Synovial fluid WBC counts alone do not help to distinguish between patients with septic and Lyme disease hip monoarthritis.
Abstract
BACKGROUND:
Patients with septic hip arthritis require surgical drainage, but they can be difficult to distinguish from patients with Lyme arthritis. The ability of synovial fluid white blood cell (WBC) counts to help discriminate between septic and Lyme arthritis of the hip has not been investigated.
METHODS:
We assembled a retrospective cohort of patients ≤21 years of age with hip monoarticular arthritis and a synovial fluid culture obtained who presented to 1 of 3 emergency departments located in Lyme disease endemic areas. Septic arthritis was defined as a positive synovial fluid culture result or synovial fluid pleocytosis (WBC count ≥50 000 cells per µL) with a positive blood culture result. Lyme arthritis was defined as positive 2-tiered Lyme disease serology results and negative synovial fluid bacterial culture results. All other patients were classified as having other arthritis. We compared median synovial fluid WBC counts by arthritis type.
RESULTS:
Of the 238 eligible patients, 26 (11%) had septic arthritis, 32 (13%) had Lyme arthritis, and 180 (76%) had other arthritis. Patients with septic arthritis had a higher median synovial fluid WBC count (126 130 cells per µL; interquartile range 83 303–209 332 cells per µL) than patients with Lyme arthritis (53 955 cells per µL; interquartile range 33 789–73 375 cells per µL). Eighteen patients (56%) with Lyme arthritis had synovial fluid WBC counts ≥50 000 cells per µL. Of the 94 patients who underwent surgical drainage, 13 were later diagnosed with Lyme arthritis.
CONCLUSIONS:
In Lyme disease endemic areas, synovial fluid WBC counts cannot always help differentiate septic from Lyme arthritis. Rapid Lyme diagnostics could help avoid unnecessary operative procedures in patients with Lyme arthritis.
What’s Known on This Subject:
Synovial fluid white blood cell (WBC) counts in patients with septic and Lyme disease knee arthritis are similar. However, the predictive ability of synovial fluid WBC counts in patients with hip monoarthritis has not been examined.
What This Study Adds:
Neither a synovial fluid WBC count ≥50 000 cells per μL nor ≥100 000 cells per μL could be used to accurately distinguish between patients with septic and Lyme arthritis of the hip. Accurate and rapid diagnostics are needed to inform initial clinical decision-making for patients with hip monoarthritis.
Septic arthritis of the hip is a severe condition that requires prompt initiation of parenteral antibiotics and surgical drainage (joint washout) to prevent permanent joint injury.1 Although it is uncommon, patients with septic arthritis can present similarly to those with Lyme arthritis or other arthritis types.2 The challenge for clinicians is to identify patients with septic arthritis without unnecessarily performing invasive procedures on (eg, arthrocentesis and arthroscopy) or treating with broad-spectrum antibiotics those patients with Lyme disease or other inflammatory arthritis. In patients who have diagnostic arthrocentesis performed, synovial fluid cell counts may be used to guide initial clinical decisions (eg, the need for operative procedures and parenteral antibiotics) before either bacterial culture or 2-tiered Lyme disease serology results are available.
In our previous 2-center cohort study, we identified the following high-risk laboratory predictors to distinguish septic arthritis from Lyme arthritis in patients with an isolated, nontraumatic, swollen knee: peripheral blood absolute neutrophil count (ANC) ≥10 000 cells per µL and erythrocyte sedimentation rate (ESR) ≥40 mm per hour.3 However, in patients who underwent arthrocentesis, the knee synovial fluid white blood cell (WBC) count and ANC did not distinguish between patients with septic and Lyme arthritis.4 Because this investigation was limited to patients with knee arthritis, the applicability to patients with hip arthritis is uncertain because Lyme disease less commonly affects the hip joint.5–7
To this end, we conducted a retrospective cohort study of patients with acute monoarticular arthritis of the hip undergoing arthrocentesis in 1 of 3 emergency departments (EDs) located in Lyme disease endemic areas. Our primary objective was to determine the ability of synovial fluid cell counts to help discriminate among septic, Lyme, and other types of hip arthritis because clinical decisions about operative intervention are often based on this cell count. Our secondary objective was to examine the sensitivity of the Kocher criteria,8 a widely validated clinical decision tool that helps identify patients with septic arthritis of the hip.
