| Soybean oil | 15 | 15 | 15 | 15 | ||||
| D,L-Methionine | 2 | 2 | 2 | 2 | ||||
| Monosodium phosphate1 | − | − | 10.5 | 10.5 | ||||
| Sand | 18 | 8.5 | 9.5 | − | ||||
| Limestone | 10 | 19.5 | 10 | 19.5 | ||||
| Sodium chloride | 1 | 1 | 1 | 1 | ||||
| Choline chloride | 2 | 2 | 2 | 2 | ||||
| Sodium bicarbonate | 3 | 3 | 1 | 1 | ||||
| Vitamin mix2 | 2 | 2 | 2 | 2 | ||||
| Mineral mix3 | 1 | 1 | 1 | 1 | ||||
| TiO2 | 5 | 5 | 5 | 5 | ||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | |
| Analyzed composition | ||||||||
| Ca, g/kg DM | 5.9 | 6.4 | 9.8 | 10.0 | 6.2 | 6.2 | 10.1 | 9.8 |
| Total P, g/kg DM | 4.5 | 4.5 | 4.3 | 4.4 | 7.5 | 7.4 | 7.4 | 7.3 |
| InsP6-P, g/kg DM | 2.9 | 2.8 | 2.9 | 2.9 | 2.9 | 2.8 | 2.8 | 2.8 |
| Myo-inositol, μmol/g DM | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Ins(1,2,3,4,5)P54, μmol/g DM | 0.5 | 0.6 | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 |
| Ins(1,2,4,5,6)P54, μmol/g DM | 1.1 | 1.2 | 1.2 | 1.2 | 1.1 | 1.1 | 1.2 | 1.2 |
| InsP64, μmol/g DM | 15.6 | 15.3 | 15.7 | 15.5 | 15.8 | 15.4 | 15.4 | 15.3 |
| Phytase Activity, FTU/kg | <50 | 1150 | <50 | 1150 | <50 | 1160 | <50 | 1230 |
1NaH2PO4 for use in foodstuff (Dr. Paul Lohmann GmbH KG, Emmerthal, Germany).
2Vitamin premix (Raiffeisen Kraftfutterwerke Süd GmbH, Würzburg, Germany), provided per kg of complete diet: 1,200 IU vitamin A, 3,000 IU vitamin D3, 30 mg DL-α-Tocopherylacetate, 2.4 mg vitamin K3, 3 mg vitamin B1, 6 mg vitamin B2, 6 mg vitamin B6, 30 μg vitamin B12, 50 mg nicotinic acid, 14 mg pantothenic acid, 1 mg folic acid, 0.1 mg biotin.
3Mineral mix (Gelamin, Gesellschaft für Tierernährung mbH, Memmingen, Germany), provided per kg of complete diet: 240 mg calcium from calcium carbonate, 100 mg manganese from manganese-(II)-oxide, 65 mg zinc from zinc-oxide, 25 mg iron from ferrous-(II)-carbonate, 7 mg copper from cupric-(II)-sulphate pentahydrate, 4 mg iodine from calcium iodate, 0.3 mg selenium from sodium selenite.
4No other InsP isomers were detected.
Sampling and Measurements
Animals and feed were weighed on d 1, 15, and 27 to determine ADFI, and to calculate ADG and feed-to-gain ratio (F:G) on a pen basis. The animals were deprived of feed 2 h before slaughter, and then 1 h before the slaughter, feeders were moved back into the pens in order to standardize intestinal fill. On d 25, one animal per pen (6 pens per treatment) was stunned with a gas mixture of 35% CO2, 35% N2, and 30% O2, killed by decapitation, and the trunk blood collected in tubes containing sodium fluoride and heparin. Blood samples were then centrifuged for 10 min at 2,000 × g to separate the plasma. On d 27, all other broilers from the same pen were stunned with the gas mixture and euthanized by CO2 asphyxiation. The number of animals per pen varied between 14 and 17 owing to other collection events in the d prior to the sample collection event described here. Digesta from the crop, proventriculus and gizzard together (prov+giz), duodenum and jejunum together (duo+jej), and the terminal part of the ileum (last two-thirds of the section between Meckel's diverticulum and 2 cm prior the ileo-ceco-colonic junction) were collected and pooled on a pen basis. The crop was clamped with an arterial clamp to prevent emptying. Crop and stomachs were then opened and upended, and digesta gently removed with a spatula without scraping the mucosa. Digesta from intestinal sections were rinsed with cold double-distilled water. All samples were immediately frozen at −20°C, freeze-dried, and pulverized by a vibrating cup mill. Pulverized samples were stored in airtight containers until further analysis at a temperature below 6°C.
Chemical Analysis
Ground feed samples were analyzed according to the official methods in Germany (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA), 2007) for DM (method no. 3.1), and CP (no. 4.1.1). Pulverized ileum digesta samples also were analyzed for CP. Pulverized feed and digesta samples were analyzed for P, Ca, and Ti using a modified method from Boguhn et al. (2009), described in detail by Zeller et al. (2015a).
The extraction and measurement of InsP3–6 isomers in feed and digesta were carried out using the method of Zeller et al. (2015a) with slight modifications. Briefly, samples were extracted twice with a solution of 0.2 M EDTA and 0.1 M sodium fluoride (pH 8.0; 4°C) for 30 min under agitation, and centrifuged after each extraction at 12,000 × g for 15 minutes. The respective supernatants were combined, and a 1-mL sample was centrifuged at 14,000 × g for 15 min, and then filtered before being centrifuged again at 14,000 × g for 30 minutes. Filtrates were analyzed using high-performance ion chromatography and UV detection at 290 nm after post-column reaction with Fe(NO3)3 in HClO4 using an ICS-3000 system (Dionex, Idstein, Germany). Some InsP3 isomers could not be identified because the specific standards were unavailable. A clear discrimination between the isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because of co-elution, and therefore the term InsP3x will be used for these InsP3 isomers of unknown proportions. InsP6 was used for quantification, and correction factors for differences in detector responses for InsP3–5 were used according to Skoglund et al. (1997). For the analysis of the InsP1–2 isomers that were analyzed solely in the ileum digesta, an extraction was performed with 0.2 M sodium fluoride at pH 8.0, and otherwise carried out as previously described for InsP3–6 isomers. Filtrates were analyzed by high-performance ion chromatography and conductivity detection using an ICS-3000 system (Dionex, Idstein, Germany). A clear discrimination between the isomers Ins(1)P1 and Ins(2)P1 was not possible because of co-elution, and therefore the term InsP1x will be used for the InsP1 isomers of unknown proportions.
For analysis of MI, samples of feed and digesta were derivatized without sample cleanup. Proteins from plasma samples were precipitated by addition of acetonitrile, and samples were lyophylized prior to derivatization. A 2-step derivatization procedure comprising oximation and silanisation was carried out. Deuterated MI was used as internal standard. MI was measured using a 5977A gas chromatograph/mass spectrometer of Agilent (Waldbronn, Germany).
