Abstract
Objective
This study evaluated the efficacy and safety of oral naldemedine 0.1 mg, 0.2 mg, or 0.4 mg once daily in patients who had opioid-induced constipation (OIC) and maintained a stable laxative regimen.
Methods
This four-week, phase 2b, randomized, double-blind placebo-controlled trial (clinicaltrials.gov identifier NCT01443403) enrolled patients on long-term opioid therapy for chronic noncancer pain with OIC. The primary efficacy end point was change in weekly spontaneous bowel movement (SBM) frequency from baseline to the last two weeks of treatment. Secondary end points included the proportion of SBM responders (patients with ≥3 SBMs/week and an increase of ≥1 SBM/week from baseline over the last 2 weeks of treatment). Safety parameters assessed included adverse events, effects on analgesia, and opioid withdrawal symptoms.
Results
Overall, 244 patients were randomized 1:1:1:1 to naldemedine 0.1 mg, 0.2 mg, 0.4 mg, or placebo. Baseline patient characteristics were comparable. Weekly SBM frequency was significantly higher with naldemedine 0.2 mg (3.37, P = 0.0014) and 0.4 mg (3.64, P = 0.0003), but not with 0.1 mg (1.98, P = 0.3504), vs placebo (1.42). The proportion of SBM responders was significantly higher with naldemedine 0.2 mg (71.2%, P = 0.0005) and 0.4 mg (66.7%, P = 0.003), but not with 0.1 mg (52.5%, P = 0.1461), vs placebo (39.3%). Treatment-emergent adverse events were generally mild to moderate in severity; incidences increased with naldemedine dose. No clinically meaningful changes in other safety parameters were observed.
Conclusion
Naldemedine 0.2 mg once daily is the optimal dose for future confirmatory trials in OIC.
Keywords: Naldemedine, Chronic Noncancer Pain, Opioid-Induced Constipation, Peripherally Acting μ-Opioid Receptor Antagonist
Introduction
Chronic pain that requires long-term treatment is reported to affect 25–44% of adults in Europe and the United States (US) [1–3]. Opioid analgesics play an important role in the management of chronic pain; in the US, they are estimated to be used by 40–47 per 1,000 individuals (approximately 13–15 million people in total) [4].
Opioids alleviate pain by acting on opioid receptors in the central and peripheral nervous systems [5]. Activation of opioid receptors in the gastrointestinal (GI) tract can result in GI adverse events (AEs). Three opioid receptors are expressed in the GI tract: κ, δ, and μ receptors. κ and δ receptors are expressed in the stomach and proximal colon, but their role in causing GI AEs is unclear [5]. μ receptors are expressed throughout the GI tract, and when opioids bind to them neural activity in the submucosal and myenteric plexuses of the enteric nervous system is decreased. This impairs GI transit and motility, decreases gut fluid secretion, and increases fluid absorption, which can lead to opioid-induced constipation (OIC), the most common side effect of opioid therapy [5]. In a population-based survey, 57% of individuals with chronic noncancer pain who used opioids experienced OIC [6]. OIC can have a negative effect on patients’ well-being. This is exemplified by a survey of 359 patients using opioids, which showed that health-related quality of life was significantly worse in those with OIC than in those without OIC [7].
Firstline treatment for OIC involves the use of laxatives; however, laxatives do not address the mechanism underlying the disorder. Furthermore, there is insufficient clinical evidence available to determine the efficacy and side effect profile of laxatives [5,8]. This suggests the need for effective treatments for OIC.
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are specifically designed to block opioid actions at peripheral μ-opioid receptors in the GI tract [5]. Two PAMORAs are approved by the US Food and Drug Administration for the treatment of OIC [9]: Naloxegol (a pegylated derivative of naloxone, delivered as oral tablets) has recently been approved for the treatment of OIC in adults with chronic noncancer pain [10], and methylnaltrexone bromide (delivered as a subcutaneous injection or as oral tablets) has been approved for the treatment of OIC in adults with chronic noncancer pain and for the treatment of OIC in adults with advanced illness on palliative care when laxatives are ineffective [11].
Naldemedine (S-297995) is a novel PAMORA being developed for the treatment of OIC in adults with chronic noncancer pain and cancer-related pain [12]. The structure of naldemedine is analogous to that of the opioid receptor antagonist naltrexone, but structural modifications limit the ability of naldemedine to cross the blood-brain barrier. In preclinical models, naldemedine potently inhibited the constipating effects of opioids without compromising pain relief or inducing centrally mediated opioid withdrawal [12]. Early clinical dose-finding studies demonstrated that 0.3 mg once daily had a good benefit-risk profile in patients with opioid-induced bowel dysfunction [13].
The objectives of the study reported here were to evaluate the efficacy and safety of three doses of oral naldemedine (0.1 mg, 0.2 mg, and 0.4 mg once daily) and define the optimal dose in patients with chronic noncancer pain who have OIC.
Methods
Study Design
This was a phase 2b, multicenter, randomized, double-blind, placebo-controlled parallel-group trial to evaluate the efficacy and safety of three different doses of oral naldemedine in patients with chronic noncancer pain receiving opioid therapy who had OIC and who maintained a stable laxative regimen throughout the study (clinicaltrials.gov identifier: NCT01443403).
