ABSTRACT
In 2015, the US Food and Drug Administration (FDA) approved the anti-proprotein convertase subtilsin/kexin type 9 (PCSK9) monoclonal antibodies, alirocumab and evolocumab, to treat patients with hypercholesterolemia and mixed dyslipidemia. Since then, considerable attention has been paid to the use of these monoclonal antibodies for the treatment of diabetic dyslipidemia with a goal of reducing the risk for cardiovascular disease. Recently, consensus statements on the clinical use of PCSK9 inhibitors in patients with type 2 diabetes mellitus, who are unable to achieve the goal of low-density lipoprotein cholesterol (<70 mg/dL or <1.8 mmol/L), have been published by panels of experts in Greece, Europe (European Society of Cardiology and European Atherosclerosis Society Task Force), and the United States (American College of Cardiology Consensus Committee). On December 1, 2017, the FDA approved evolocumab to prevent heart attack, stroke, and coronary revascularization. In this article, we review recent advances concerning the pathophysiology of diabetic dyslipidemia, the physiology of PCSK9, the mechanisms of action of PCSK9 inhibitors, clinical trials examining PCSK9 inhibitors in type 2 diabetes, and perspectives of nonstatin therapy in the treatment of diabetic dyslipidemia.
KEYWORDS: Alirocumab, cardiovascular risk, diabetic dyslipidemia, evolocumab, low-density lipoprotein cholesterol, proprotein convertase subtilisin/kexin type 9, triglyceride
In 2015, the US Food and Drug Administration (FDA) approved the first anti-proprotein convertase subtilsin/kexin type 9 (PCSK9) monoclonal antibodies, alirocumab and evolocumab, to treat patients with hypercholesterolemia and mixed dyslipidemia. The FDA stated that elevated low-density lipoprotein (LDL) cholesterol is a risk factor for cardiovascular diseases. For several years, the regulatory agency has used a reduction in LDL cholesterol as a validated surrogate of cardiovascular risk for several lipid-altering drugs to support approval.1,2 In this regard, alirocumab and evolocumab significantly lowered LDL cholesterol from baseline relative to the comparators.1,2 Since then, considerable attention has been paid to the use of the two monoclonal antibodies for targeting PCSK9 as a treatment for diabetic dyslipidemia with the goal of reducing the risk of cardiovascular disease.3,4 Clinical phase 3 trials of alirocumab and evolocumab have demonstrated that both drugs substantially decrease LDL cholesterol and non–high-density lipoprotein cholesterol (non-HDL-C) levels in patients with type 2 diabetes mellitus.5–10 There has been a growing opinion that PCSK9 inhibitors may be used safely among adults with diabetes mellitus who are statin intolerant and have high cardiovascular risk.11,12 Hence, the introduction of PCSK9 inhibitors offers a nonstatin therapeutic option for the treatment of type 2 diabetic patients who are unable to achieve their LDL cholesterol goals (<70 mg/dL or <1.8 mmol/L) with statin therapy. On December 1, 2017, the FDA approved evolocumab to prevent heart attack, stroke, and coronary revascularization.13 Here we review advances regarding the pathophysiology of diabetic dyslipidemia, the physiology of PCSK9, the mechanisms of action for PCSK9 inhibitors, clinical trials examining PCSK9 inhibitors in patients with type 2 diabetes, and nonstatin therapies for the treatment of diabetic dyslipidemia.
PATHOPHYSIOLOGY OF DIABETIC DYSLIPIDEMIA
Both insulin resistance and hyperglycemia play a significant role in the pathophysiology of diabetic dyslipidemia. The pathogenesis of diabetic dyslipidemia has been ascribed to abnormalities in the quantity, quality, and kinetics of lipoprotein.14–16 Quantitative derangements include an increase in triglyceride levels and a decrease in HDL cholesterol levels. Qualitative abnormalities include a higher participation of large very low-density lipoprotein (VLDL) 1, small density LDL particles, triglyceride-rich VLDL 1, and triglyceride-rich HDL 2, and kinetic abnormalities include an increased production of VLDL 1, decreased VLDL catabolism, and increased HDL catabolism. Therefore, VLDL 1 has become a primary focus in the pathophysiology of diabetic dyslipidemia. In addition, hyperglycemia likely acts as the driving force behind the overproduction of VLDL 1, and the function of free fatty acids appears to act as an inducer to increase VLDL production because hepatic fatty acid availability regulates VLDL-triglyceride production.