Analysis of AA was performed according to Rodehutscord et al. (2004). In brief, samples were oxidized in an ice bath using a mixture of hydrogen peroxide, phenolic formic acid solution, and phenol. Then, samples were hydrolyzed at 113°C for 24 h in a mixture containing hydrochloric acid and phenol. Norleucine was used as an external standard. AA were separated and detected using an L-8900 Amino Acid Analyzer (VWR, Hitachi Ltd, Tokyo, Japan). Methionine and cysteine were determined as methionine sulfone and cysteic acid, respectively. The concentrations of tyrosine, histidine, and phenylalanine may be affected to some extent by the oxidation procedure (Mason et al., 1980).
Feed samples were analyzed for phytase activity by Enzyme Services and Consultancy (Ystrad Mynach, Wales, UK) using the analytical method of the enzyme producer (pH 4.5; 60°C), followed by transferring the results to the commonly used FTU per kilogram of feed by a validated transfer factor.
Calculations and Statistical Analyses
ADG, ADFI, and F:G were calculated for the experimental period from d 15 to 27 on a pen basis, and then divided by the number of remaining animals per pen. Dead animals were taken into account by the calculation of their ADG and ADFI per d of life.
The disappearance of InsP6, P, Ca, CP, and AA was calculated based on the analyzed concentrations of InsP6, P, Ca, CP, AA, and Ti in feed and digesta. The following generally accepted equation was used:
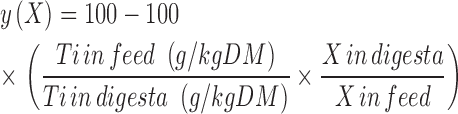 |
Where: y(X) is the disappearance of X in %; and X is InsP6, P, Ca, CP, or AA in grams per kilogram DM. InsP6 disappearance was not calculated for prov+giz because the marker did not represent the particles in these segments properly owing to different particle sizes (Zeller et al., 2015a).
The concentrations of disappeared P and Ca (y) was calculated as follows:
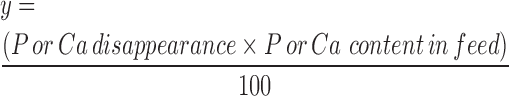 |
Where: y is in grams per kilogram DM, P or Ca disappearance is in %, and P or Ca content in feed is in grams per kilogram DM.
After testing data for normal distribution, data that were not normally distributed were either log or square transformed. Table values in this work are presented as the treatment means of the untransformed data and the pooled SEM. All data were analyzed in a 3-factorial analysis of variance using the MIXED procedure of the software package SAS (version 9.3; SAS Institute Inc., Cary, NC). For all traits analyzed in this experiment, with the exception of blood, samples were pooled on a pen basis, and thus the pen was considered to be the experimental unit. In the case of the blood that was obtained from individual birds, the bird was considered to be the experimental unit. The following model was chosen: Yijk = μ + αi + βj + γk+ (αβ)ij + (αγ)ik + (βγ)jk + (αβγ)ijk + εijk, where Yijk = response variable, μ = overall mean, αi = effect of P addition, βj = effect of Ca addition, γk = effect of phytase addition, and all possible interactions among the effects and εijk = residual error. Replicates ( = blocks) were considered to be random effects. Statistical significance was declared at P < 0.05.
RESULTS
Performance Traits
The initial BW of broiler chicks on d 15 was 556 g, and did not differ among treatments (P = 0.248). The P × Ca × Phy interaction was significant for BW at 27 d, ADFI, ADG, and F:G (P < 0.05, respectively; Table 2). The highest BW at 27 d, ADG and ADFI, and the lowest F:G were observed in the treatment P−Ca−Phy+ and in treatments with supplemented P. The supplementation of phytase increased animal performance in the treatments without the addition of P, but not in treatments with P addition. Performance traits were the lowest in animals receiving the treatment P−Ca+Phy−.
Table 2.
Interaction of P, Ca, and phytase on performance traits of broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| BW d 27, g | 1,528b | 1,640a | 1,270c | 1,509b | 1,625a | 1,646a | 1,608a | 1,640a | 19.0 | *** | *** | *** | *** | *** | * | * |
| ADFI, g/d | 120c | 125a,b | 99e | 116d | 128a | 128a | 124b,c | 127a,b | 1.4 | *** | *** | *** | *** | *** | *** | * |
| ADG, g/d | 80b | 88a | 59c | 79b | 89a | 90a | 87a | 90a | 1.2 | *** | *** | *** | *** | *** | *** | ** |
| F:G, g/g | 1.50b | 1.42d | 1.67a | 1.47b,c | 1.44c,d | 1.42d | 1.43d | 1.41d | 0.01 | *** | *** | *** | *** | *** | *** | *** |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
a–eMeans within a row not showing a common superscript differ (P < 0.05).
InsP6 Disappearance
In the crop, InsP6 disappearance was low when no phytase was supplemented (Table 3). An interaction of Ca × Phy (P = 0.048) was caused by a higher rate of InsP6 disappearance owing to Ca supplementation in the absence of phytase, whereas treatments Ca−Phy+ and Ca+Phy+ did not differ. In the duo+jej, a P × Phy interaction (P = 0.008) was detected, indicating that the effects of phytase were larger when P was supplemented. In the ileum, the interaction P × Ca × Phy (P < 0.001) was caused by decreased InsP6 disappearance in P+Ca-Phy-, and even more so in P+Ca+Phy− in comparison to P−Ca−Phy− or P−Ca+Phy−; however, all differences were lost when phytase was added.
Table 3.