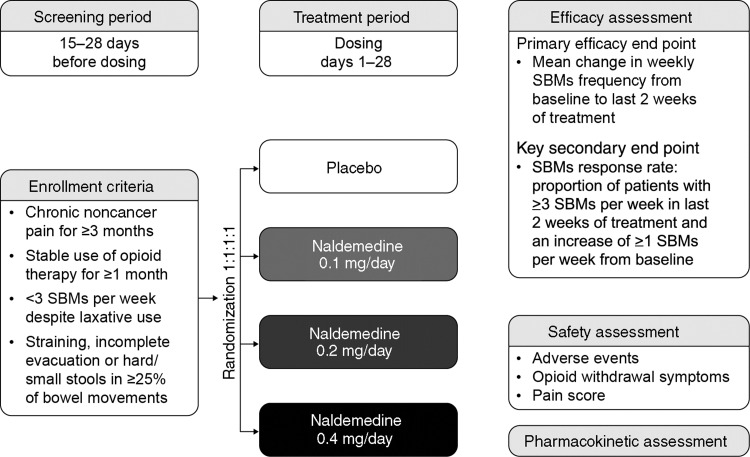
Patients age 18 years or older were screened for 15–28 days (Figure 1). Those who met the eligibility criteria and completed a Bowel Movement and Constipation Assessment (BMCA) diary on a daily basis for at least 14 days were enrolled in the treatment period. Patients were then randomized 1:1:1:1 to receive oral naldemedine 0.1 mg, 0.2 mg, or 0.4 mg or placebo once daily for 28 days and were followed up for a further 28 days to complete safety assessments. Patients were randomly assigned to one of the four treatment groups using an interactive voice response system, which assigned a unique number to each patient and was used to identify the patient in all data systems.
Figure 1.
Trial design. SBMs = spontaneous bowel movement.
Ethics Approval
The trial was approved by the appropriate institutional review boards, and it complied with the ethical principles of the Declaration of Helsinki and good clinical practice. All patients provided written informed consent.
Eligibility Criteria
Patients’ eligibility to participate in the study was assessed during screening. To be eligible, patients were required to: have a documented medical history of chronic noncancer pain for at least three months before screening; be taking a stable dose of a full opioid agonist equivalent to at least 30 mg oral morphine daily for one month or longer before screening; and have self-reported ongoing symptoms of OIC, defined as fewer than three spontaneous bowel movements (SBMs) per week despite a stable regimen of laxatives and one or more of the following symptoms in at least 25% of bowel movements: straining, feeling of incomplete evacuation, and/or hard/small stools, defined as Bristol Stool Scale (BSS) score lower than 3.
Patients were required either to maintain a stable laxative regimen throughout the study (defined as any combination of laxatives that had been taken consistently in the 28 days before the start of the study) or not to use any laxatives.
The following exclusion criteria were applied: evidence of clinically significant GI disease, bowel dysfunction, bowel obstruction, or pelvic disorder that may cause constipation; a history of chronic constipation before starting analgesic medication or nonopioid causes of bowel dysfunction that may have contributed to constipation; severe constipation that had not been appropriately managed, such that the patient was at immediate risk of developing serious related complications; initiation of a new treatment regimen for OIC or a prokinetic agent within 28 days of screening; cancer treatment within the past five years; history or presence of any clinically important abnormality, medical condition, or use of concomitant medication(s), that could have interfered with the study; medically significant cardiovascular, respiratory, hepatic, renal or thyroid dysfunction, or a history of human immunodeficiency virus infection; any medical or psychiatric condition that may have compromised the ability of the patient to understand and comply with the study protocol; current use of any prohibited medication, including opioid receptor antagonists, partial agonists, fentanyl, or meperidine; the inability to take oral medication; any history of illegal drug use in the past five years; surgery within one month of screening or planned surgery during study treatment that would, in the opinion of the investigators, have affected the study results; any relevant allergies; treatment with an investigational study drug in the 30 days before screening; or previous exposure to naldemedine. There were no specific exclusion criteria for patients with persistent or uncontrolled pain or with disruptions to the blood-brain barrier.
Efficacy Assessments
The BMCA diary was completed on a daily basis for the 28 days of treatment. As part of this diary record, patients also assessed the consistency of stools using BSS. To minimize the potential for overestimating the frequency of BMs, all passages of stool with a score of at least 1 on the BSS that occurred within a two-hour time frame were classified as a single BM. The baseline mean number of SBMs was calculated from the data collected in the last two weeks of screening, before the first dose of study drug was administered. Any BMs in the 24 hours after use of rescue laxatives were not considered to be spontaneous.
The primary efficacy end point was the mean change in weekly SBM frequency from baseline to the last two weeks of the treatment period. Secondary efficacy end points were:
the change in weekly SBM frequency from baseline to weeks 1, 2, 3, and 4;
the change in weekly frequency of BMs, complete BMs (CBM), and complete SBMs (CSBM, defined as an SBM accompanied by the feeling of complete evacuation) from baseline to the last two weeks of the treatment period;
the proportions of SBM and CSBM responders (defined as patients with ≥3 SBMs/CSBMs per week in the last two weeks of the treatment period and an increase of ≥ 1 SBMs/CSBMs per week from baseline);
change in weekly frequency of SBMs without straining from baseline to the last two weeks of the treatment period;
change in abdominal bloating score from baseline to the last two weeks of the treatment period (this score was assessed daily for the past 24 hours and could range from 0 to 4, where 0 = absent or no bloating, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe bloating);
change in abdominal discomfort score from baseline to the last two weeks of the treatment period (this score was assessed daily for the past 24 hours and could range from 0 to 4, where 0 = no abdominal discomfort, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe discomfort);
the proportion of patients with an improvement in their global satisfaction score at the end of treatment on day 29 (assessed by the patient selecting one of the following descriptors: markedly worsened, moderately worsened, slightly worsened, unchanged, slightly improved, moderately improved, or markedly improved);
time to first SBM and CSBM after initial treatment administration;
the incidence of SBMs and of CSBMs in the four, eight, 12, and 24 hours after initial treatment administration;
change in the number of days with SBMs and with CSBMs per week from baseline to the last two weeks of the treatment period;
change in weekly frequency of SBMs rated 3 or 4 on the BSS from baseline to the last two weeks of the treatment period;
change in the weekly frequency of false starts of BMs from baseline to the last two weeks of the treatment period;
change in the weekly frequency of rescue laxative use from baseline to the last two weeks of the treatment period;
the frequency of rescue laxative use during the treatment period.