PHYSIOLOGY OF PCSK9
Remarkable advances have been achieved since PCSK9's first description in 2003,17 rendering it a promising treatment for diabetic dyslipidemia. Cutting-edge discoveries, such as genetic variants of gain-of-function18 and loss-of-function mutations19 in the PCSK9 gene, aid in the understanding of PCSK9's role in regulating cholesterol homeostasis by exerting opposite effects on LDL cholesterol levels and cardiovascular risks.20 PCSK9 originates from hepatocytes, and it is an endogenous inhibitor of the hepatic LDL receptor. After PCSK9 binds to the LDL receptor, the LDL receptor degrades in the endosomes and lysosomes, promoting their clearance in the hepatocyte and preventing the recycling of the LDL receptor back to the cell surface, ultimately increasing plasma LDL cholesterol concentrations21 (Figure 1a).
Figure 1.
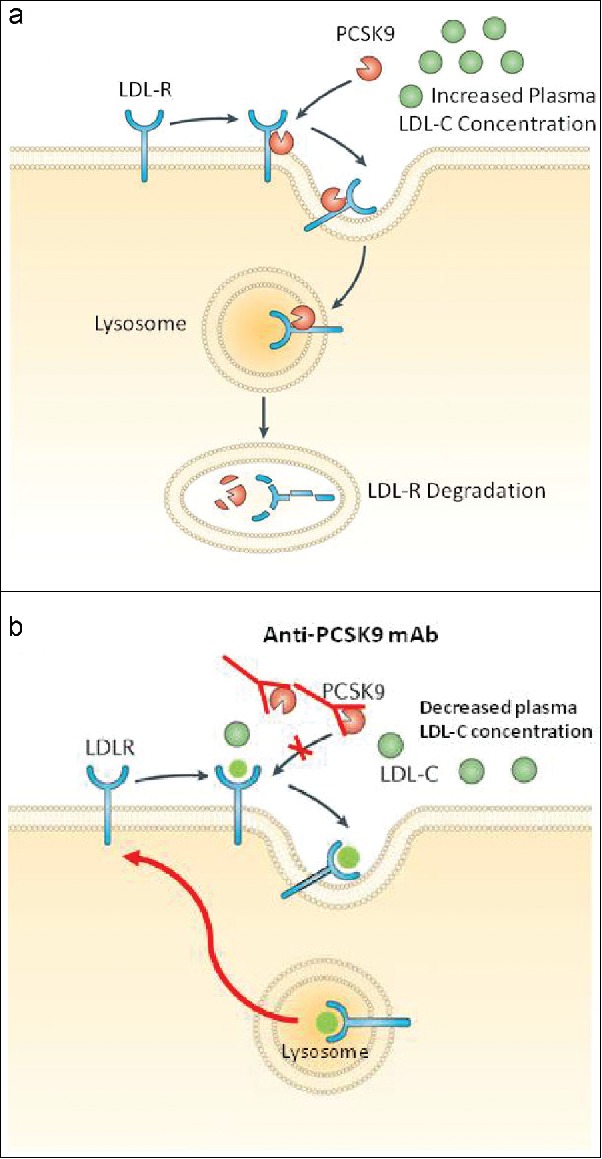
(a) Prevention of low-density lipoprotein receptor (LDL-R) recycling by proprotein convertase subtilisin/kexin type 9 (PCSK9), causing an increase in plasma low-density lipoprotein cholesterol (LDL-C) concentration. (b) Mechanism of action of anti-proprotein convertase subtilsin/kexin type 9 (PCSK9) monoclonal antibody (mAb). The anti-PCSK9 mAb binds to PCSK9, preventing it from binding to the LDL-R. Through inhibition of PCSK9, the LDLR is recycled to the hepatic cell surface and may continue to clear LDL-C particles, causing a decrease in plasma LDL-C concentration. Modified with permission from Tecson et al.21
MECHANISM OF ACTION OF PCSK9 INHIBITORS
PCSK9 binds to the LDL receptor, resulting in its degradation in the endosomes and lysosomes; however, pharmacological inhibition of PCSK9 prevents its binding.22,23 Figure 1b illustrates that the inhibition of PCSK9 reduces the degradation of LDL receptors, allowing for more LDL receptor recycling to the surface of hepatocytes, which increases the clearance of LDL receptors and substantially lowers plasma LDL cholesterol as well as apolipoprotein B (Apo B)-100.23 It is noteworthy that PCSK9 inhibitors may induce further LDL cholesterol reduction and generally improve the lipid profile in patients with diabetes.4
CLINICAL PHASE 3 TRIALS IN PATIENTS WITH TYPE 2 DIABETES WITH PCSK9 INHIBITORS