Interaction of P, Ca, and phytase on InsP6, P and Ca disappearance up to the crop, duodenum/jejunum, and terminal ileum of broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| InsP6 disappearance, % | ||||||||||||||||
| Crop | 2 | 68 | 12 | 61 | 3 | 66 | 4 | 71 | 5.03 | n.s. | † | *** | n.s. | n.s. | * | † |
| Duo/Jej | 46 | 81 | 39 | 73 | 36 | 76 | 16 | 66 | 2.44 | *** | *** | *** | † | ** | n.s. | n.s. |
| Ileum | 56b | 87a | 54b | 77a | 40c | 87a | 21d | 77a | 2.76 | *** | *** | *** | *** | *** | ** | *** |
| P disappearance | ||||||||||||||||
| Duo/Jej, % | 20e | 44b,c | 11f | 39c,d | 46b | 61a | 38d | 45b,c | 2.68 | *** | *** | *** | * | *** | n.s. | * |
| Duo/Jej, g/kg DM | 0.9e | 2.0d | 0.5f | 1.7d | 3.5b | 4.5a | 2.8c | 3.2b | 0.15 | *** | *** | *** | *** | * | n.s. | * |
| Ileum, % | 48 | 68 | 41 | 60 | 62 | 76 | 48 | 59 | 1.65 | *** | *** | *** | ** | ** | n.s. | n.s. |
| Ileum, g/kg DM | 2.2 | 3.1 | 1.7 | 2.7 | 4.6 | 5.6 | 3.6 | 4.3 | 0.09 | *** | *** | *** | *** | n.s. | n.s. | n.s. |
| Ca disappearance | ||||||||||||||||
| Duo/Jej, % | 37b,c | 40a,b | 38a,b | 39a,b | 37b,c | 41a | 35c | 29d | 1.20 | *** | *** | n.s. | *** | * | *** | ** |
| Duo/Jej, g/kg DM | 2.2f | 2.5d,e | 3.8a,b | 3.9a | 2.3e,f | 2.5d | 3.5b | 2.8c | 0.09 | *** | *** | n.s. | *** | *** | *** | ** |
| Ileum, % | 57a,b | 53b,c | 55a,b | 50c | 51c | 57a | 41d | 38d | 1.22 | *** | *** | n.s. | *** | ** | ** | * |
| Ileum, g/kg DM | 3.3 | 3.4 | 5.4 | 5.0 | 3.1 | 3.6 | 4.1 | 3.7 | 0.09 | *** | *** | n.s. | *** | n.s. | *** | n.s. |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
a–fMeans within a row not showing a common superscript differ (P < 0.05).
P and Ca Disappearance
The P × Ca × Phy interaction was significant for P disappearance (percent and grams per kilogram DM) in the duo+jej (P = 0.030 and P = 0.017, respectively; Table 3). This interaction was the lowest in the treatment P−Ca+Phy−, and increased by P or phytase or both. In the ileum, a P × Phy interaction (P = 0.004) was caused by increased P disappearance with P and phytase supplementation, whereby all treatments with phytase addition did not differ. The supplementation of P increased P disappearance only when no Ca was supplemented, resulting in a Ca × P interaction (P = 0.003).
The P × Ca × Phy interaction was significant for Ca disappearance (percent) in the duo+jej (P = 0.006) and ileum (P = 0.033; Table 3). In the duo+jej, Ca disappearance was increased by phytase in the treatment P+Ca−, but decreased by phytase in the treatment P+Ca+. In the ileum, Ca disappearance (percent) was decreased by P supplementation when no phytase was present. It was increased by phytase in treatment P+Ca− and decreased by phytase in P−Ca+.
Inositol Phosphate Isomers
The concentration of the main InsP5 isomer in the degradation pathway of the added phytase—Ins(1,2,3,4,5)P—in the crop was affected by the interaction P × Phy (P < 0.001; Table 4), resulting in a greater decrease by phytase when no P was present. The concentration of Ins(1,2,5,6)P4, the main InsP4 isomer in the degradation pathway of the added phytase, was increased by P (P = 0.001) and phytase addition (P < 0.001). The concentration of InsP3x in the crop was increased by P addition (P < 0.001). Although not all factors could be evaluated statistically, as some values were below the limit of detection, there was an observable phytase effect, as InsP3x was detectable only in the presence of phytase. In the prov+giz, although a statistical evaluation was not possible, a phytase effect was obvious for Ins(1,2,4,5,6)P5 and Ins(1,2,3,4,5)P5, which were detected only when phytase was not present, and for Ins(1,2,5,6)P4, which was detected only in the presence of phytase (Table 4). The concentration of Ins(1,2,5,6)P4 was increased by Ca and highest in the treatments P+Ca- and P+Ca+, resulting in a P × Ca interaction (P = 0.026). In the duo+jej, the concentrations of Ins(1,2,3,4,5)P5 and Ins(1,2,5,6)P4 were higher in the treatment P+Ca+ than in other treatments, which resulted in a P × Ca interaction (P = 0.049 and P < 0.001, respectively; Table 5). In the ileum, the concentration of Ins(1,2,3,4,5)P5 was increased by the supplementation of P (P = 0.036) or Ca (P = 0.024; Table 6). The increase in Ins(1,2,5,6)P4 as a result of phytase or P supplementation was larger when Ca was supplemented, leading to a Ca × Phy (P < 0.001) and P × Ca interaction (P = 0.002). As in duo+jej, the concentration of InsP3x in the ileum was decreased by phytase in the treatment P−Ca+, and increased by phytase in the treatment P+Ca+, resulting in a P × Ca × Phy interaction (P < 0.001). Ins(1,2)P2 concentrations in the digesta of the terminal ileum were higher in P−Ca+ than in P+Ca− and P+Ca+ when no phytase was present, and increased with P or P and Ca combined when phytase was present, resulting in a P × Ca × Phy interaction (P = 0.004). The addition of phytase resulted in a significant increase in the concentration of InsP1x; however, these effects were greater in the presence of P supplementation, which caused a P × Phy interaction (P = 0.003).
Table 4.
Interaction of P, Ca, and phytase on concentrations of InsP isomers (μmol/g DM) in the digesta of the crop and the proventriculus/gizzard of broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca- | P−Ca+ | P+Ca- | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| Crop | ||||||||||||||||
| InsP6 | 14.1 | 4.8 | 13.9 | 5.8 | 14.6 | 4.9 | 14.6 | 4.4 | 0.74 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Ins(1,2,4,5,6)P5 | 1.2 | 0.2 | 1.2 | 0.3 | 1.2 | 0.2 | 1.2 | 0.2 | 0.04 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Ins(1,2,3,4,5)P5 | 0.8 | 0.2 | 0.7 | 0.2 | 0.6 | 0.4 | 0.7 | 0.3 | 0.04 | n.s. | n.s. | *** | n.s. | *** | n.s. | n.s. |
| Ins(1,2,3,4,6)P5 | 0.2 | n.d.4 | 0.2 | n.d. | 0.2 | n.d. | 0.3 | n.d. | 0.02 | n.s. | n.s. | . | n.s. | . | . | . |
| Ins(1,2,5,6)P4 | 0.2 | 3.3 | 0.2 | 2.7 | n.d. | 4.8 | <LOQ5 | 4.8 | 0.38 | ** | n.s. | *** | n.s. | . | n.s. | . |
| InsP3x5 | n.d. | 1.0 | n.d. | 0.8 | n.d. | 1.5 | n.d. | 1.6 | 0.10 | *** | n.s. | . | n.s. | . | . | . |
| Proventriculus and gizzard | ||||||||||||||||
| InsP6 | 7.2 | n.d. | 6.4 | 0.4 | 7.1 | 0.3 | 6.6 | 0.9 | 0.23 | † | n.s. | *** | n.s. | n.s. | ** | . |
| Ins(1,2,4,5,6)P5 | 0.5 | n.d. | 0.4 | n.d. | 0.4 | n.d. | 0.4 | n.d. | 0.03 | n.s. | n.s. | . | † | . | . | . |
| Ins(1,2,3,4,5)P5 | 0.2 | n.d. | 0.2 | n.d. | 0.2 | n.d. | 0.2 | n.d. | 0.03 | n.s. | n.s. | . | n.s. | . | . | . |
| Ins(1,2,5,6)P4 | n.d. | 0.5 | n.d. | 1.1 | n.d. | 1.7 | n.d. | 1.9 | 0.21 | *** | ** | . | * | . | . | . |
| InsP3x6 | n.d. | n.d. | n.d. | 0.4 | n.d. | 0.7 | n.d. | 0.6 | 0.09 | † | n.s. | . | . | . | . | . |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4n.d., not detectable in the majority of samples.