Methods
Study Design
We conducted a retrospective medical record review of patients aged ≤21 years who presented to 1 of 3 pediatric EDs, each located in Lyme endemic areas. The study period varied by participating sites on the basis of electronic database availability: Boston Children’s Hospital (January 2007–September 2017), Hasbro Children’s Hospital (December 2010–August 2017), and Yale New Haven Children’s Hospital (February 2013–June 2017). The study protocol was approved by the institutional review board at each participating institution with permission for data sharing.
Patient Population
We included patients with hip monoarticular arthritis who underwent arthrocentesis as part of the initial diagnostic evaluation. To identify potentially eligible cases, we screened the electronic databases at each participating site to identify hip synovial fluid cultures obtained within 48 hours of an ED encounter. We manually reviewed all identified medical records to determine patient eligibility. Patients were excluded if any of the following were identified: involvement of multiple joints, hip trauma or surgery within 30 days of presentation, or a prosthetic hip joint. If a patient had >1 hip synovial fluid culture obtained on >1 occasion during the study period, we included only the first eligible ED encounter.
Data Collection
We developed a manual of operations to standardize study procedures as well as the medical record review process. Data extraction was conducted at Boston Children’s Hospital (A.H.D. and L.E.N.), Rhode Island Hospital/Hasbro Children's Hospital (A.C.G. and K.G.), and Yale New Haven Children’s Hospital (T.J.L. and P.L.A.). We abstracted clinical factors, demographics, laboratory and microbiology results, radiology study results, hospital disposition, as well as any operative interventions. We defined peak Lyme season as any encounter between June 1 and October 31.9 Patients who had received any antibiotics within 72 hours before diagnostic arthrocentesis were classified as pretreated. Data were entered into research electronic data capture tools hosted at Boston Children’s Hospital.10
Outcome Measures
We defined a case patient with septic arthritis as a patient with a joint fluid culture result that was positive for a bacterial pathogen or a joint fluid pleocytosis (synovial fluid WBC count ≥50 000 cells per μL) with a blood culture result that was positive for a bacterial pathogen.11–15 We classified the following bacterial species a priori as contaminants: Bacillus species, Corynebacterium species, Micrococcus species, Streptococcus viridans and Staphylococcus epidermidis.16 We defined a case patient with Lyme arthritis as a patient with a positive 2-tier Lyme serology result who did not meet the criteria for septic arthritis.3 A positive 2-tiered serology result required a positive enzyme-linked immunoassay first-tier test followed by a supplemental immunoblot interpreted by using Centers of Disease Control and Prevention standard criteria.17 Because immunoglobulin M (IgM) alone can yield a false-positive result after 30 days from the initial infection18,19 and arthritis is a late-stage manifestation of Lyme disease, we required a positive immunoglobulin G (IgG) result to classify a patient as having Lyme arthritis. Patients who did not meet the criteria for either septic or Lyme arthritis were classified as having other inflammatory arthritis. This group included patients with pelvic or femoral osteomyelitis with a reactive synovial fluid effusion as well as those with transient synovitis. Poststreptococcal arthritis was diagnosed in patients with a history of β-hemolytic Streptococcus infection in the absence of an alternate diagnosis and was also classified as other inflammatory arthritis.
Kocher Criteria
The Kocher criteria, a previously validated clinical prediction rule used for the initial evaluation of patients with irritable hip, includes the following 4 predictors: inability to bear weight on the affected limb, presence of fever (documented temperature ≥38.5°C), peripheral WBC count ≥12 000 cells per mm3, and ESR ≥40 mm per hour.8,12 Patients with 2 or more of the predictors are classified as being at high risk of septic arthritis (defined at 40%) and should undergo diagnostic arthrocentesis.8 Patients who were missing 1 or more Kocher criteria were excluded from this subanalysis unless they had 2 or more predictors present. We calculated the sensitivity and positive predictive value (PPV) of a Kocher score ≥2 for septic arthritis.
Statistical Analysis
We compared proportions using χ2 tests and continuous variables using the Wilcoxon rank test. We calculated 95% confidence intervals (CIs) using an exact binomial distribution. First, we compared the clinical and laboratory characteristics of patients by arthritis type: septic, Lyme disease, and other inflammatory arthritis. Next, we used a scatterplot to depict the synovial fluid WBC counts and ANCs by arthritis type. Using a common clinical threshold for septic arthritis, we examined the arthritis types of patients with 2 common synovial fluid WBC count cut-points (≥50 000 cells per µL and ≥100 000 cells per µL).8,13
We used SPSS version 23.0 for all analyses (IBM SPSS Statistics, IBM Corporation).