Diabetic dyslipidemia is characterized by elevated LDL cholesterol, the predominance of small density LDL particles, elevated triglycerides, and decreased HDL cholesterol.14 The primary goal of the pharmacological management of diabetic dyslipidemia is to reduce the levels of LDL cholesterol, and the secondary goal focuses on reducing non-HDL cholesterol and Apo B levels.4 To further reduce cardiovascular risk, management should include attempts to decrease triglycerides and increase HDL cholesterol. It has been proposed that non-HDL cholesterol is a therapeutic target for mixed dyslipidemia (e.g., non-HDL cholesterol <130 mg/dL [3.3 mmol/L] for high risk; <100 mg/dL [2.6 mmol/L] for very high risk).5 Because patients with diabetic dyslipidemia have higher risks for developing both cardiovascular disease and metabolic disease, current medications (e.g., statins, ezetimibe, fibrates, etc.) are at times unsuccessful in achieving the recommended goals for LDL cholesterol (<70 mg/dL), non-HDL cholesterol (<100 mg/dL), and Apo B (<80 mg/dL).4 Therefore, the discovery of PCSK9 inhibitors has been a promising addition to the list of potential treatment options. Table 1 presents the lipid changes in patients with type 2 diabetes and mixed dyslipidemia treated with PCSK9 inhibitors in selected clinical trials after 2015.
Table 1.
Lipid changes in patients with diabetes treated with PCSK9 inhibitors in selected clinical trials
| References | Trial | No. of NCT | Anti-PCSK9 antibody | No. of patients | Duration (weeks) | LDL-C (%) | Non-HDL-C (%) | TC (%) | TG (%) | Lp(a) (%) | HDL-C (%) | Apo B (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taskinen et al.5 | Five phase 3 trials | Alirocumab | 675 DM with dyslipidemia | 24 | −57% | −45% | ||||||
| Leiter et al.6 | ODYSSEY COMB II | 01644188 | Alirocumab | 148 diabetes mellitus | 24 | −49% | −41% | −15% | −20% | +8% | −30% | |
| Muller-Wieland et al.8 | ODYSSEY DM-INSULIN | 02585778 | Alirocumab | 287 T1DM | 24 | −49%a | −38% | −27% | −6% | −19% | +8% | −33% |
| Muller-Wieland et al.8 | ODYSSEY DM-INSULIN | 02585778 | Alirocumab | 49 T1DM | 24 | −49%a | −46% | −14% | −30% | −23% | +11% | −39% |
| Sattar et al.9 | Three phase 3 trials | 01763866 | Evolocumab | 46 T2DM | 12 | −60% | −55% | −38% | −23% | −31% | +7% | |
| 01763918 | ||||||||||||
| 01763905 | ||||||||||||
| Sabatine et al.10 | FOURIER | 01764633 | Evolocumab | 11,031 DM | 48 | −57% |
PCSK9 indicates proprotein convertase subtilsin/kexin type 9; NCT, ClinicalTrials.gov number; LDL-C, low density lipoprotein cholesterol; non-HDL-C, non–high-density lipoprotein cholesterol; TC, total cholesterol; TG, total triglycerides; Lp(a), lipoprotein (a); HDL-C, high-density lipoprotein cholesterol; Apo B, apolipoprotein B; FOURIER, Further cardiovascular Outcomes Research with PCSK9 Inhibition in subjects with Elevated Risk; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Data collected at week 12.