Safety Assessments
Adverse events (AEs) were recorded from the time of informed consent until the end of the follow-up period (28 days after the last dose of treatment). The duration, severity (mild, moderate, severe), relationship to the study drug (not related, possibly related, probably related, definitely related, as assessed by the investigators), action taken, outcome, and seriousness of each AE were recorded. A serious AE (SAE) was defined as any AE occurring at any dose that resulted in any of the following outcomes: death, life-threatening AE, hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, or a congenital anomaly/birth defect.
Patients were asked to rate their pain using the 11-point numerical rating scale (NRS) [14], where 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, and 7–10 = severe pain. The NRS was completed prior to the initiation of study treatment (during screening and predose on day 1 of treatment) and at several time points after treatment initiation (one hour postdose on day 1, predose on days 8, 15, 22, and at any time during the visit on day 29 of treatment).
Patients were assessed for opioid withdrawal symptoms using the clinical opiate withdrawal scale (COWS), an 11-item questionnaire [15]. The COWS assessment was administered prior to the initiation of study treatment (during screening and predose on day 1 of treatment) and at several time points after treatment initiation (one hour postdose on day 1 and predose on days 8, 15, 22, and 29 of treatment).
Clinical laboratory safety tests (chemistry, hematology, and urinalysis), physical examinations, and 12-lead electrocardiography were conducted, and vital signs were recorded as part of the safety assessment.
Pharmacokinetic Assessments
The pharmacokinetic profile of naldemedine was determined in a subset of patients from whom plasma samples were collected on day 1 at 0 (predose) and one hour (±5 minutes), two hours (±10 minutes), four hours (±15 minutes), and eight hours (±30 minutes) postdose, and day 2 at 24 hours (±1 hour) postdose. Plasma samples were also collected from 12 patients on day 28 at 0 (predose) and one hour (±5 minutes), two hours (±10 minutes), four hours (±15 minutes), and eight hours (±30 minutes) postdose, and day 29 at 24 hours (±1 hour) postdose.
The following parameters were recorded: maximum observed plasma concentration (Cmax); time to Cmax (Tmax); area under the plasma concentration time curve from time 0 to the time point of the last measurable concentration in the dose interval (24 hours; AUC0–τ); and apparent terminal elimination half-life.
Statistical Analyses
Based on pairwise comparison with placebo, a sample size of 212 (53 subjects per treatment group) was required to provide greater than 80% power to detect a treatment difference of at least 2.1 in the primary end point of change from baseline in the number of SBMs per week (at a two-sided significance level of 0.05 and assuming a standard deviation of 3.8). A total target sample size of 240 subjects (60 per treatment group) was determined, taking into consideration a 10% dropout rate.
The modified intent-to-treat population comprised all randomized patients who received the study drug and for whom at least one postdose primary efficacy assessment had been completed. The safety population comprised all patients who received the study drug. The per protocol population comprised all patients who received the last dose of treatment and did not have major protocol violations (defined as those that could potentially affect the efficacy or safety conclusions of the study; these violations were determined prior to unblinding the study database).
For the primary end point, the mean change in weekly SBM frequency in each naldemedine dose group was compared with that of the placebo group, based on an analysis of covariance model, with frequency of SBMs per week at baseline as a covariate. Naldemedine dose groups were compared with the placebo group sequentially in descending order of dose.
As a secondary end point, the mean change in weekly SBM frequency was also compared between the different naldemedine dose groups. Mean change in SBM frequency from baseline to weeks 1, 2, 3, and 4 was compared using a mixed-effects repeated measure model, which included the frequency of SBMs per week at baseline as a covariate, and treatment group, week, and week-by-treatment group interaction as fixed effects. The variance-covariance between-weeks matrix was unstructured. The differences in the proportion of SBM and CSBM responders between each naldemedine dose group and the placebo group, and frequencies of SBMs or CSBMs in the four, eight, 12, and 24 hours following initial treatment administration, were compared using a χ2 test. No corrections for multiplicity were performed for assessment of secondary end points. Summary statistics regarding the mean abdominal bloating score and discomfort score, and the corresponding change from baseline, were calculated by treatment group. Patients’ global satisfaction scores were tabulated by treatment group. The change in weekly BM, CBM, and CSBM frequency, change in weekly frequency of SBMs without straining, change in the number of days with SBMs and with CSBMs per week, and change in weekly frequency of SBMs rated 3 or 4 on the BSS were analyzed in a similar manner to the primary analysis, using repeated measure analysis. For the times to first SBM or to first CSBM after initial treatment administration, the distributions of times for each treatment group were compared using a generalized Wilcoxon test. Summary statistics regarding the change in frequency of false starts of BMs, weekly frequency of rescue laxative use, and frequency of rescue laxative use during the treatment period were calculated by treatment group. The change in frequency of rescue laxative use in each naldemedine dose group was compared with that in the placebo group using a Wilcoxon rank-sum test.
