Children with penicillin allergy were tested, found to receive negative test results, and, subsequently, tolerated penicillin without serious adverse or allergic reactions.
Abstract
BACKGROUND:
Penicillin allergy is commonly reported in the pediatric emergency department. We previously performed 3-tier penicillin allergy testing on children with low-risk symptoms, and 100% tolerated a penicillin challenge without an allergic reaction. We hypothesized that no serious allergic reactions would occur after re-exposure to penicillin and that prescription practices would change after testing.
METHODS:
We performed a follow-up case series of 100 children whose test results were negative for penicillin allergy. Research staff administered a brief follow-up phone survey to the parent and primary care provider of each patient tested. We combined the survey data and summarized baseline patient characteristics and questionnaire responses. We then completed a 3-tier economic analysis from the prescription information gathered from surveys in which cost savings, cost avoidance, and potential cost savings were calculated.
RESULTS:
A total of 46 prescriptions in 36 patients were reported by the primary care provider and/or parents within the year after patients were tested for penicillin allergy. Twenty-six (58%) of the prescriptions filled were penicillin derivatives. One (4%) child developed a rash 24 hours after starting the medication; no child developed a serious adverse reaction after being given a penicillin challenge. We found that the cost savings of delabeling patients as penicillin allergic was $1368.13, the cost avoidance was $1812.00, and the total potential cost savings for the pediatric emergency department population was $192 223.00.
CONCLUSIONS:
Children with low-risk penicillin allergy symptoms whose test results were negative for penicillin allergy tolerated a penicillin challenge without a severe allergic reaction developing. Delabeling children changed prescription behavior and led to actual health care savings.
What’s Known on This Subject:
Many children present to the pediatric emergency department with a reported penicillin allergy. The majority of children with reported penicillin allergy have low-risk symptoms and could tolerate an oral penicillin challenge without an allergic reaction.
What This Study Adds:
Children deemed low risk who were tested for penicillin allergy tolerated the medication within the following year without serious adverse or allergic reactions. Delabeling children changed prescription behavior and led to health cost-savings.
Penicillin allergy is the most commonly reported medication allergy and is frequently reported in children who present to the pediatric emergency department (PED).1–6 The majority of children with reported penicillin allergy could likely tolerate penicillin without having an allergic or adverse reaction.7 The labeling of a patient with a penicillin allergy has many negative effects that include increased health risks and prescription costs. Macy and Contreras8 found that patients with a reported penicillin allergy history “spend significantly more time in the hospital, are exposed to significantly more antibiotics associated with Clostridium difficile and Vancomycin-Resistant Enterococci and are associated with increased hospital use.” Additionally, delabeling patients reported to have penicillin allergy can lead to decreased prescription costs with low rates of subsequent adverse reactions.9
With our previous work, we found that 76% of the children presenting to a PED with penicillin allergy reported by families had exclusively low-risk allergy symptoms and, therefore, a low pretest probability for true immunoglobulin E–mediated allergy.10 After this initial study, we performed standard 3-tier penicillin allergy testing on 100 of the children presenting with low-risk symptoms.11 All 100 received negative test results for penicillin allergy after a 500-mg oral challenge with amoxicillin. Families were told of the lack of true allergy, but what was not known was whether families and primary care providers (PCPs) would alter treatment of the children on the basis of this testing.
Our goals with this project were to evaluate the effect that the testing had on prescribing practices in PCP offices for children who received negative test results for penicillin allergy and evaluate how comfortable parents and doctors were with delabeling. Additionally, we wanted to document any adverse reactions and/or allergic symptoms that occurred with any subsequent penicillin prescriptions. We hypothesized that no serious allergic reactions after re-exposure to penicillin would occur to a child deemed nonallergic by our testing process and that we would show that it changed prescription practices and lead to significant cost savings.
Methods
Study Design
We performed a follow-up case series of 100 children with reported penicillin allergy who were tested without cost to them and found to receive negative test results for penicillin allergy. All 100 children had been classified as having low-risk symptoms of allergy on a penicillin allergy questionnaire and tested negative for penicillin allergy on oral challenge. Calling families and PCPs for the families to understand subsequent care of the children was determined by the institutional review board to not be human subjects research.