A pooled subgroup analysis of five phase 3 trials showed that treatment of patients with type 2 diabetes with mixed dyslipidemia with 150 mg alirocumab every 2 weeks for 24 weeks reduced LDL cholesterol levels by 57% and reduced non-HDL cholesterol by 45%. Adverse events included nasopharyngitis/upper respiratory infection (≥5%) and injection site reaction (3%). The safety of alirocumab was unaffected by the presence of mixed dyslipidemia. Hence, alirocumab substantially improved LDL cholesterol and non-HDL cholesterol, and non-HDL cholesterol may be used as a therapeutic target for mixed dyslipidemia.5 In 2017, three ODYSSEY program studies of alirocumab were conducted.6–8 The ODYSSEY COMBO II trial included 148 patients with type 2 diabetes treated with alirocumab.6 This 104-week, double-blind trial enrolled patients with documented atherosclerotic cardiovascular disease and baseline LDL cholesterol ≥70 mg/dL (1.8 mmol/L). Patients received 150 mg every 2 weeks. At 24 weeks, the average LDL cholesterol levels were reduced by 49%, non-HDL cholesterol by 41%, Apo B by 31%, lipoprotein (a) by 20%, and triglycerides by 15% from baseline. Additionally, HDL cholesterol increased by 8%.6 The study showed that over a 104-week period, alirocumab consistently resulted in greater LDL cholesterol reduction than ezetimibe for patients with or without diabetes, with a similar safety profile, regardless of baseline diabetes status.6 The ODYSSEY-DM-INSULIN phase 3b/4 trial8 and the ODYSSEY-DM-DYSLIPIDEMIA trial7 have completed and results were presented at the American Diabetes Association in San Diego in June 2017. Since then, the ODYSSEY DM-INSULIN results have been officially published.6 It randomized 441 participants with type 2 diabetes and 76 with type 1 diabetes with high cardiovascular risk and LDL-C ≥70 mg/dL (≥1.8 mmol/L) to receive either subcutaneous alirocumab or placebo in a 2:1 ratio every 2 weeks for 24 weeks. A significant reduction in LDL-C was detected for patients with type 1 and type 2 diabetes receiving alirocumab when compared to placebo (47.8% ± 6.5% for type 1 diabetes and 49.0% ± 2.7% for type 2 versus placebo; both P < 0.0001). Statistically significant reductions in non-HDL cholesterol and apo B were also found when compared to placebo (P values < 0.0001) without important safety concerns. The final publication for the ODYSSEY DM-DYSLIPIDEMIA trial is awaited to fill the knowledge gap regarding mixed hyperlipidemia, the most common lipid disorder among diabetics. The trial's aim was to enroll 420 participants with type 2 diabetes, mixed hyperlipidemia (non-HDL-C ≥100 mg/dL and triglycerides ≥150 and <500 mg/dL despite maximally tolerated statin therapy), and documented atherosclerotic cardiovascular disease or at least two cardiovascular risk factors. Patients were randomized to alirocumab or placebo in a 2:1 ratio. Results presented to date include 413 patients recruited with the primary efficacy endpoint reported as a 33.3% reduction in non-HDL-C in the alirocumab group compared to fenofibrate (P < 0.0001).8
Similarly, evolocumab markedly improved lipid profiles in 246 patients with type 2 diabetes.9 At week 12, LDL cholesterol levels were reduced from baseline by 60%, non-HDL cholesterol by 55%, total cholesterol by 38%, total triglycerides by 23%, and lipoprotein (a) by 31%. Additionally, HDL cholesterol increased by 7% (Table 1). These data revealed the promising efficacy of evolocumab as a treatment for type 2 diabetes. Adverse events of injection site reaction and myalgia were similar across all treatment arms. Recently, Sabatine and colleagues reported the results of a prespecified secondary analysis from the Further cardiovascular Outcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER) trial in patients with or without diabetes treated with evolocumab (140 mg every 2 weeks or 420 mg once per month).10 The FOURIER trial consisted of 11,031 patients with diabetes (40%) and 16,533 patients without diabetes (60%), with median baseline LDL-C concentrations of 92 mg/dL (2.3 mmol/L). At 48 weeks, evolocumab yielded a mean reduction in LDL cholesterol of 57% (95% confidence interval [CI], 56–58; P < 0.0001), non-HDL cholesterol by 50% (95% CI, 49–51; P < 0.0001), Apo B by 48% (95% CI, 46–48), and triglycerides by 16% (95% CI, 14–18, P < 0.0001) among patients with diabetes. Consistent