5<LOQ, not quantifiable in the majority of samples.
6At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
Table 5.
Interaction of P, Ca, and phytase on concentrations of InsP isomers (μmol/g DM) in the digesta of the duodenum/jejunum of broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| InsP6 | 13.5 | 5.0 | 17.1 | 7.4 | 17.8 | 7.0 | 25.1 | 10.0 | 0.74 | *** | *** | *** | † | ** | * | n.s. |
| Ins(1,2,4,5,6)P5 | 0.3d | 0.3d | 0.5c | 0.4c,d | 0.5c | 0.3d | 1.4a | 0.7b | 0.05 | *** | *** | *** | *** | *** | *** | * |
| Ins(1,2,3,4,5)P5 | 0.6 | 0.9 | 0.8 | 0.9 | 0.8 | 0.9 | 1.1 | 1.6 | 0.11 | ** | ** | * | * | n.s. | n.s. | n.s. |
| Ins(1,2,3,4,6)P5 | 0.2 | n.d.4 | 0.3 | n.d. | 0.3 | n.d. | 0.4 | n.d. | 0.02 | *** | * | . | n.s. | . | . | . |
| Ins(1,2,5,6)P4 | n.d. | 1.1 | n.d. | 1.1 | n.d. | 0.9 | 0.3 | 2.8 | 0.20 | ** | *** | *** | *** | . | . | . |
| Ins(1,2,3,4)P4 | 0.4b | 0.1c | 0.8a | 0.1c | 0.6b | 0.2c | 0.5b | 0.4b | 0.07 | n.s. | * | *** | † | * | n.s. | ** |
| InsP3x5 | 0.3c | 0.3c | 0.7b | 0.3c | 0.5b,c | 0.5b,c | 0.3c | 1.8a | 0.10 | *** | *** | ** | * | *** | ** | *** |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4n.d., not detectable in the majority of samples.
5At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
a–dMeans within a row not showing a common superscript differ (P < 0.05).
Table 6.
Interaction of P, Ca, and phytase on concentrations of InsP isomers (μmol/g DM) in the digesta of the terminal ileum of broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| InsP6 | 17.4 | 5.6 | 20.7 | 10.3 | 26.7 | 5.7 | 35.6 | 10.6 | 1.29 | *** | *** | *** | n.s. | *** | n.s. | n.s. |
| Ins(1,2,4,5,6)P5 | 0.3c | 0.4c | 0.7b | 0.7b | 0.7b | 0.3c | 2.0a | 0.8b | 0.09 | *** | *** | *** | *** | *** | * | * |
| Ins(1,2,3,4,5)P5 | 1.1 | 1.2 | 1.0 | 1.6 | 1.6 | 0.8 | 1.9 | 2.0 | 0.25 | * | * | n.s. | n.s. | n.s. | † | n.s. |
| Ins(1,2,3,4,6)P5 | 0.5 | n.d.4 | 0.4 | n.d. | 0.7 | n.d. | 0.8 | n.d. | 0.04 | *** | n.s. | . | † | . | . | . |
| Ins(1,2,5,6)P4 | n.d. | 1.4 | n.d. | 1.7 | 0.2 | 0.9 | 0.6 | 4.9 | 0.42 | * | ** | *** | ** | . | *** | . |
| Ins(1,2,3,4)P4 | 0.8 | n.d. | 1.1 | 0.3 | 1.1 | 0.2 | 0.9 | 0.5 | 0.11 | n.s. | † | *** | † | * | * | . |
| Ins(1,5,6)P3 | <LOQ5 | 0.2 | <LOQ | <LOQ | 0.2 | 0.2 | 0.2 | 0.2 | 0.02 | n.s. | n.s. | n.s. | . | . | n.s. | . |
| InsP3x6 | 0.6b,c | 0.4c | 1.1b | 0.4c | 0.7b,c | 0.6b,c | 0.5b,c | 3.3a | 0.19 | *** | *** | n.s. | * | *** | ** | *** |
| Ins(1,2)P2 | 1.2b,c | 0.4d | 2.0b | 0.5c,d | 1.1c,d | 2.0b | 0.6c,d | 3.1a | 0.25 | ** | * | n.s. | n.s. | *** | n.s. | ** |
| InsP1x7 | 1.3 | 2.2 | 1.6 | 2.2 | 1.0 | 3.8 | 0.9 | 3.2 | 0.37 | n.s. | n.s. | *** | n.s. | ** | n.s. | n.s. |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4n.d., not detectable in the majority of samples.
5<LOQ, not quantifiable in the majority of samples.
6At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
7At least one of the following isomers: Ins(1)P1, Ins(2)P1.
a–dMeans within a row not showing a common superscript differ (P < 0.05).
Myo-inositol
The concentration of MI in the prov+giz was significantly reduced by the addition of P only in the presence of Ca, resulting in a P × Ca interaction (Table 7). Further, the concentrations were reduced by P addition, but this effect was repealed by the addition of phytase, resulting in a P × Phy interaction. The concentration of MI in the duo+jej was decreased by P and Ca supplementation, and increased by the addition of phytase (P < 0.001, respectively). In the ileum, a higher decrease in MI by Ca supplementation was detected in the presence of phytase supplementation, leading to a Ca × Phy interaction (P = 0.022). The MI concentration in blood plasma was increased by phytase (P < 0.001) and decreased by Ca (P = 0.001). A positive relationship (R2 = 0.72; Figure 1) between the MI concentrations in the ileum and in blood plasma was detected.
Table 7.
Interaction of P, Ca, and phytase on myo-inositol concentration in the digesta of proventriculus/gizzard, duodenum/jejunum, terminal ileum, and in blood plasma of broiler chickens fed the experimental diets from d 15 to d 25 (blood) or 27 (digesta).1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca− | P−Ca+ | P+Ca− | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| Prov/Giz4 | 1.1 | 1.3 | 1.0 | 1.4 | 0.7 | 1.7 | 0.5 | 1.2 | 0.11 | ** | ** | *** | ** | *** | n.s. | † |
| Duo/Jej4 | 12.7 | 18.9 | 9.2 | 15.8 | 9.3 | 16.8 | 5.5 | 12.9 | 0.57 | *** | *** | *** | n.s. | n.s. | n.s. | n.s. |
| Ileum4 | 10.1 | 16.6 | 5.7 | 11.3 | 5.6 | 14.6 | 3.2 | 6.5 | 0.92 | *** | *** | *** | n.s. | n.s. | * | n.s. |
| Blood5 | 0.24 | 0.45 | 0.18 | 0.32 | 0.28 | 0.45 | 0.22 | 0.32 | 0.03 | n.s. | ** | *** | n.s. | n.s. | n.s. | n.s. |
1Data are given as treatment means; n = 7 pens (blood plasma myo-inositol n = 6 individuals).