Research staff called the listed parent or legal guardian (hereafter termed parent) and PCPs of each child to determine care practices and any subsequent antibiotic usage or adverse events related to the use of antibiotics. The parents were asked to complete a brief 2-minute phone survey (Table 1). Only 1 parent completed the survey per patient. Research staff recorded the date on which the call was made in relation to when the child was tested to calculate the duration of follow-up from testing. The survey included 6 items that assessed parental knowledge of their child’s current medical record pertaining to allergies and their child’s penicillin allergy testing results. Additional questions included whether their child had been prescribed and filled or taken antibiotics since being tested and if any allergic reaction had occurred. Parents reported their comfort level with their child receiving a penicillin antibiotic after the negative allergy test results and whether they had discussed the results of their negative allergy test with their PCPs. The PCP for each child was called to complete a 4-question follow-up survey. Questions included whether a penicillin allergy was noted within the medical record and whether an antibiotic had been prescribed since allergy testing. If an antibiotic had been prescribed, any symptoms of allergic reaction were noted. Results from both surveys were uploaded to a secure, online database by using Research Electronic Data Capture, hosted at the Medical College of Wisconsin.12
TABLE 1.
Parent and PCP Questions
| Parent Questionnaire | PCP Questionnaire |
|---|---|
| “What allergies, if any, are currently listed in your child’s medical record?” | “Within your medical records does this child have a reported allergy to penicillin or cephalosporins?” |
| “Are you aware of the results of your child’s penicillin testing?” | “Were you notified that this child was tested for penicillin allergy in the last year, and was found to be negative after an oral challenge?” |
| “Your child was tested for penicillin allergy and found to be negative. Did you discuss the research findings with your child’s primary care doctor?” | “Has the child been prescribed any penicillin antibiotics or cephalosporins in the past year?” |
| “How comfortable would you be if your child was given a penicillin antibiotic now?” | “If yes, after being prescribed the antibiotic did the child have any reported reaction to the antibiotic?” |
| “Has your child taken any antibiotics since being tested for penicillin allergy?” | |
| “If yes, did the child have any reaction after taking the antibiotic?” |
We combined data from the PCP and parent surveys to identify children who received an antibiotic. By using merged data from 2 surveys, antibiotic prescriptions were identified as follows: If the name of the class of antibiotic was the same in both surveys, the prescription was counted once. If the prescription classes of antibiotics were different, we assumed that different antibiotics were filled and that families may have gotten their prescription from an urgent care or emergency department and not their PCP’s office.
Data Analysis
Descriptive characteristics were used to summarize the baseline patient characteristics of the children who had received an antibiotic since receiving negative test results for penicillin allergy. For the economic analysis, the following 8 different prescription brands were listed in the PCP and parent surveys: amoxicillin, amoxicillin and clavulanate, azithromycin, clindamycin, cefadroxil, cefdinir, penicillin, and cephalexin. All brand names were switched to their generic name. We conducted 3 levels of economic cost savings analysis on the basis of the following assumptions: amoxicillin is the treatment of choice for otitis media, strep throat, and pneumonia; and the majority of children who were prescribed amoxicillin in this cohort would have likely been placed on cefdinir in the face of a reported allergy. Because cephalexin and clindamycin are used to treat skin infections, the 2 children prescribed these 2 medications were likely not prescribed them because of a penicillin allergy, and these were not included in the economic cost savings and cost avoidance analysis.
To calculate antibiotic cost, we took the median age of children in this study, which was 8 years old, and assumed that the majority of children at this age would be prescribed liquid antibiotics. We used www.cdc.gov/growthcharts and found that the 50th weight-for-age percentile in an 8-year-old boy or girl is 26 kg.13 We used the Web site www.goodrx.com to calculate the amount of standard “oral solution” used to treat otitis media.14 We used the median retail cost because of the wide fluctuations in retail price and presence of outliers with antibiotic prescription. The retail price used was “without coupon” price.