Results
Study Population
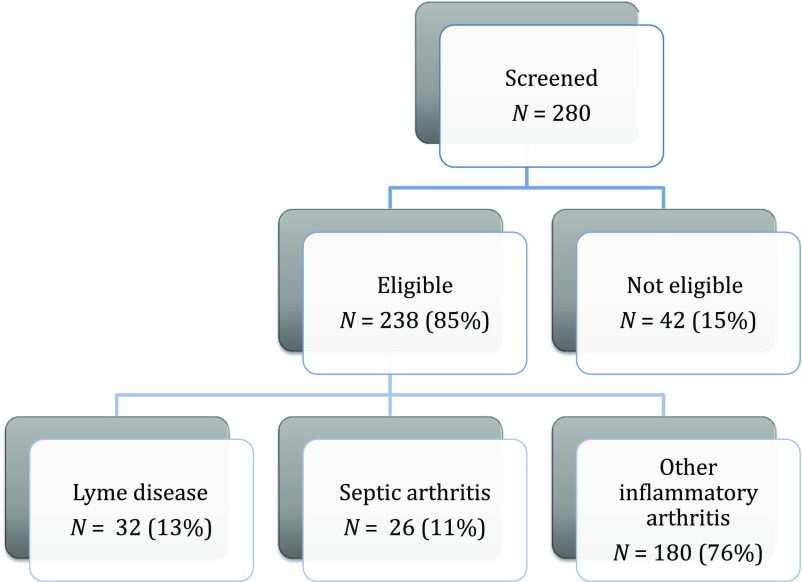
Of the 280 potentially eligible cases screened, 238 (85%) met study inclusion criteria (Fig 1). The number of study patients varied across sites (range 29–162 patients). The median patient age was 6.0 years (interquartile range [IQR] 4.0–8.6 years), and 132 (55%) were of male sex. Of the included patients, 6 had previously been diagnosed with Lyme disease (median time 8 months before ED presentation), 2 had a known immunodeficiency (hypogammaglobulinemia), and 3 were on immunomodulatory medications (mycophenolate, prednisone, or infliximab). Of the study patients, 100 (42%) had documented fever and 23 (10%) had been pretreated with any antibiotic in the 72 hours before diagnostic arthrocentesis.
FIGURE 1.
Study population.
Arthritis Types
Twenty-six patients (11%) had septic arthritis, 32 (13%) had Lyme arthritis, and 180 (76%) had other inflammatory arthritis. The diagnosis of septic arthritis was made with a positive result for synovial fluid culture alone (n = 18; 69% of cases with septic arthritis), synovial fluid pleocytosis and a positive blood culture result (n = 1; 4%), and both a positive synovial fluid result and blood culture result (n = 7; 27%). Seventeen of the 26 patients with septic hip arthritis (65%) had a positive synovial fluid Gram-stain result for bacteria. The septic arthritis cases were caused by the following bacterial pathogens: Staphylococcus aureus (24 patients) and Kingella kingae (2 patients).
Of the 238 study patients, 198 (83%) had Lyme disease testing performed, of which 44 had a positive first-tier test result and 32 had Lyme arthritis. Of these, 16 (50%) had a positive result for IgG alone, and 16 (50%) had both a positive IgG result and IgM result. Of the 14 patients with Lyme arthritis who had a synovial fluid Lyme polymerase chain reaction test obtained, 4 had a positive Lyme disease polymerase chain reaction test result (sensitivity 29%; 95% CI 8%–58%). Two patients with Lyme arthritis had a history of previous Lyme disease. One additional patient with septic arthritis had a positive 2-tiered Lyme disease serology result, which the treating clinician considered to be evidence of previous Lyme disease. Patients with Lyme arthritis presented to the participating EDs throughout the year (54% outside the peak Lyme season of June to October).