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4μmol/g DM.
5mmol/L.
Figure 1.
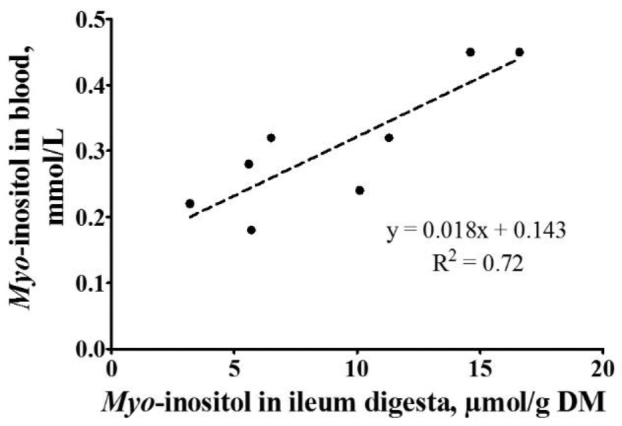
Relationship between myo-inositol concentration in the ileum and myo-inositol concentration in the blood plasma of broilers. The dots represent the treatment means with 7 replicates (pens) for the ileum data and 6 replicates (individuals) for the blood data.
Prececal Crude Protein and Amino Acid Disappearance
The prececal disappearance of Met, Lys, and Thr ranged between 82 and 86%, 74 and 79% and 61 and 66%, respectively (Table 8). Prececal disappearance of CP and all AA was increased by phytase supplementation (P < 0.01). No effects of Ca supplementation were found, and the effect of P was only significant for Cys (P = 0.048).
Table 8.
Interaction of P, Ca, and phytase on prececal CP and AA disappearance (%) in broiler chickens fed the experimental diets from d 15 to d 27.1
| Dietary treatment2 | P-value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P−Ca- | P−Ca+ | P+Ca- | P+Ca+ | Pooled | P | Ca | Phy | P × Ca | P × Phy | Ca × | P × Ca | |||||
| Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | Phy− | Phy+ | SEM | Phy | × Phy | ||||||
| CP | 69 | 74 | 69 | 74 | 73 | 74 | 71 | 74 | 1.3 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Arg | 81 | 83 | 81 | 84 | 82 | 84 | 82 | 83 | 0.9 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| His4 | 69 | 74 | 69 | 74 | 71 | 73 | 71 | 74 | 1.4 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Ile | 70 | 75 | 70 | 75 | 74 | 75 | 72 | 75 | 1.5 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Leu | 71 | 76 | 71 | 76 | 74 | 76 | 74 | 76 | 1.6 | n.s. | n.s. | *** | n.s. | † | n.s. | n.s. |
| Lys | 75 | 79 | 74 | 78 | 76 | 79 | 74 | 77 | 1.3 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Met | 84 | 86 | 82 | 85 | 84 | 85 | 84 | 85 | 1.1 | n.s. | n.s. | ** | n.s. | n.s. | n.s. | n.s. |
| Phe4 | 72 | 77 | 72 | 77 | 75 | 77 | 75 | 77 | 1.5 | n.s. | n.s. | *** | n.s. | † | n.s. | n.s. |
| Thr | 61 | 66 | 61 | 66 | 64 | 66 | 63 | 66 | 1.6 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Val | 68 | 73 | 69 | 74 | 72 | 73 | 70 | 73 | 1.5 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Ala | 69 | 74 | 68 | 74 | 72 | 73 | 71 | 74 | 1.6 | n.s. | n.s. | ** | n.s. | † | n.s. | n.s. |
| Asp | 69 | 73 | 68 | 73 | 71 | 73 | 69 | 72 | 1.3 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Cys | 51 | 58 | 52 | 59 | 53 | 59 | 56 | 60 | 1.6 | * | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Glu | 77 | 81 | 76 | 81 | 79 | 80 | 78 | 80 | 1.2 | n.s. | n.s. | *** | n.s. | † | n.s. | n.s. |
| Gly | 63 | 68 | 63 | 68 | 65 | 68 | 64 | 68 | 1.4 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Pro | 70 | 75 | 70 | 75 | 70 | 74 | 73 | 76 | 1.4 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Ser | 67 | 72 | 66 | 72 | 69 | 71 | 69 | 72 | 1.5 | n.s. | n.s. | *** | n.s. | n.s. | n.s. | n.s. |
| Tyr4 | 69 | 75 | 69 | 74 | 73 | 74 | 72 | 75 | 1.5 | † | n.s. | *** | n.s. | † | n.s. | n.s. |
1Data are given as treatment means; n = 7 pens.
2Calculated composition: P-, 4.1 g P/kg DM; P+, 6.9 g P/kg DM; Ca-, 6.2 g Ca/kg DM: Ca+, 10.3 g Ca/kg DM; Phy-, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 P-values are indicated as follows: n.s., P ≥ 0.10; †, P < 0.10; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4The concentrations of tyrosine, histidine, and phenylalanine may be affected to some extent by the oxidation procedure (Mason et al., 1980).
DISCUSSION
InsP6, P, and Ca Disappearance
This study confirms the high potential of broilers to degrade phytate (56% InsP6 disappearance up to the terminal ileum) and absorb the released P (48% P disappearance up to the terminal ileum) when fed diets significantly deficient in both P and Ca. Thereby, the results obtained are in good agreement with previous studies reviewed by Rodehutscord and Rosenfelder (2016).
Regarding the crop, the low level of InsP6 disappearance in broilers fed a corn-soybean meal-based feed without phytase supplementation has been previously described by Zeller et al. (2016). InsP6 disappearance in the crop was higher in the treatments without phytase when Ca was supplemented. This might indicate a Ca-dependent activation of phytases (Choi et al., 2001; Oh et al., 2001) produced by microorganisms in the crop, or may indicate a shift towards a more suitable pH for either microorganisms or enzymes. In birds from the same experiment, Borda-Molina et al. (2016) found Lactobacillus to be the most abundant genus in crop digesta, and observed a tendency towards a higher abundance of L. taiwanensis in birds fed the treatment P−Ca+ in comparison to the other treatments. Presently, it is not possible to evaluate whether a causal relationship existed. However, Ca or P had no significant effect in the crop when diets were supplemented with microbial phytase.