The incidences of treatment-emergent AEs (TEAEs, defined as AEs with an onset date between the first dose of study drug and 14 days after the last dose of study drug), treatment-related AEs (possibly, probably, or definitely related to study drug), and SAEs were compared between each of the naldemedine dose groups and the placebo group using Fisher’s exact test. Changes in NRS pain scores and COWS scores were analyzed using Welch’s t test.
Results
Baseline Patient Characteristics
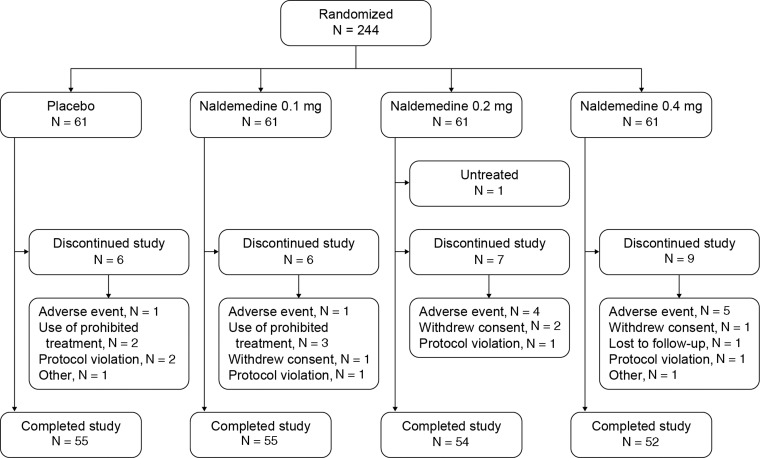
In total, 244 patients were randomized 1:1:1:1 to the naldemedine 0.1 mg, 0.2 mg, 0.4 mg, or the placebo groups (Figure 2). Baseline patient characteristics were comparable across the different treatment groups (Table 1). The mean total daily dose of opioids at baseline was not significantly different across treatment groups (P = 0.8635). Subjects were required to maintain a stable laxative regimen throughout the study. The proportion of subjects using concomitant, regular laxative agents during the study was similar across the placebo and naldemedine dose groups (72.1% [44/61], 75.4% [46/61], 78.0% [46/59], and 71.9% [41/57] for the placebo, naldemedine 0.1 mg, 0.2 mg, and 0.4 mg groups, respectively).
Figure 2.
Study flow diagram. Modified intent-to-treat population: placebo, N = 61; 0.1 mg cohort, N = 61; 0.2 mg cohort, N = 59; 0.4 mg cohort, N = 57. Safety population: placebo, N = 61; 0.1 mg cohort, N = 61; 0.2 mg cohort, N = 60; 0.4 mg cohort, N = 61. Per protocol population: placebo, N = 54; 0.1 mg cohort, N = 51; 0.2 mg cohort, N = 53; 0.4 mg cohort, N = 45.
Table 1.
Baseline characteristics of the trial population (modified intent-to-treat population)
| Naldemedine |
||||
|---|---|---|---|---|
| Placebo(N = 61) | 0.1 mg/day(N = 61) | 0.2 mg/day(N = 59) | 0.4 mg/day(N = 57) | |
| Age, y | 53.1 (10.9) | 49.5 (9.7) | 50.7 (11.4) | 54.1 (11.2) |
| Female, N (%) | 45.0 (73.8) | 47.0 (77.0) | 38.0 (64.4) | 37.0 (64.9) |
| Race, N (%) | ||||
| White | 49 (80.3) | 49 (80.3) | 47 (79.7) | 51 (89.5) |
| Black | 9 (14.8) | 11 (18.0) | 12 (20.3) | 6 (10.5) |
| Other | 3 (4.9) | 1 (1.6) | 0 | 0 |
| Ethnicity, N (%) | ||||
| Hispanic or Latino | 4 (6.6) | 6 (9.8) | 2 (3.4) | 4 (7.0) |
| Not Hispanic or Latino | 57 (93.4) | 55 (90.2) | 57 (96.6) | 53 (93.0) |
| Body mass index, kg/m2 | 29.8 (7.2) | 29.6 (6.3) | 32.0 (8.1) | 30.6 (5.6) |
| No. of SBMs/wk | 1.22 (0.72) | 1.51 (0.82) | 1.52 (0.92) | 1.20 (0.95) |
| No. of CSBMs/wk | 0.38 (0.53) | 0.49 (0.70) | 0.52 (0.70) | 0.39 (0.67) |
| Equivalent daily morphine dose, mg/day | 146.5 (212.5) | 120.6 (206.7) | 124.3 (158.6) | 125.3 (143.2) |
All data are mean (standard deviation) unless otherwise stated.
CSBM = complete spontaneous bowel movement; SBM = spontaneous bowel movement.