with improvements in patients' lipid profiles, evolocumab significantly lowered the risk of heart attack by 27% (P < 0.001), stroke by 21% (P = 0.01), and coronary revascularization by 22% (P < 0.001). Further, evolocumab did not increase the risk of diabetes development (hazard ratio 1.05, 95% CI, 0.94–1.17) or worsen glycemia, over a median of 2.2 years of follow-up,10,24 regardless of baseline glycemic status. The results of the FOURIER trial presented a striking contrast compared to studies with statins in which hemoglobin A1c levels increased by about 0.12% in patients with diabetes, body weight increased (0.24 kg higher), and there was a 9% increase in the risk of new-onset diabetes.10,24 As a result, the FOURIER trial came to a conclusion that evolocumab lowered LDL-C and significantly reduced cardiovascular risk with similar relative efficacy in patients with and without diabetes mellitus.10 These data led to the FDA's approval of evolocumab for the prevention of heart attack, stroke, and coronary revascularization on December 1, 2017.13
A panel of experts in internal medicine, endocrinology, and cardiology in Greece proposed that PCSK9 inhibitors (alirocumab and evolocumab) could be used in patients with diabetes with known cardiovascular disease or chronic kidney disease (estimated glomerular filtration rate ≤60 mL/min/1.73 m2 and/or albuminuria ≥3 months) or other target organ damage and LDL-C ≥100 mg/dL while on maximally tolerated lipid-lowering therapy and lifestyle modification.11 The European Society of Cardiology and European Atherosclerosis Society Task Force consensus recommended that anti-PCSK9 monoclonal antibodies may be considered in patients with diabetes who are at very high risk and have target organ damage (even without a history of atherosclerotic cardiovascular disease and without meeting the LDL-C goal on optimum lipid-lowering therapy including statin and ezetimibe).12 Recently, the updated 2016 American College of Cardiology Consensus Committee recommendations stated that if a statin-ezetimibe combination does not achieve a 50% reduction in LDL-C or if LDL-C remains >70 mg/dL, then a PCSK9 monoclonal antibody should be considered after a patient-physician discussion.25 These new proposals provide physicians the opportunity to choose nonstatin therapeutic options to treat patients with type 2 diabetes who are unable to achieve LDL-C targets via statins.
PERSPECTIVES OF NONSTATIN THERAPY IN TREATMENT OF DIABETIC DYSLIPIDEMIA
The current hypothesis is that PCSK9-targeted therapy includes not only monoclonal antibodies but also antisense oligonucleotides, small interfering RNAs, small molecules, and many others.26 Because these anti-PCSK9 antibodies have already demonstrated their importance, a great effort has been made by pharmaceutical companies to develop additional PCSK9 inhibitors.27 In 2017, Pfizer Inc.27 and Genentech Inc.28 reported the efficacy and safety of two new anti-PCSK9 antibodies, bococizumab27 and RG765228; however, those trials were not specifically designed for subjects with diabetic dyslipidemia. It should be emphasized that different types of anti-PCSK9 drugs have distinct mechanisms of action.26 For example, alirocumab, evolocumab, and bococizumab lower LDL-C by blocking the PCSK9-mediated downregulation of the LDL receptor, whereas the mechanism for antisense oligonucleotides is via inhibition of PCSK9 expression.27 Unlike alirocumab and evolocumab, which are fully human monoclonal antibodies, bococizumab is a humanized immunoglobulin 2 monoclonal antibody.27 A new report in 2017 showed that bococizumab significantly reduced concentrations of total LDL, total small LDL, and small VLDL particles from baseline when compared to placebo. Additionally, bococizumab increased the size of LDL, VLDL, and HDL particles.27 However, in the pivotal SPIRE Trials program, there was a loss of efficacy over time with bococizumab due to antidrug antibodies. A phase 1 study of RG7652 (Roche Genentech), another fully human monoclonal antibody, revealed a substantial and sustained reduction of LDL-C, with an acceptable safety profile and minimal immunogenicity.28 In conclusion, PCSK9 inhibitors are useful for the treatment of diabetic dyslipidemia. Research regarding the risk of developing diabetes mellitus following long-term exposure to PCSK9 inhibitors is warranted.