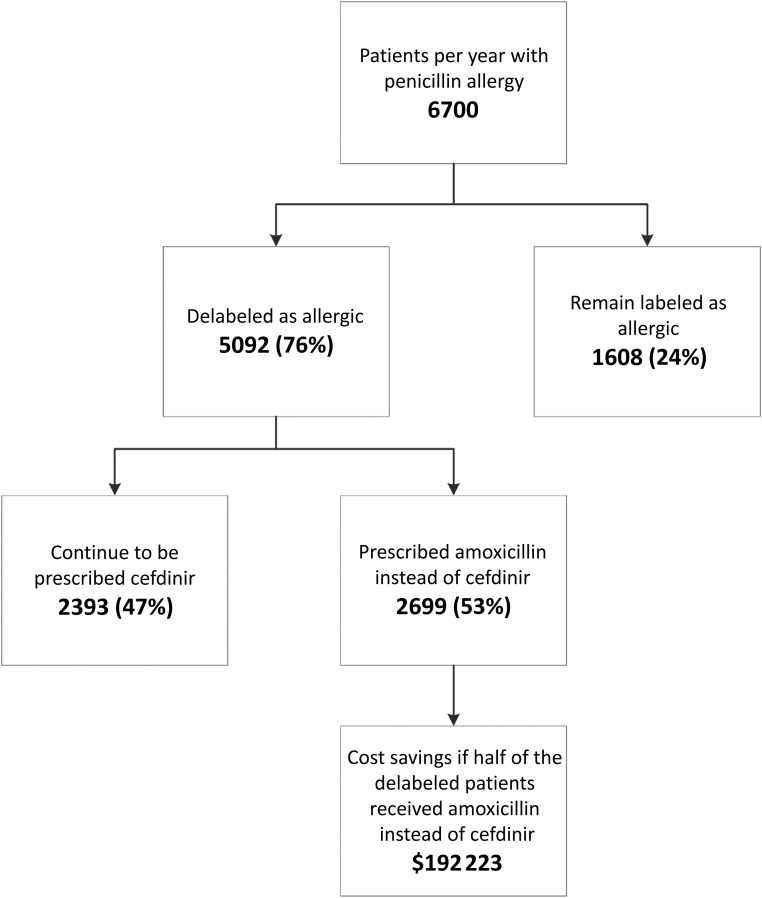
To calculate cost savings, we determined the total expected antibiotic cost by first calculating the expected average nonpenicillin antibiotic course cost in the follow-up cohort and assumed that the same distribution would have been given for all prescriptions. We then calculated the actual antibiotic cost in the follow-up cohort and subtracted this value from the total expected antibiotic cost. Second, we calculated the cost avoidance assuming that patients who filled the 24 amoxicillin and/or penicillin prescriptions would have filled cefdinir in its place. Lastly, we extrapolated the total potential cost savings generated by the ∼6700 patients per year treated in a large tertiary care PED who report penicillin allergy. This number is based on an estimated 10% of families who report penicillin allergy in a PED whose volume is 67 000 patients seen per year. In doing this third level of analysis, we made the following 2 assumptions: (1) 76% of patients with low-risk symptoms would likely be delabeled as penicillin-allergic10, and (2) 53% of the delabeled patients would be prescribed amoxicillin and/or penicillin instead of a cefdinir without penicillin allergy.
Results
One hundred families were called for parent survey completion; 81 (81%) completed the follow-up questionnaire. Seventy-three (90%) parents reported that they were aware of their child’s penicillin allergy testing results. Sixty-five (80%) parents reported that they notified their PCP of their child’s negative penicillin allergy test results. One hundred PCPs were called for PCP survey completion; 98 (98%) completed the follow-up questionnaire. Eight-two (84%) PCPs reported that they were not notified by patient families that their child was tested and found to receive negative test results for penicillin allergy. Fifty-one (52%) children were still reported to have a penicillin allergy in the PCP medical record. The median length of time from allergy testing to follow-up was 1 year for both parents and PCPs. Of those patients, 24 (69%) were white, 5 (14%) were African American, and 4 (11%) were Hispanic.
Of the 81 parents who were asked how comfortable they would be to have their child receive a penicillin antibiotic, 59 (73%) reported that they would be “comfortable” or “very comfortable,” 19 (24%) would be “somewhat comfortable,” and 3 (4%) would be “not comfortable.” Each of the 22 families who reported that they were either “somewhat comfortable” or “not comfortable” was asked the reason for their discomfort. Of those, 17 (74%) cited they were fearful that their child may have a repeat allergic reaction after re-exposure to a penicillin antibiotic.
Thirty-six patients had filled at least 1 prescription, and 10 patients had filled 2 prescriptions for a total number of 46 prescriptions (Fig 1). The median (interquartile range) age of children who had received an antibiotic since allergy testing was 8 years old (6–12).
FIGURE 1.
Prescriptions since antibiotic testing. PMD, primary medical doctor.