Efficacy
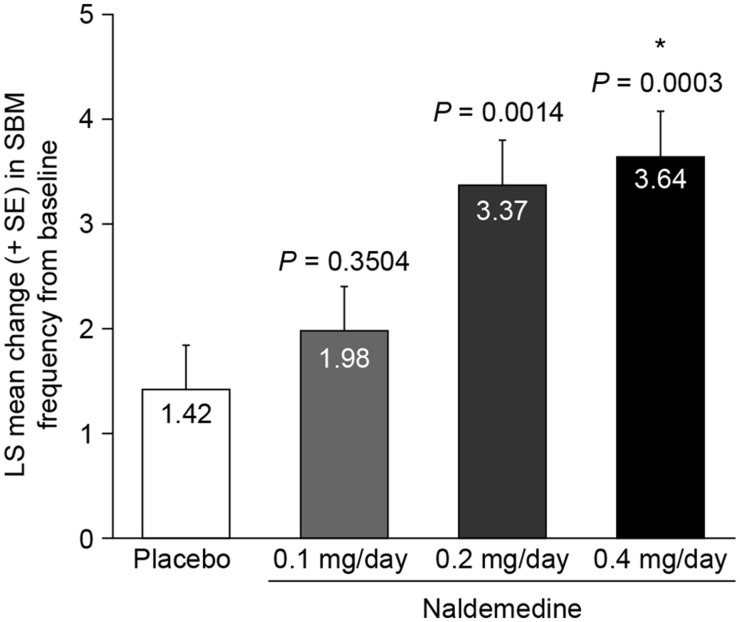
There was a statistically significant increase from baseline to the last 2 weeks of treatment in mean weekly SBM frequency (the primary end point) in the naldemedine 0.2 mg group (least squares [LS] mean increase 3.37 ± standard error [SE] 0.43 SBMs per week) and the 0.4 mg group (3.64 ± 0.44) compared with the placebo group (1.42 ± 0.42, P = 0.0014, P = 0.0003, respectively) (Figure 3). The difference in mean weekly SBM frequency between the 0.2 mg and 0.4 mg doses was not statistically significant (P = 0.6657). The increase in mean weekly SBM frequency in the naldemedine 0.1 mg group was not significant compared with placebo (1.98 ± 0.42, P = 0.3504).
Figure 3.
Primary efficacy end point: least squares mean change in weekly spontaneous bowel movement frequency from baseline to the last two weeks of the treatment period (modified intent-to-treat population). Exact P values in the figure are compared with placebo. *P = 0.6657 for naldemedine 0.4 mg vs 0.2 mg daily. LS = least squares; SBM = spontaneous bowel movement; SE = standard error.
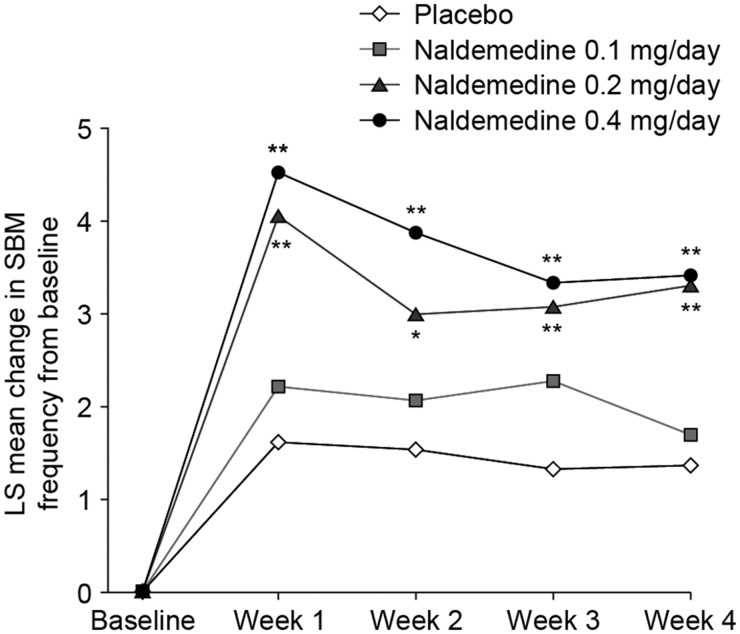
Compared with the placebo group, the naldemedine 0.2 mg and 0.4 mg groups demonstrated a significant increase in mean SBM frequency from baseline to each week of the four-week treatment period (0.2 mg group, P < 0.01 for weeks 1, 3, and 4 and P < 0.05 for week 2 vs placebo; 0.4 mg group, P < 0.01 for weeks 1–4) (Figure 4). Naldemedine 0.1 mg was not significantly different from placebo at any time point.
Figure 4.
Least squares mean change in weekly spontaneous bowel movement frequency from baseline to weeks 1, 2, 3, and 4 (modified intent-to-treat population). *P < 0.05; **P < 0.01 vs placebo. LS = least squares; SBM = spontaneous bowel movement.
CSBM weekly frequency increased significantly from baseline to the last two weeks of the treatment period in the naldemedine 0.2 mg group (LS mean increase 2.69 ± 0.35) and 0.4 mg group (2.44 ± 0.36) compared with the placebo group (0.99 ± 0.35, P = 0.0007, P = 0.0039, respectively) (Figure 5A). The difference in mean weekly CSBM frequency between the 0.2 mg and 0.4 mg dose groups was not significant (P = 0.6117). There was also no significant difference between the naldemedine 0.1 mg group (1.49 ± 0.344, P = 0.3077) and the placebo group.
Figure 5.
A) Least squares mean change in weekly complete spontaneous bowel movement (CSBM) frequency from baseline to the last two weeks of the treatment period. *P = 0.6117 for naldemedine 0.4 mg vs 0.2 mg once daily. B) Proportion of SBM responders. *P = 0.5989 for naldemedine 0.4 mg vs 0.2 mg once daily. C) Proportion of CSBM responders. *P = 0.9872 for naldemedine 0.4 mg vs 0.2 mg once daily. Modified intent-to-treat population shown for (A), (B), and (C). Exact P values in figures are compared with placebo. CSBM = complete spontaneous bowel movement; LS = least squares; SBM = spontaneous bowel movement; SE = standard error.
The proportion of SBM responders was significantly higher with naldemedine 0.2 mg (71.2%) and 0.4 mg (66.7%) than with placebo (39.3%, P = 0.0005, P = 0.003, respectively) (Figure 5B). The difference in the proportion of SBM responders between the naldemedine 0.2 mg and 0.4 mg doses was not significant (P = 0.5989). The proportion of SBM responders in the naldemedine 0.1 mg group was not significantly different from the proportion in the placebo group (52.5%, P = 0.1461).
