ABSTRACT
A young woman presented with an anterior ST elevation myocardial infarction shortly after synthetic cannabis use. This report highlights the cardiovascular risks of these drugs.
KEYWORDS: K2, myocardial infarction, synthetic cannabinoids
Since the early 2000s, the use of synthetic cannabinoids (SCBs), also known as K2, Spice, Mojo, Cloud 9, herbal incense, and many others, has increased behind natural marijuana, also known as D9-tetrahydrocannabinol (THC).1 There are numerous SCB structures and new ones continue to be synthesized. Different structures and formulations of the drug have variable toxicities and unknown contaminants. The unpredictability of these drugs combined with their inability to be detected on routine urine drug screens pose a large public health concern.2,3 There are several reports of acute myocardial infarction triggered by synthetic marijuana use; most of these have been very young patients with normal-appearing coronary arteries on angiography.4,5 We describe a 30-year-old woman with a recent exposure to K2 who presented with an acute anterior ST segment elevation myocardial infarction (STEMI) with left anterior descending artery (LAD) occlusion.
CASE PRESENTATION
A 30-year-old morbidly obese black woman without significant medical problems presented to the emergency room with a 5-day history of bilateral finger contractures associated with arm cramping that radiated to the chest. The evening of admission she had similar symptoms associated with shortness of breath, diaphoresis, nausea, and vomiting followed by syncope. She denied any personal or family history of heart disease. She admitted to routine marijuana and K2 use for the past 5 months, including K2 use the evening of presentation. She also admitted to drinking a gallon of vodka on occasion during social events, but she only drank about 2 oz of liquor the evening prior to presentation.
On admission, she was obese with a body mass index of 53.4 kg/m2. Her blood pressure was 174/104 mm Hg, heart rate was 104 beats per minute, respiratory rate was 20 breaths per minute, and temperature was 97.3°F. Her troponin was 0.34 ng/mL with a peak of 54.36 ng/mL. Creatinine was 1.58 mg/dL, and white blood cell count was 15,500/μL. Hemoglobin A1c and thyroid-stimulating hormone were 5.3% and 1.91 mIU/L, respectively. Tests for HIV, antinuclear antibodies, cardiolipin antibodies, beta 2 glycoprotein antibodies, and phosphatidylserine antibodies were negative. Her urine drug screen was positive for cannabinoids, opiates, and benzodiazepines. Electrocardiogram showed sinus rhythm; ST segment elevation in leads I, II, aVL, V2, V3, V4, V5, V6; and ST segment depression in leads III, aVR, and aVF (Figure 1).
Figure 1.
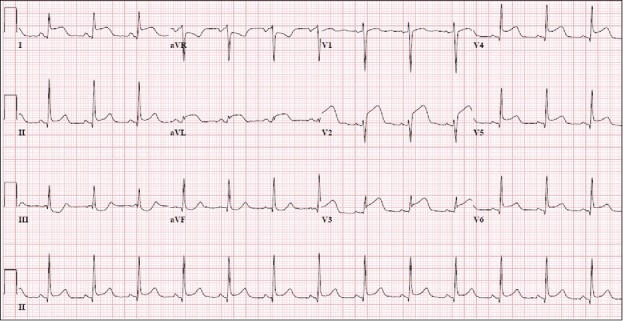
Electrocardiogram showed sinus rhythm; ST segment elevation in leads I, II, aVL, V2, V3, V4, V5, V6; and ST segment depression in leads III, aVR, and aVF.
Left-sided heart catheterization showed a left ventricular end diastolic pressure of 20 mm Hg and a left ventricular ejection fraction of 50% with anterolateral and apical akinesis. Coronary angiography showed an eccentric stenosis and a large near-occlusive filling defect compatible with thrombus in the proximal LAD (Figure 2a). It also showed occlusion of distal LAD, likely a result of embolization from the proximal lesion. We performed manual thrombectomy of proximal (Pronto V3 thrombus extraction catheter) and distal (Pronto LP thrombus extraction catheter) LAD, which provided significant improvement in the appearance of the proximal area but just partial improvement in distal LAD. Thrombectomy was followed by placement of 3.5 × 16 mm and 4.0 × 16 mm Promus Premier stents in proximal LAD. Final angiogram without a wire showed an optimal result in the proximal LAD (Figure 2b) but persistence of thrombotic occlusion in the very distal LAD.
Figure 2.
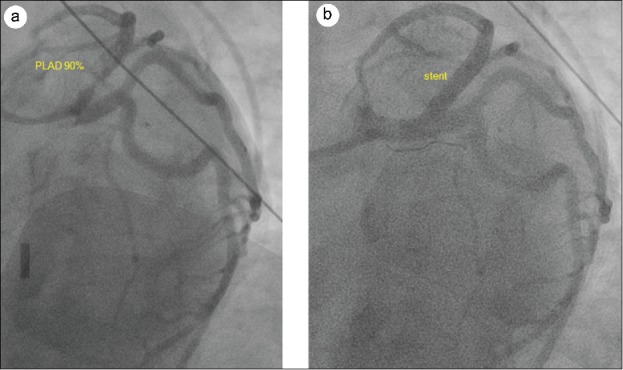
(a) Coronary angiography showed an eccentric stenosis due to plaque rupture and a large near-occlusive filling defect compatible with thrombus in the proximal left anterior descending artery. (b) Proximal left anterior descending artery after stent placement.