The most commonly prescribed antibiotic was amoxicillin and/or penicillin (n = 24; 52%), followed by azithromycin (n = 13; 28%), cefdinir (n = 6; 13%), amoxicillin and clavulanic acid (n = 2; 4%), and cefadroxil (n = 1; 2%). Of the penicillin derivative prescriptions filled, 1 (4%) child developed a rash ∼24 hours after starting amoxicillin and was relabeled as penicillin allergic. This occurred after that child’s first prescription for amoxicillin.
The cost savings totaled $1368.13 (Table 2). The cost avoidance amounted to $1812.00. Lastly, we extrapolated the total potential cost savings generated by the ∼6700 patients per year with a reported penicillin allergy in our PED (Fig 2). The total cost savings for the entire pediatric patients who could potentially benefit from the penicillin delabeling would amount to $192 223.00.
TABLE 2.
Cost Savings in Patients Delabeled as Penicillin Allergic
| Total Prescriptions Filled | Median Price per Prescription | Average Nonpenicillin Cost | Real Antibiotic Cost | Costa Savings | Costb Avoidance | |
|---|---|---|---|---|---|---|
| Amoxicillin = penicillin (preferred drug) | 24 | $10.00 | $70.00 | $240.00 | $1368.13 | $1812.00 |
| Azithromycin | 13 | $66.00 | — | $858.00 | — | — |
| Cefdinir | 6 | $85.50 | — | $513.00 | — | — |
| Amoxicillin and clavulanic acid | 2 | $106.00 | — | $212.00 | — | — |
| Cefadroxil | 1 | $29.10 | — | $29.10 | — | — |
| Total | 46 | — | $3220.23 | $1852.10 | $1368.13 | $1812.00 |
—, not applicable.
Total expected antibiotic course cost minus the real antibiotic course cost.
Cost savings avoided by assuming that patients who filled the 24 amoxicillin and/or penicillin prescriptions would have filled cefdinir in its place.
FIGURE 2.
Extrapolated total cost savings of delabeling patients with penicillin allergy.
Discussion
In this follow-up project, we found that within 1 year of being tested for allergy, 36 children from our testing population had received 26 penicillin-derivative prescriptions and 1 (4%) child developed a rash ∼24 hours after starting the medication and was relabeled as penicillin allergic. Consistent with our hypothesis, no child suffered a serious allergic reaction after re-exposure to the medication.
In our initial study, each child underwent a gold standard 3-tier penicillin allergy testing process. After the negative test result, a discussion with family members occurred explaining their child’s ability to take penicillin in the future. Additionally, the child’s label of penicillin allergy was removed from the hospital medical record. When contacting families a median of 1 year later, we found 10% of families were not aware that their child had received negative test results for penicillin allergy and was able to receive the medication. We believe that this reveals the importance of providing clear and succinct discharge paperwork in which providers explain the implications of allergy testing.
In addition, we found that 28% of children’s families were either only “somewhat comfortable” or “not comfortable” with receiving a penicillin antibiotic after being delabeled as allergic. The majority of families cited that their reason for discomfort was concern that another allergic reaction may occur. It is interesting to note that all patients who were tested for penicillin allergy had low-risk symptoms of allergy, such as rash, that often developed days after initial exposure to the antibiotic. Therefore, it is likely that they were never allergic in the first place. However, it reveals the powerful effect a labeled allergy can have on a family and the difficulty in reassuring families even with low-risk symptoms and negative results for allergy testing.
The inclusion of a child’s health care team, including PCPs and their pharmacy going forward, is vital to the success of delabeling children as penicillin allergic. With our initial study, we altered the hospital medical record report of allergy and relied on the family to notify the PCP of allergy testing results. Our current project revealed that ˃80% of PCPs were not notified of allergy testing results and over half still had the allergy documented in the chart. We assume that the majority of patient pharmacies were also likely not notified of a negative allergy test result and that in the future the addition of this practice would be helpful in delabeling patients in a more comprehensive fashion. Dissemination of results, therefore, needs to be sent directly to the entire health care team who can then reinforce the lack of a penicillin allergy and shift treatment to equally effective low-cost treatments.
This study reveals the actual and potential financial savings for patients delabeled as penicillin allergic. Within 1 year of testing 100 patients, more than half of the 46 prescriptions issued after delabeling were penicillin derivatives. In that time, we found cost savings that amounted to $1368.13 and cost avoidance of $1812. Although these numbers may not be substantially large, the potential savings when applied to an annual volume of 67 000 visits increased to an estimated cost savings of $192 223. Additionally, these numbers reflect estimates from 1 hospital system and do not reflect the potential savings that may occur in a multicenter trial. In summary, we believe that real-time penicillin allergy delabeling in the PED would be a safe alternative to penicillin skin testing and lead to substantial cost savings in health care throughout the United States.