The proportion of CSBM responders was significantly higher with naldemedine 0.2 mg (45.8%) and 0.4 mg (45.6%) than with placebo (21.3%, P = 0.0045, P = 0.005, respectively) (Figure 5C). The difference in the proportion of CSBM responders between the 0.2 mg and 0.4 mg doses was not significant (P = 0.9872). The proportion of CSBM responders in the naldemedine 0.1 mg group was not significantly different from that in the placebo group (29.5%, P = 0.2984).
Patients in the naldemedine 0.2 mg and 0.4 mg dose groups had significantly greater increases in the mean weekly frequency of SBMs without straining (0.2 mg group, LS mean ± SE increase 2.92 ± 0.39; 0.4 mg group, 3.21 ± 0.40) compared with the placebo group (0.85 ± 0.38, P < 0.05 vs placebo for both dose groups). There was no significant difference between the naldemedine 0.1 mg group (1.54 ± 0.38) and the placebo group (Table 2).
Table 2.
Secondary end point results (modified intent-to-treat population)
| Naldemedine |
||||
|---|---|---|---|---|
| Placebo(N = 61) | 0.1 mg/day(N = 61) | 0.2 mg/day(N = 59) | 0.4 mg/day(N = 57) | |
| Increase in weekly frequency of SBMs without straining, LS mean (SE)† | 0.85 (0.38) | 1.54 (0.38) | 2.92* (0.39) | 3.21* (0.40) |
| Decrease in abdominal bloating score, mean (SD)† | 0.40 (0.69) | 0.46 (0.76) | 0.54 (0.72) | 0.57 (0.77) |
| Decrease in abdominal discomfort score, mean (SD)† | 0.42 (0.73) | 0.51 (0.79) | 0.50 (0.70) | 0.42 (0.99) |
| Proportion of patients with an improvement in global satisfaction score, % | 50.00‡ | 72.41*,‡ | 91.23*,‡ | 83.02*,‡ |
LS = least squares; SBM = spontaneous bowel movement; SD, standard deviation and SE, standard error.
P < 0.05 vs placebo.
From baseline to the last two weeks of the treatment period.
Proportion based on total number of patients with available data (placebo, N = 58; naldemedine 0.1 mg, N = 58; naldemedine 0.2 mg, N = 57; naldemedine 0.4 mg, N = 53).
There were no significant differences in the mean change in abdominal bloating score in any of the treatment groups compared with the placebo group (mean [SD] decrease 0.46 [0.76] in the 0.1 mg group; 0.54 [0.72] in the 0.2 mg group; 0.57 [0.77] in the 0.4 mg group, and 0.40 [0.69] in the placebo group) (Table 2). Similarly, there were no significant differences in the mean change in abdominal discomfort score in any of the treatment groups compared with placebo (mean [SD] decrease 0.51 [0.79] in the 0.1 mg group; 0.50 [0.70] in the 0.2 mg group; 0.42 [0.99] in the 0.4 mg group; 0.42 [0.73] in the placebo group) (Table 2).
The proportion of patients with an improvement in global satisfaction score at the end of treatment (those selecting slightly, moderately, or markedly improved) was significantly higher in all three treatment groups than in the placebo group (0.1 mg group, 72.41%; 0.2 mg group, 91.23%; 0.4 mg group, 83.02%; placebo, 50.00%; P < 0.05 for all doses compared with placebo) (Table 2). Additional secondary end point results are summarized in Supplementary Table S1.
Safety
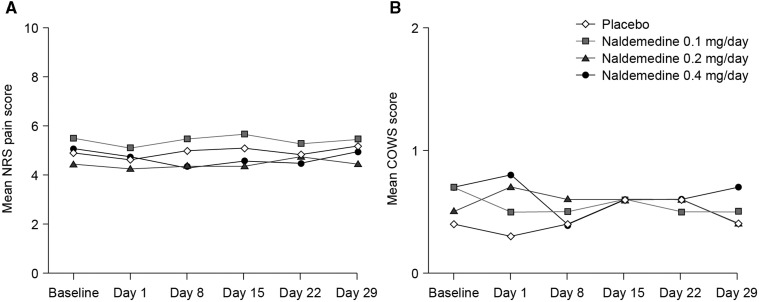
The overall incidence of TEAEs was similar across the treatment groups. The most common TEAEs were GI disorders, including abdominal pain, diarrhea, flatulence, and nausea (Table 3). Most TEAEs were mild to moderate in severity. The incidence of treatment-related AEs increased with naldemedine dose. Ten patients discontinued from the study owing to a TEAE: one in the 0.1 mg group (chest pain, recorded as an SAE); four in the 0.2 mg group (flatulence, upper abdominal pain, diarrhea, abdominal pain); and five in the 0.4 mg group (diarrhea [N = 3], dyspnea [N = 1], abdominal pain plus diarrhea [N = 1]). There were no clinically meaningful changes from baseline in NRS pain scores or COWS scores relative to placebo in any of the naldemedine groups (Figure 6).
Table 3.