Up to the terminal ileum and in the absence of added phytase, a decrease in InsP6 disappearance was observed in the treatment P+Ca−. It was further decreased in the treatment P+Ca+, while Ca alone had no effect. The effect of P on endogenous phytases, and thus InsP degradation, has been considered to be an end product inhibition (Greiner et al., 1993; Angel et al., 2002; Olukosi and Fru-Nji, 2014; Zeller et al., 2015b). The low InsP6 disappearance in the presence of combined P and Ca supplementation also was shown by Manangi and Coon (2008). In their work, with 25-day-old broilers in 3 experiments, the numerically lowest fecal phytate-P disappearance (2 to 4%) without the addition of phytase was found in the treatments with 0.9% dietary Ca and 0.33 to 0.45% non-phytate P (NPP). It is possible that the added P inhibited the endogenous phytases, and the added Ca then precipitated the accumulated InsP6 (Angel et al., 2002).
Up to the terminal ileum and when phytase was supplemented, there was no significant difference in InsP6 disappearance between the treatments in the present study. Thus, it seems that the level of the phytase used in this study was sufficient to diminish the negative effects of the mineral supplements. However, the numerically lower values of InsP6 disappearance in the phytase treatments P−Ca+ and P+Ca+ (77%), in comparison to the other phytase treatments (87%), is an indication of an impact on degradation by Ca supplementation also in the presence of phytase as reported by Qian et al. (1996). The disappearance of P up to the duo+jej was significantly lowest in the treatment with Ca supplementation alone, and also was decreased by Ca supplementation up to the terminal ileum. This indicates a precipitation of free and released P owing to complex building with Ca (Hamdi et al., 2015) in the small intestine, where the increasing pH along the digestive tract reduces mineral solubility, possibly leading to the decreased growth of the birds. A reduction in P disappearance owing to increased dietary Ca levels has been previously reported by Angel et al. (2002), Tamim et al. (2004), Amerah et al. (2014), and Hamdi et al. (2015). Ca supplementation also decreased the ADFI and ADG of birds, even over the short application period in this study. As the F:G was also significantly reduced in this treatment in comparison to the other treatments, the poorer performance is not only a result of the reduced ADFI, but also of the reduction in P disappearance, which is therefore explainable by mechanisms other than phytate degradation (e.g., the formation of Ca-phosphates, as previously stated by Olukosi and Fru-Nji (2014)). The formation of Ca-phosphate complexes also may explain why phytase supplementation improved performance in the P−Ca+ treatment, although not to the level of the other phytase treatments.
Increasing dietary P and Ca to commonly fed levels decreased InsP6 disappearance, demonstrating that phytate degradation is likely much lower under commercial conditions than levels measured in low P and Ca digestibility trials. Such effects on P disappearance were not quite as dramatic, suggesting that InsP6 and P disappearance are not well correlated. This reinforces the need to analyze the complete pattern of phytate degradation in order to better understand the effect of mineral and phytase addition.
Inositol Phosphate Isomers and Myo-inositol
To the best of our knowledge, this is the first study to demonstrate that all InsP esters with their positional isomers are involved in the complete degradation of InsP6 to MI in the ileum digesta of broilers. Furthermore, although MI has come into focus in recent yr, there have been very limited data reported on MI concentrations in the small intestine of broilers (Beeson et al., 2017) and pigs (Kühn et al., 2016; Laird et al., 2016).
In the crop, treatments with P addition had higher concentrations of Ins(1,2,5,6)P4 and InsP3x than treatments without added P. This indicates that these degradation steps are inhibited by P. However, the concentrations of InsP isomers in the crop and stomachs were mainly affected by phytase supplementation. The decrease in the concentrations of the InsP6 and InsP5 isomers owing to phytase led to higher concentrations of InsP4 and InsP3 isomers, as previously shown by Zeller et al. (2016).
In the ileum, the InsP isomers were affected differently by the different mineral supplementations. Without phytase supplementation, the treatment P−Ca+ had lower InsP6 and higher Ins(1,2)P2 and InsP1x, but identical MI concentrations in comparison to the P+Ca− treatment. This could be the result of P having a more pronounced effect than Ca on the endogenous microbial or epithelial phytases dephosphorylating InsP6, whereas Ca may have a greater effect on the endogenous phosphatases degrading the lower InsP isomers. However, since intestinal MI concentrations are a balance between the rates of formation from InsP1 and absorption, such generalizations need to be treated with caution. In the P+Ca+ treatment without phytase, concentrations of InsP6 and InsP5 were higher than in the other non-phytase treatments, indicating a negative effect of the combined supplements on the first 2 steps of dephosphorylation by endogenous microbial and epithelial phytases.
In the ileum in treatments with supplemented phytase, the concentrations of the InsP isomers primarily produced by the added phytase (Ins(1,2,3,4,5)P5 and Ins(1,2,5,6)P4), and also the concentration of InsP3x and InsP2, were again numerically highest in the P+Ca+ treatment. Higher InsP concentrations can indicate slower degradation, and thus the lower MI concentrations observed in the ileum in the P+Ca+ treatments are a logical consequence. This indicates negative effects of the combined supplementation of P and Ca on all degradation steps in the presence of phytase. The in vitro study by Sommerfeld et al. (2017) supports these outcomes, where corn-soybean meal-based diets with varying concentrations of Ca and P were incubated in a simulation of the poultry digestive tract. InsPs were less degraded in the treatments with the supplementation of either P and Ca combined, or high P alone, in the presence of the Quantum Blue phytase. Of the phytase treatments in the ileum, the treatment P−Ca+ had slightly higher InsP6 and lower MI concentrations than P−Ca−, indicating that Ca supplementation affected only the InsP6 concentration, and not any further degradation steps from InsP5 down to MI. This could be an indication of the direct inhibition of the added microbial phytase by Ca, as described by McCuaig et al. (1972); however, it is more likely the impact of Ca on phytate accessibility, as the lower InsPs were further degraded without any Ca interference. Given that the level of the measured InsP1–2 and InsP1–3 isomers represented nearly 25 and 35%, respectively, of the total InsPs in the ileum digesta of the P+Ca+Phy+ treatment, it is clear that the complete degradation cascade—including the lowest InsPs—should be followed when evaluating the efficacy of phytases.