For the next 24 hours postcatheterization, in addition to her ongoing nausea and vomiting, the patient had difficult-to-control hypertension and tachycardia. The remainder of her hospital course was uneventful.
DISCUSSION
SCBs are associated with cardiovascular toxicity. The most common cardiovascular effect is sinus tachycardia, often in conjunction with hypertension, as seen in our patient.3,6 These illicit drugs have also been associated with myocardial infarction. In 2011, there were four reported cases of STEMIs in 16-year-old boys after K2 use.4,5 The subjects of these cases were young teenagers without other cardiovascular risk factors or past medical history. All of the above cases that underwent coronary angiography showed no evidence of coronary artery disease; therefore, their myocardial infarctions were attributed to coronary vasospasm related to SCB use rather than occlusive disease. To our knowledge, this is the first case that describes significant coronary artery disease following the use of SCBs.
The mechanism for how these drugs affect the heart and cardiovascular system is still not well understood. SCBs and THC both bind to cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2). These receptors are G-coupled receptors that, when stimulated, result in decreased levels of cyclic adenosine monophosphate. SCBs, however, are direct agonists of the CB1 receptor, whereas THC is a partial agonist.7 SCBs therefore bind to these receptors with higher affinity and subsequently have a higher rate of toxicity than THC.
Though these receptors are found mostly in the brain and peripheral tissues, such as the liver, lungs, and kidneys, there are studies describing CB1 receptor activity related to the heart and vasculature.7 Sugamura et al studied the therapeutic effects of CB1 receptor blockade in coronary artery disease. They showed that CB1 receptor blockade significantly reduced atheroma formation and may be therapeutically beneficial for atherogenesis.8 One could assume that if CB1 blockade decreases the burden of atherosclerotic disease, then CB1 agonists such as K2 could increase the burden of atherosclerotic disease. Similarly, Deusch et al studied the procoagulatory effects of THC in humans and found that THC increased glycoprotein IIB/IIIA and P-selectin expression on platelets, indicating that marijuana may have a procoagulatory effect.9 Moreover, CB1 and CB2 receptors were noted via western blot to be present on platelets.9 However, the specific effects that these receptors have on platelets are not known. More research is still needed to determine the specific procoagulatory effects of SCBs.
From the social policy point of view, there is an urgent need to address the “K2 epidemic” in large urban centers like Dallas, where the number of K2-related emergency calls has increased by 177%.10
References
- 1.Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of spice products (synthetic cannabinoids). Drug Alcohol Depend. 2012;120:238–241. doi.org/ 10.1016/j.drugalcdep.2011.07.011. PMID:21835562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forrester MB, Haywood T. Geographic distribution of synthetic cannabinoid exposures reported to Texas poison centers. Am J Drug Alcohol Abuse. 2012;38:603–608. doi.org/ 10.3109/00952990.2012.670339. PMID:22571605. [DOI] [PubMed] [Google Scholar]
- 3.Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59–62. doi.org/ 10.1097/MAJ.0000000000000466. PMID:26132518. [DOI] [PubMed] [Google Scholar]
- 4.McKeever RG, Vearrier D, Jacobs D, LaSala G, Okaneku J, Greenberg MI. K2—not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: a case report. J Med Toxicol. 2015;11:129–131. doi.org/ 10.1007/s13181-014-0424-1. PMID:25154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622–e1627. doi.org/ 10.1542/peds.2010-3823. PMID:22065271. [DOI] [PubMed] [Google Scholar]
- 6.Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54:1–13. doi.org/ 10.3109/15563650.2015.1110590. PMID:26567470. [DOI] [PubMed] [Google Scholar]
- 7.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi.org/ 10.1016/S0163-7258(97)82001-3. PMID:9336020. [DOI] [PubMed] [Google Scholar]
- 8.Sugamura K, Sugiyama S, Fujiwara Y, et al.. Cannabinoid 1 receptor blockade reduces atherosclerosis with enhances reverse cholesterol transport. J Atheroscler Thromb. 2010;17:141–147. doi.org/ 10.5551/jat.2865. PMID:20124735. [DOI] [PubMed] [Google Scholar]
- 9.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth Analg. 2004;99:1127–1130. doi.org/ 10.1213/01.ANE.0000131505.03006.74. PMID:15385362. [DOI] [PubMed] [Google Scholar]
- 10.Wilonsky R. Dallas struggling to solve its K2 epidemic as the walking dead roam downtown. http://www.dallasnews.com. Published September19, 2017 Accessed November12, 2017. [Google Scholar]