Numbers and proportions of patients reporting TEAEs (safety population)
| Naldemedine |
||||
|---|---|---|---|---|
| Placebo(N = 61) | 0.1 mg/day(N = 61) | 0.2 mg/day(N = 60) | 0.4 mg/day(N = 61) | |
| TEAE | 31 (50.8) | 25 (41.0) | 30 (50.0) | 34 (55.7) |
| Serious TEAE | 0 (0.0) | 2 (3.3) | 0 (0.0) | 1 (1.6) |
| Treatment-related TEAE | 10 (16.4) | 10 (16.4) | 15 (25.0) | 24* (39.3) |
| Any GI TEAE | 8 (13.1) | 13 (21.3) | 15 (25.0) | 21 (34.4) |
| Abdominal pain | 1 (1.6) | 3 (4.9) | 5 (8.3) | 9 (14.8) |
| Diarrhea | 3 (4.9) | 3 (4.9) | 3 (5.0) | 11 (18.0) |
| Flatulence | 2 (3.3) | 3 (4.9) | 3 (5.0) | 2 (3.3) |
| Nausea | 1 (1.6) | 1 (1.6) | 4 (6.7) | 3 (4.9) |
All data are N (%).
GI = gastrointestinal; TEAE = treatment-emergent adverse event.
P < 0.01 vs placebo.
Figure 6.
A) Numerical rating scale pain scores and B) clinical opiate withdrawal scale scores (safety population). COWS = clinical opiate withdrawal scale; NRS = numerical rating scale.
Changes in chemistry, hematology, and urinalysis parameters from baseline to the end of the treatment period were similar for all treatment groups, and there were no clinically notable differences between the treatment groups. Mean changes in vital signs were minimal, and no clinically relevant changes were observed in any treatment group. There were no clinically meaningful changes in physical examination parameters from baseline to the end of the treatment period in any treatment group. Mean changes in electrocardiogram parameters were generally small, and no clinically relevant changes were observed in any treatment group.
Pharmacokinetic Profile
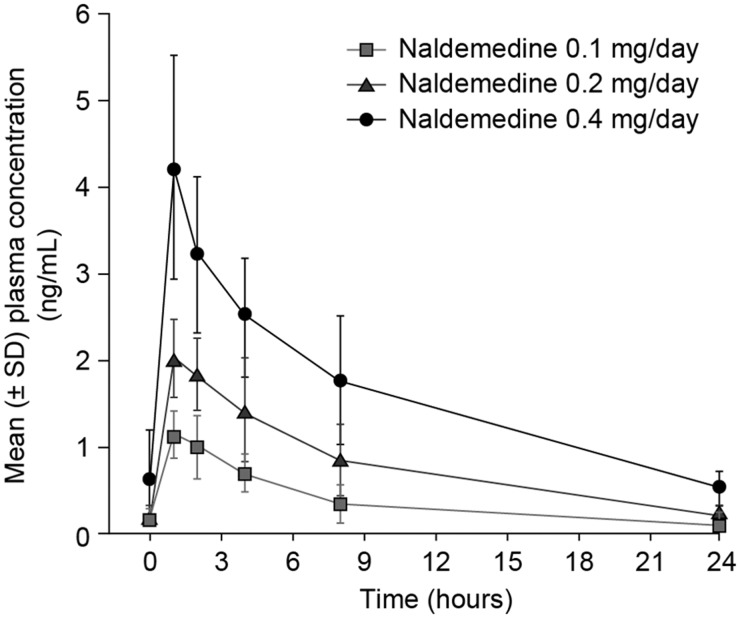
Naldemedine was rapidly absorbed following multiple 0.1 mg–0.4 mg doses, with a median Tmax of 1.0 hour (Figure 7 and Table 4). The geometric mean Cmax and AUC0-τ of naldemedine increased in proportion to the dose over the range of 0.1–0.4 mg following single and multiple doses.
Figure 7.
Mean plasma concentration time profile for naldemedine on day 28. SD = standard deviation.
Table 4.
Pharmacokinetic profile of naldemedine
| N | Cmax, ng/mL | Tmax, h | AUC0–τ, ng•h/mL | t½,z, h | |
|---|---|---|---|---|---|
| Naldemedine 0.1 mg/day | 4 | 1.15 (25.8) | 1.03 (1.00–1.97) | 9.677 (33.7) | 8.64 (16.4) |
| Naldemedine 0.2 mg/day | 4 | 2.00 (22.7) | 1.00 (1.00–1.03) | 16.94 (46.6) | 7.11 (28.7) |
| Naldemedine 0.4 mg/day | 4 | 4.03 (32.3) | 1.00 (1.00–1.08) | 31.72 (11.4) | 10.8 (31.5) |
All values are given as geometric mean (percentage coefficient of variation) other than Tmax, which is given as median (range). AUC0–τ, area under the concentration time curve from time 0 to the time point of the last measurable concentration in the dose interval.
Cmax = maximum observed plasma concentration Tmax = time to Cmax t½,z = apparent terminal elimination half-life
Discussion
Many patients with chronic noncancer pain who develop OIC do not achieve satisfactory relief of OIC symptoms using diet, laxatives, or stool softeners; thus, an effective approach is needed for these patients [5,8]. In this phase 2b, double-blind placebo-controlled trial, the efficacy and safety of the novel PAMORA naldemedine, administered at once-daily doses of 0.1, 0.2, and 0.4 mg, was evaluated in patients with chronic noncancer pain who had OIC and were receiving opioid therapy. The trial met its primary end point, demonstrating that naldemedine 0.2 mg or 0.4 mg once daily significantly increased the mean weekly frequency of SBMs from baseline to the last two weeks of the treatment period, compared with placebo. Change in mean weekly frequency of SBMs in the naldemedine 0.1 mg group was not significantly different from that in the placebo group. Based on the overall efficacy and safety profiles demonstrated in this study, naldemedine 0.2 mg once daily has been selected for future clinical trials in OIC.