References
- 1.US Food and Drug Administration, Office of Medical Products and Tobacco, Center for Drug Evaluation and Research, Division of Metabolism and Endocrinology Products Briefing information for the June 9, 2015, meeting of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC). BLA 125559, Praluent (alirocumab) injection. https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm449863.htm. Updated June 5, 2015 Accessed November15, 2017. [Google Scholar]
- 2.US Food and Drug Administration, Office of Medical Products and Tobacco, Center for Drug Evaluation and Research, Division of Metabolism and Endocrinology Products Briefing information for the June 10, 2015, meeting of the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC). https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm450071.htm. Updated June 8, 2015 Accessed November15, 2017. [Google Scholar]
- 3.Khavandi M, Duarte F, Ginsberg HN, Reyes-Soffer G. Treatment of dyslipidemias to prevent cardiovascular disease in patients with type 2 diabetes. Curr Cardiol Rep. 2017;19:7. doi: 10.1007/s11886-017-0818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippatos TD, Florentin M, Georgoula M, Elisaf MS. Pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol. 2017;10:187–200. doi: 10.1080/17512433.2017.1263565. [DOI] [PubMed] [Google Scholar]
- 5.Taskinen MR, Del Prato S, Bujas-Bobanovic M, Louie MJ, Lorenzato C, Colhoun HM. Alirocumab in individuals with diabetes and mixed dyslipidemia: pooled analyses of five phase 3 trials. Paper resented at: International Diabetes Federation World Congress; August 2015 http://conference.idf.org/IDF2015/CM.NET. Accessed November15, 2017. [Google Scholar]
- 6.Leiter LA, Zamorano JL, Bujas-Bobanovic M, Louie MJ, Lecorps G, Cannon CP. Lipid-lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub-analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989–996. doi: 10.1111/dom.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiter LA, Cariou B, Müller-Wieland D, et al.. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM-INSULIN randomized trial. Diabetes Obes Metab. 2017;19(12):1781–1792. doi: 10.1111/dom.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Wieland D, Leiter LA, Cariou B, Letierce A, Colhoun HM, Del Prato S. Design and rationale of the ODYSSEY DM-DYSLIPIDEMIA trial: lipid-lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidaemia at high cardiovascular risk. Cardiovasc Diabetol. 2017;16:70. doi: 10.1186/s12933-017-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol. 2016;4:403–410. doi: 10.1016/S2213-8587(16)00003-6. [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, Ferrari GM De. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. doi: 10.1016/S2213-8587(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 11.Achimastos A, Alexandrides T, Alexopoulos D, Athyros V, Bargiota A, Bilianou E. Expert consensus on the rational clinical use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. Hormones (Athens). 2016;15:8–14. [DOI] [PubMed] [Google Scholar]
- 12.Landmesser U, Chapman MJ, Farnier M, et al; European Atherosclerosis Society. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245–2255. [DOI] [PubMed] [Google Scholar]
- 13.Amgen FDA approves Amgen's Repatha® (evolocumab) to prevent heart attack and stroke. http://www.amgen.com. Accessed December13, 2017.
- 14.Rocha NA, East C, Zhang J, McCullough PA. ApoCIII as a Cardiovascular Risk Factor and Modulation by the Novel Lipid-Lowering Agent Volanesorsen. Curr Atheroscler Rep. 2017;19:62. doi: 10.1007/s11883-017-0697-3. PMID:29124482. [DOI] [PubMed] [Google Scholar]
- 15.Soran H, Schofield JD, Adam S, Durrington PN. Diabetic dyslipidaemia. Curr Opin Lipidol. 2016;27:313–322. doi: 10.1097/MOL.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 16.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 20.Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res. 2008;49:399–409. doi: 10.1194/jlr.M700443-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Tecson KM, Panettiere-Kennedy KS, Won JI, Garg P, Olugbode O, McCullough PA. Relation between proprotein convertase subtilisin/kexin type 9 and directly measured low-density lipoprotein cholesterol. Proc (Bayl Univ Med Cent). 2017;30:16–20. doi: 10.1080/08998280.2017.11929514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- 23.Page MM, Watts GF. PCSK9 inhibitors—mechanisms of action. Aust Prescr. 2016;39:164–167. doi: 10.18773/austprescr.2016.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotta LA, Griffin SJ. PCSK9 inhibition and type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5:926–927. doi: 10.1016/S2213-8587(17)30321-2. [DOI] [PubMed] [Google Scholar]
- 25.Adhyaru BB, Jacobson TA. Role of non-statins, LDL-C thresholds, and special population considerations: a look at the updated 2016 ACC Consensus Committee recommendations. Curr Atheroscler Rep. 2017;19:29. doi: 10.1007/s11883-017-0666-x. [DOI] [PubMed] [Google Scholar]
- 26.He NY, Li Q, Wu CY, Ren Z, Gao Y, Pan LH. Lowering serum lipids via PCSK9-targeting drugs: current advances and future perspectives. Acta Pharmacol Sin. 2017;38:301–311. doi: 10.1038/aps.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan H, Gumbiner B, Joh T, Riel T, Udata C, Forgues P. Effects of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition with bococizumab on lipoprotein particles in hypercholesterolemic subjects. Clin Ther. 2017;39:2243–2259.e5. doi: 10.1016/j.clinthera.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Baruch A, Luca D, Kahn RS, Cowan KJ, Leabman M, Budha NR. A phase 1 study to evaluate the safety and LDL cholesterol-lowering effects of RG7652, a fully human monoclonal antibody against proprotein convertase subtilisin/kexin type 9. Clin Cardiol. 2017;40:503–511. doi: 10.1002/clc.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]