Of the 180 patients with other inflammatory arthritis, the treating clinicians made the following diagnoses: 5 patients (3%) had proximal femoral or pelvic osteomyelitis, 1 (0.5%) had poststreptococcal arthritis (antiStreptolysin O titer 405 IU/mL), and 1 had Legg–Calvé–Perthes disease (0.5%). The remaining 173 were diagnosed with transient synovitis by the treating clinicians. Of these, 18 (10%) had been pretreated with an antibiotic before diagnostic arthrocentesis.
Kocher Criteria
We were able to calculate a Kocher score for 233 study patients (98%). The prevalence of septic arthritis increased with the number of Kocher predictors present (Table 1). Of the 26 patients with septic arthritis, 23 had ≥2 Kocher criteria (sensitivity 88%; 95% CI 70%–98%). Importantly, 18 patients with Lyme arthritis (56% of patients with Lyme arthritis) had ≥2 Kocher criteria.
TABLE 1.
PPV of Kocher Score for Septic Arthritis
| Kocher Score | Published Likelihood of Septic Arthritis, % | No. Casesa | Septic Arthritis (N = 26), n (%) | PPV for Cut-point, % (95% CI) |
|---|---|---|---|---|
| 0 | 0 | 16 | 0 (0) | 11 (7–16) |
| 1 | 3 | 74 | 3 (12) | 12 (8–17) |
| 2 | 40 | 85 | 7 (27) | 16 (10–23) |
| 3 | 93 | 46 | 11 (42) | 28 (17–41) |
| 4 | 99 | 12 | 5 (19) | 42 (15–72) |
| Total | — | 233 | 26 (100) | — |
—, not applicable.
Patients with missing data for the Kocher criteria without 2 or more high-risk predictors did not have a Kocher score calculated (n = 5).
Comparison Among Arthritis Types
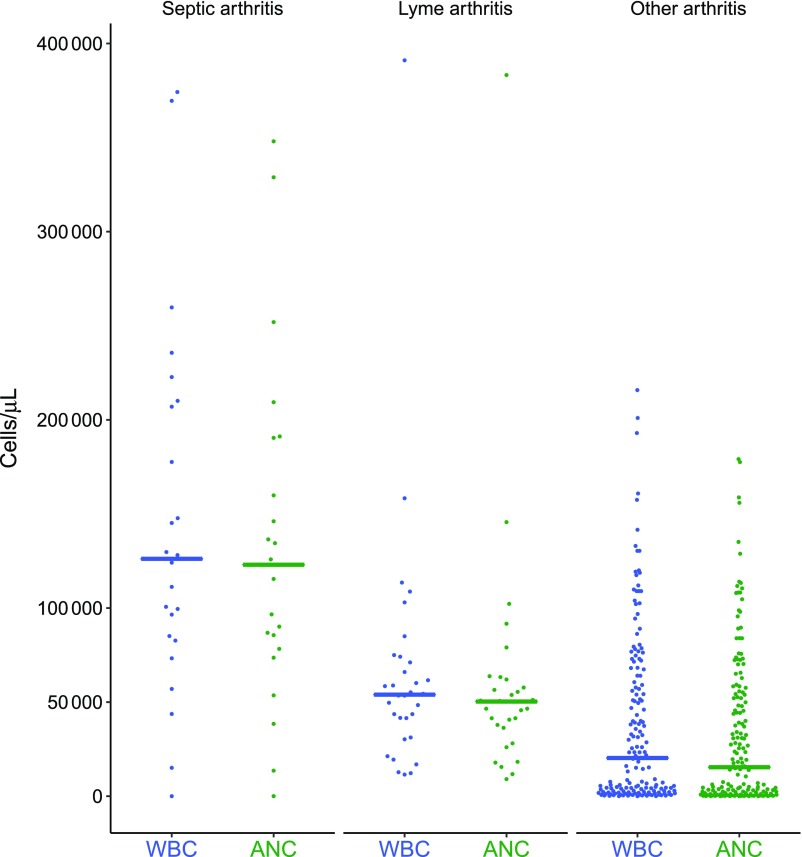
Next, we compared clinical and laboratory findings of patients with septic, Lyme, and other inflammatory arthritis (Table 2). Patients with Lyme arthritis presented throughout the year. We had available synovial fluid WBC counts for 221 patients (94.2%). Patients with septic arthritis had higher median synovial fluid WBC counts and ANCs when compared with patients with either Lyme or other arthritis (Fig 2). However, 5 patients (19% of those with septic arthritis) had a synovial fluid WBC count <50 000 cells per μL, and 11 patients had a synovial fluid count <100 000 cells per µL. Of the 92 patients with a synovial fluid WBC count ≥50 000 cells per µL, 18 (43%) had Lyme arthritis, and of 41 with synovial fluid ≥100 000 cells per µL, 5 (12%) had Lyme arthritis.