The results of MI concentrations in the duo+jej, ileum, and blood—which were increased by phytase—suggests that the animal has the ability to fully dephosphorylate phytate. According to the literature, it is unclear whether non-ruminants are able to fully degrade phytate, as thus far, all 3- and 6-phytases have been shown to be incapable of splitting the phosphate group off of the C2 atom on the phytate molecule (Wyss et al., 1999; Selle and Ravindran, 2007). However, it seems that endogenous microbial or epithelial phosphatases can break the P-group from this position, thus leading to complete dephosphorylation. The higher concentrations of MI in the treatments with added phytase can be explained by the higher degradation of InsP6 to lower InsP isomers, which can then be further degraded by endogenous microbial or epithelial phosphatases to MI. The positive relationship between the MI concentrations in ileum and blood is an indication of the efficient absorption of MI. Ca supplementation decreased MI concentrations in the ileum and blood plasma. This effect on the MI concentration in the ileum may be the result of the inhibition of endogenous phosphatases, and thus reduced InsP degradation as previously described. The effect of Ca on blood MI levels could have been caused by the direct effect of Ca on MI transporters from the intestine into the blood, or if lower InsPs are absorbable, the impact of Ca on epithelial dephosphorylation, which has not yet been studied. MI is thought to have several biological functions, including roles in cell survival and growth, lipid metabolism, and insulin sensitivity (Huber, 2016; Lee and Bedford, 2016). In experiments in which either phytase or free MI were added to diets, an increase in the performance of broilers and feed efficiency was observed, depending on the age of the birds (Żyła et al., 2004; Cowieson et al., 2013). Therefore, phytase studies should also consider MI as a relevant part of phytase efficiency, especially when very high doses of phytase are applied.
Crude Protein and Amino Acids
No effect of the added minerals on the disappearance of proteins or AA until the terminal ileum was found. Proteins and AA are known to form binary complexes with phytate under acidic conditions, or ternary phytate-cation-protein-complexes in the pH prevailing in the small intestine (Yi et al., 1996; Ravindran et al., 2000). Therefore, Ca ions are likely to act as bridges between phytate and proteins. As it has been shown that supplementation with dietary P and Ca reduce InsP6 disappearance (Shastak et al., 2014; Zeller et al., 2015b), the hypothesis was that the supplementation of P or Ca also would affect the disappearance of AA. This hypothesis has been rejected. This confirms the results of Wilkinson et al. (2014), who also reported no interactions between Ca and NPP—added to broiler diets in several concentrations—on the prececal digestibility of most AA measured. However, these results contradict the results of Ravindran et al. (2000), who found a phytase × NPP interaction for most AA studied, where the response of phytase was increased in response to low NPP diets. As Ravindran et al. (2000) used dietary concentrations of 400 and 800 FTU/kg of an Aspergillus phytase, it is possible that the higher dose of the modified E. coli phytase used in the present study—which diminished all mineral effects on InsP6 disappearance—also could have diminished the potential effects of different concentrations of NPP on apparent AA digestibility. Amerah et al. (2014) reported a decrease in AA digestibility with increasing dietary Ca. This also could not be confirmed in the present study.
There was a clear effect of phytase on the disappearance of CP and AA (between 2 and 6% in comparison to the non-phytase treatments). This is in agreement with the results of Ravindran et al. (2000), Rutherfurd et al. (2002), and Amerah et al. (2014). However, such effects are not consistent throughout all studies. In some broiler trials, no effect of phytase on AA digestibility was observed (Sebastian et al., 1997; Peter and Baker, 2001; Rodehutscord et al., 2004). While there could be several reasons for this, the reader is referred to the reviews of Selle et al. (2000, 2012) for information on how phytase can increase AA digestibility along with factors influencing such a response, as the focus of the present study is to elucidate the effects of P or Ca supplementation.
We conclude that the InsP6 disappearance owing to endogenous phytases and phosphatases was mainly reduced by the addition of P, and to a greater extent by combined P and Ca supplementation. The supplementation of 1,500 FTU phytase/kg diminished the negative effects of the mineral supplements on InsP6 disappearance, although there was an indication that higher Ca levels undermined the benefit of the phytase. However, supplementation with P and Ca combined clearly impeded the degradation of the lower InsP esters in the digestive tract. Additionally, phytase supplementation increased the concentration of MI in the digestive tract and blood. Supplementation with 1,500 FTU phytase/kg feed increased the disappearance of AA up to the terminal ileum with no effect of P or Ca supplementation. Thus, in phytase assays investigating AA digestibility, dietary levels of P or Ca can be reduced without effects on AA digestibility.
Acknowledgements
Vera Sommerfeld was supported with a doctoral scholarship of the Studienstiftung des deutschen Volkes, which is gratefully acknowledged.
REFERENCES
- Amerah A. M., Plumstead P. W., Barnard L. P., Kumar A.. 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 93:906–915. [DOI] [PubMed] [Google Scholar]
- Angel R., Tamim N. M., Applegate T. J., Dhandu A. S., Ellestad L. E.. 2002. Phytic acid chemistry: Influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 11:471–480. [Google Scholar]
- Beeson L. A., Walk C. L., Bedford M. R., Olukosi O. A.. 2017. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 96:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguhn J., Baumgärtel T., Dieckmann A., Rodehutscord M.. 2009. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 63:337–342. [DOI] [PubMed] [Google Scholar]
- Borda-Molina D., Vital M., Sommerfeld V., Rodehutscord M., Camarinha-Silva A.. 2016. Insights into broilers' gut microbiota fed with phosphorus, calcium, and phytase supplemented diets. Front. Microbiol. 7:2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. M., Suh H. J., Kim J. M.. 2001. Purification and properties of extracellular phytase from Bacillus sp. KHU-10. J. Protein Chem. 20:287–292. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Acamovic T., Bedford M. R.. 2004. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. Br. Poult. Sci. 45:101–108. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Ptak A., Mackowiak P., Sassek M., Pruszynska-Oszmalek E., Żyła K., Swiatkiewicz S., Kaczmarek S., Józefiak D.. 2013. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 92:2124–2134. [DOI] [PubMed] [Google Scholar]
- Eeckhout W., de Paepe M.. 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47:19–29. [Google Scholar]
- Gesellschaft für Ernährungsphysiologie 1999. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler). 1st ed DLG Verlag, Frankfurt am Main, Germany. [Google Scholar]
- Greiner R., Konietzny U., Jany K.-D.. 1993. Purification and characterization of two phytases from Escherichia coli. Arch. Biochem. Biophys. 303:107–113. [DOI] [PubMed] [Google Scholar]