Naldemedine 0.4 mg produced a slightly greater increase in mean weekly SBM frequency than naldemedine 0.2 mg, but the difference was not significant, suggesting no increase in efficacy with this higher dose. The effects of naldemedine 0.2 mg and 0.4 mg vs placebo were maintained throughout the four-week study period.
Benefits were observed with naldemedine 0.2 mg and 0.4 mg across multiple secondary end points in the current study. Change in CSBM frequency and the proportion of SBM and CSBM responders showed significant improvements compared with placebo; this was not seen with naldemedine 0.1 mg. There was again no significant difference between the two higher doses, suggesting that increasing the dose above 0.2 mg per day does not provide any additional benefit.
Straining to pass a BM and a feeling of incomplete evacuation are key symptoms used to determine OIC [5]. Naldemedine 0.2 mg and 0.4 mg reduced these symptoms, as demonstrated by significant differences in relevant secondary end points (increase in the proportion of CSBM responders and the mean weekly frequency of SBMs without straining) compared with placebo.
There were no significant changes in the abdominal bloating and abdominal discomfort scores between baseline and the end of the treatment period in this study. The reasons for these findings are not clear. A possible explanation is that the scales used in the current study were not sufficiently sensitive to detect small changes in abdominal bloating and discomfort. To the best of our knowledge, there is not a validated scale for an objective assessment of bloating in patients with constipation.
Patients in all three dose groups were significantly more satisfied with treatment at the end of the current study than those receiving placebo: over 90% of patients receiving naldemedine 0.2 mg, but only 50% of those receiving placebo, were satisfied.
All doses of naldemedine were well tolerated, and no safety concerns emerged in this study. The overall incidence of TEAEs was similar across treatment groups. The incidence of treatment-related AEs increased with naldemedine dose. The highest number of treatment-related AEs was reported in patients treated with naldemedine 0.4 mg. This suggests a dose-related response, with a better safety profile being demonstrated for naldemedine 0.2 mg than for naldemedine 0.4 mg. The most frequently reported treatment-related AEs were GI disorders, including abdominal pain, diarrhea, flatulence, and nausea. These AEs are expected based on the mechanism of action of the drug and its expected effect on μ-opioid receptors expressed in the enteric nervous system.
Naldemedine had no effect on the analgesic property of opioids (as assessed by NRS pain scores) and did not cause opioid withdrawal (as assesed by COWS scores), consistent with its peripheral action. The pharmacokinetic profile of naldemedine demonstrated that it is rapidly absorbed, with an apparent terminal elimination half-life (7.11–10.8 hours) that supports once-daily dosing.
Study Limitations
This trial had some limitations: the use of a self-recorded diary to determine subjective outcomes, including straining, constipation, patient satisfaction, and pain may have introduced some bias. Such diaries are, however, a routine and necessary element of all OIC trials. Larger trials with longer duration of treatment are needed to confirm the results of this study.
Conclusion
Administration of the PAMORA naldemedine at doses of 0.2 mg and 0.4 mg once daily for four weeks was efficacious in relieving OIC in patients with chronic noncancer pain who were taking opioids long term. The 0.2 mg dose was shown to have a better safety profile than the 0.4 mg dose. Treatment with naldemedine did not compromise analgesia or cause opioid withdrawal symptoms. Naldemedine 0.2 mg once daily will be used in future confirmatory trials in OIC.
Supplementary Material
Acknowledgments
Medical writing support (editorial and graphics) was provided by Noëlle L O’Regan PhD of PharmaGenesis London, London, UK and was funded by Shionogi.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
References
- 1. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH.. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 2010;11:1230–9. [DOI] [PubMed] [Google Scholar]
- 2. Loyland B, Miaskowski C, Wahl AK, Rustoen T.. Prevalence and characteristics of chronic pain among long-term social assistance recipients compared to the general population in Norway. Clin J Pain 2010;26:624–30. [DOI] [PubMed] [Google Scholar]
- 3. Ohayon MM, Stingl JC.. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res 2012;46:444–50. [DOI] [PubMed] [Google Scholar]
- 4. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: A multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: Results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32. [DOI] [PubMed] [Google Scholar]
- 7. Bell T, Annunziata K, Leslie JB.. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: Findings from the National Health and Wellness Survey. J Opioid Manag 2009;5:137–44. [DOI] [PubMed] [Google Scholar]
- 8. Brenner DM, Chey WD.. An evidence-based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Supplements 2014;2:38–46. [Google Scholar]
- 9. Webster LR. Opioid-induced constipation. Pain Med 2015;16(suppl 1):S16–21. [DOI] [PubMed] [Google Scholar]
- 10.AstraZeneca. Movantik prescribing information. Available at: http://www.azpicentral.com/movantik/movantik.pdf#page=1 (accessed January 2017).
- 11.Salix. Relistor prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208271s000lbl.pdf (accessed January 2017).
- 12. Kanemasa T, Koike K, Arai T, et al. Effects of naldemedine: A peripherally acting mu-opioid receptor antagonist in rat models of opioid-induced constipation. Am J Gastroenterol 2015;110:1322. [Google Scholar]
- 13. Webster LR, Nagata T, Yamada T, Arjona Ferreira JC. A phase 2a, randomized, double-blind, placebo-controlled, single ascending-dose study to evaluate the safety and efficacy of naldemedine in patients with chronic non-cancer pain and opioid-induced bowel dysfunction. Presented in poster format at the 35th Annual Scientific Meeting of the American Pain Society, May 11–14, 2016, Austin, TX, USA; 2016.
- 14. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 15. Wesson DR, Ling W.. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 2003;35: 253–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.