This project is limited in that not all participants who were tested for penicillin allergy were able to be contacted for follow-up. Therefore, we cannot guarantee that a severe allergic reaction did not occur in those who we could not follow-up. However, between combined family and PCP surveys, only 1 child was unable to have some degree of follow-up, and it is unlikely that a severe allergic reaction occurred without any notification to the study team. This project is also limited in that we had to make several assumptions within our economic analysis to calculate cost savings, cost avoidance, and potential estimated cost savings. This was not a cost-effectiveness analysis; neither the cost of testing, which would vary by choice of oral challenge alone or 3-tier testing, nor the long-term savings from delabeling were considered.
Conclusions
Children with low-risk penicillin allergy symptoms who received negative test results for penicillin allergy tolerate penicillin antibiotics without severe allergic reactions developing. Delabeling of children changed prescriber behavior in the year after testing, leading to more penicillin prescriptions. This change in prescribing led to actual and potential savings that occurred after delabeling patients as penicillin allergic. Further improvements in the effectiveness of penicillin allergy testing can be realized by ensuring adequate communication of label removal to, importantly, the child’s PCP but also the entire health care team.
Acknowledgments
Dr Elizabeth Phillips acknowledges the National Institutes of Health (1P50GM115305-01, 1P30AI110527-01A1, 5T32AI007474-20) and the National Health and Medical Research Council of Australia. We thank the Medical College of Wisconsin Clinical and Translational Science Institute for statistical support. We also thank Duke Wagner and the emergency department research assistants Jaimie Voss, Erica Gleason, Lauren Thomas, Nichole Graves, Brittany Cords, Jenna Hattab, Rebecca Farley, and Jennifer Kovac for their survey administration efforts.
Glossary
- PCP
primary care provider
- PED
pediatric emergency department
Footnotes
Dr Vyles conceptualized and designed the study, analyzed and interpreted data collected, and drafted the initial manuscript; Drs Chiu, Phillips, and Brousseau designed the study and analyzed and interpreted data collected; Drs Routes and Castells analyzed and interpreted data collected; Dr Kibicho analyzed and interpreted data collected and performed the economic analysis; and all authors critically reviewed the manuscript and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the American Academy of Pediatrics Section on Emergency Medicine Ken Graff Award and a Children’s Hospital of Wisconsin Foundation Vice Innovation award. This publication used research electronic data capture and was supported by the National Institutes of Health Clinical and Translational Science Institute grant UL1 RR025780. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Mendelson LM. Adverse reactions to β-lactam antibiotics. Immunol Allergy Clin North Am. 1998;18(4):745–757 [Google Scholar]
- 2.Nicklas R, Bernstein IL, Li JT, et al. β-lactam antibiotics: the diagnosis and management of anaphylaxis. J Allergy Clin Immunol. 1999;101:S498–S501 [Google Scholar]
- 3.Surtees SJ, Stockton MG, Gietzen TW. Allergy to penicillin: fable or fact? BMJ. 1991;302(6784):1051–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr JR. Penicillin allergy: a study of incidence as reported by patients. Br J Clin Pract. 1994;48(1):5–7 [PubMed] [Google Scholar]
- 5.Ahlstedt S. Penicillin allergy—can the incidence be reduced? Allergy. 1984;39(3):151–164 [DOI] [PubMed] [Google Scholar]
- 6.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160(18):2819–2822 [DOI] [PubMed] [Google Scholar]
- 7.Sogn DD, Evans R III, Shepherd GM, et al. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152(5):1025–1032 [PubMed] [Google Scholar]
- 8.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790–796 [DOI] [PubMed] [Google Scholar]
- 9.Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998;102(2):281–285 [DOI] [PubMed] [Google Scholar]
- 10.Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau DC. Parent-reported penicillin allergy symptoms in the pediatric emergency department. Acad Pediatr. 2017;17(3):251–255 [DOI] [PubMed] [Google Scholar]
- 11.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy testing in children with low-risk penicillin allergy symptoms. Pediatrics. 2017;140(2):e20170471. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention; National Center for Health Statistics Average weight for 8-year-old child. Available at: http://www.cdc.gov/growthcharts. Accessed August 30, 2017
- 14.Cost of antibiotics. Available at: www.goodrx.com. Accessed August 30, 2017