- Hamdi M., López-Vergé S., Manzanilla E. G., Barroeta A. C., Pérez J. F.. 2015. Effect of different levels of calcium and phosphorus and their interaction on the performance of young broilers. Poult. Sci. 94:2144–2151. [DOI] [PubMed] [Google Scholar]
- Huber K. 2016. Cellular myo-inositol metabolism. Pages 53–60 in Phytate Destruction. Walk C. L., Kühn I., Stein H. H., Kidd M. T., Rodehutscord M., eds., Wageningen Academic Publishers, Wageningen. [Google Scholar]
- Irvine R. F., Schell M. J.. 2001. Back in the water - The return of the inositol phosphates. Nat. Rev. Mol. Cell Bio. 2:327–338. [DOI] [PubMed] [Google Scholar]
- Kühn I., Schollenberger M., Männer K.. 2016. Effect of dietary level on intestinal phytate degradation and bone mineralization in growing pigs. J. Anim. Sci. 94:264–267. [Google Scholar]
- Laird S., Kühn I., Wilcock P., Miller H. M.. 2016. The effects of phytase on grower pig growth performance and ileal inositol phosphate degradation. J. Anim. Sci. 94:142–145. [Google Scholar]
- Lee S. A., Bedford M. R.. 2016. Inositol - An effective growth promoter? World's Poult. Sci. J. 72:743–760. [Google Scholar]
- Lei X. G., Porres J. M.. 2007. Phytase and inositol phosphates in animal nutrition: Dietary manipulation and phosphorus excretion by animals. Pages 133–149 in Inositol phosphates. Turner B. L., Richardson A. E., Mullaney E. J., eds., CAB International, Wallingford, UK, Cambridge, MA. [Google Scholar]
- Manangi M. K., Coon C. N.. 2008. Phytate phosphorus hydrolysis in broilers in response to dietary phytase, calcium, and phosphorus concentrations. Poult. Sci. 87:1577–1586. [DOI] [PubMed] [Google Scholar]
- Mason V. C., Rudemo M., Bech-Andersen S.. 1980. Hydrolysate preparation for amino acid determinations in feed constituents 6. The influence of phenol and formic acid on the recovery of amino acids from oxidized feed proteins. Z. Tierphysiol. Tierernahr. Futtermittelkd. 43:35–48. [PubMed] [Google Scholar]
- McCuaig L. W., Davies M. I., Motzok I.. 1972. Intestinal alkaline phosphatase and phytase of chicks: Effect of dietary magnesium, calcium, phosphorus and thyroactive casein. Poult. Sci. 51:526–530. [DOI] [PubMed] [Google Scholar]
- Oh B.-C., Chang B. S., Park K.-H., Ha N.-C., Kim H.-K., Oh B.-H., Oh T.-K.. 2001. Calcium-dependent catalytic activity of a novel phytase from Bacillus amyloliquefaciens DS11. Biochem. 40:9669–9676. [DOI] [PubMed] [Google Scholar]
- Olukosi O. A., Fru-Nji F.. 2014. The interplay of dietary nutrient level and varying calcium to phosphorus ratios on efficacy of a bacterial phytase: 2. Ileal and total tract nutrient utilization. Poult. Sci. 93:3044–3052. [DOI] [PubMed] [Google Scholar]
- Peter C. M., Baker D. H.. 2001. Microbial phytase does not improve protein-amino acid utilization in soybean meal fed to young chickens. J. Nutr. 131:1792–1797. [DOI] [PubMed] [Google Scholar]
- Qian H., Kornegay E. T., Denbow D. M.. 1996. Phosphorus equivalence of microbial phytase in turkey diets as influenced by calcium to phosphorus ratios and phosphorus levels. Poult. Sci. 75:69–81. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Bryden W. L.. 1999. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 78:699–706. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Selle P. H., Bryden W. L.. 2000. Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorous levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. Br. Poult. Sci. 41:193–200. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Kapocius M., Timmler R., Dieckmann A.. 2004. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 45:85–92. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Rosenfelder P.. 2016. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. Pages 15–32 in Phytate Destruction. Walk C. L., Kühn I., Stein H. H., Kidd M. T., Rodehutscord M., eds., Wageningen Academic Publishers, Wageningen. [Google Scholar]
- Rodehutscord M., Rückert C., Maurer H. P., Schenkel H., Schipprack W., Bach Knudsen K. E., Schollenberger M., Laux M., Eklund M., Siegert W., Mosenthin R.. 2016. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 70:87–107. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S. M., Chung T. K., Moughan P. J.. 2002. The effect of microbial phytase on ileal phosphorus and amino acid digestibility in the broiler chicken. Br. Poult. Sci. 43:598–606. [DOI] [PubMed] [Google Scholar]
- Sebastian S., Touchburn S. P., Chavez E. R., Lague P. C.. 1997. Apparent digestibility of protein and amino acids in broiler chickens fed a corn-soybean diet supplemented with microbial phytase. Poult. Sci. 76:1760–1769. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Cowieson A. J., Cowieson N. P., Ravindran V.. 2012. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 25:1–17. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Ravindran V.. 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135:1–41. [Google Scholar]
- Selle P. H., Ravindran V., Caldwell A., Bryden W. L.. 2000. Phytate and phytase: Consequences for protein utilisation. Nutr. Res. Rev. 13:255–278. [DOI] [PubMed] [Google Scholar]
- Shastak Y., Zeller E., Witzig M., Schollenberger M., Rodehutscord M.. 2014. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 93:2548–2559. [DOI] [PubMed] [Google Scholar]
- Skoglund E., Carlsson N.-G., Sandberg A.-S.. 1997. Determination of isomers of inositol mono- to hexaphosphates in selected foods and intestinal contents using high-performance ion chromatography. J. Agric. Food Chem. 45:431–436. [Google Scholar]
- Sommerfeld V., Schollenberger M., Hemberle L., Rodehutscord M.. 2017. Modification and application of an in vitro assay to examine inositol phosphate degradation in the digestive tract of poultry. J. Sci. Food Agric. 97:4219–4226. [DOI] [PubMed] [Google Scholar]
- Tamim N. M., Angel R., Christman M.. 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358–1367. [DOI] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 2007. Handbuch der landwirtschaftlichen Versuchs und Untersuchungsmethodik (VDLUFA–Methodenbuch), vol. III: Die Chemische Untersuchung von Futtermitteln. 1st ed.VDLUFA, Darmstadt, Germany. [Google Scholar]
- Walk C. L., Santos T. T., Bedford M. R.. 2014. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 93:1172–1177. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. J., Bradbury E. J., Thomson P. C., Bedford M. R., Cowieson A. J.. 2014. Nutritional geometry of calcium and phosphorus nutrition in broiler chicks. The effect of different dietary calcium and phosphorus concentrations and ratios on nutrient digestibility. Animal 8:1080–1088. [DOI] [PubMed] [Google Scholar]
- Wyss M., Brugger R., Kronenberger A., Rémy R., Fimbel R., Oesterhelt G., Lehmann M., van Loon A. P.. 1999. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): Catalytic properties. Appl. Environ. Microbiol. 65:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z., Kornegay E. T., Denbow D. M.. 1996. Effect of microbial phytase on nitrogen and amino acid digestibility and nitrogen retention of turkey poults fed corn-soybean meal diets. Poult. Sci. 75:979–990. [DOI] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M.. 2015a. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M.. 2016. Dietary effects on inositol phosphate breakdown in the crop of broilers. Arch. Anim. Nutr. 70:57–71. [DOI] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L. E., Rodehutscord M.. 2015b. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018–1029. [DOI] [PubMed] [Google Scholar]
- Żyła K., Mika M., Stodolak B., Wikiera A., Koreleski J., Swiatkiewicz S.. 2004. Towards complete dephosphorylation and total conversion of phytates in poultry feeds. Poult. Sci. 83:1175–1186. [DOI] [PubMed] [Google Scholar]