TABLE 2.
Patient Characteristics by Arthritis Type
| Characteristics | Septic Arthritis | Lyme Arthritis | Other Arthritis |
|---|---|---|---|
| (N = 26) | (N = 32) | (N = 180) | |
| Demographics | |||
| Age, y, median (IQR) | 7.5 (5.6–10.7) | 7.9 (4.0–11.3) | 5.6 (4.0–10.7)a |
| Male sex, n/N (%) | 12/26 (46) | 14/32 (44) | 106/180 (59) |
| Race, n/N (%) | |||
| White | 22/24 (92) | 22/29 (76) | 122/163 (75) |
| African American | 0/24 (0) | 2/29 (7) | 4/163 (2) |
| Other | 2/24 (8) | 5/29 (17) | 34/163 (21) |
| Hispanic ethnicity, n/N (%) | 1/23 (4) | 2/22 (9) | 25/148 (17) |
| Presentation during peak Lyme season, n/N (%) | 16/26 (62) | 15/32 (47) | 74/180 (41) |
| Clinical history, n/N (%) | |||
| Presence of fever | 22/26 (85)a | 9/32 (28) | 69/180 (38) |
| Antibiotic pretreatment | 4/26 (15) | 1/32 (3) | 18/180 (10) |
| Inability to bear wt | 25/26 (96) | 24/26 (92) | 135/170 (79) |
| Laboratory studies | |||
| Peripheral WBC count (cells per μL × 103),a median (IQR) | 10.8 (8.5–12.8) | 10.5 (9.3–13.2) | 11.0 (8.8–14.6) |
| Peripheral ANC count (cells per μL × 103),a median (IQR) | 7.8 (5.4–9.5) | 6.8 (5.9–9.0) | 7.2 (5.0–10.0) |
| ESR, mm/h, median (IQR) | 59 (40–84)a | 36 (25–55) | 27 (14–47) |
| CRP, mg/dL, median (IQR) | 10.4 (7.2–17.1)a | 2.0 (1.2–5.0) | 1.8 (0.3–4.6) |
| Synovial fluid WBC count, cells per µL, median (IQR) | 126 130a (8300–209 332) | 53 954a (33 789–73 375) | 20 267a (2500–64 012) |
| Synovial fluid ANC, cells per μL, median (IQR) | 123 000 (78 392–190 440) | 50 290 (36 333–62 040) | 15 362 (1645–54 196) |
| Positive blood culture result, n/N (%) | 8/22 (36)a | 0/32 (0) | 4/180 (2) |
| Positive Gram-stain synovial fluid result, n/N (%) | 17/26 (65)a | 0/32 (0) | 0/180 (0) |
| Positive synovial fluid culture result, n/N (%) | 25/26 (96)a | 0/32 (0) | 0/180 (0) |
| Radiology studies performed, n/N (%) | |||
| Radiograph | 24/26 (92) | 30/32 (94) | 155/180 (86) |
| Ultrasound | 21/26 (81) | 29/32 (91) | 166/180 (92) |
| MRI | 12/26 (46) | 7/32 (22) | 49/180 (27) |
| Clinical management | |||
| Arthroscopic joint irrigation | 26 (100)a | 13 (41) | 55 (31) |
| Hospitalized | 26 (100)a | 26 (81) | 113 (63) |
Statistical difference using pairwise comparisons (P < .05).
FIGURE 2.
Scatter plot of synovial fluid WBC counts and ANCs by arthritis type (line drawn at the median in cells/microliter).
Overall, 94 (39%) had surgical drainage of the affected hip, which included all 26 patients with septic arthritis as well as 13 patients with Lyme arthritis (41% of those with Lyme arthritis) and 55 patients with inflammatory arthritis (31% of those with inflammatory arthritis). Overall, 165 (69% of all study patients) were hospitalized with an overall median duration of hospital stay of 4 days (IQR 2–6 days). The duration of hospital stay for those who had an operative procedure was 5 days (IQR 4–7 days).
Discussion
We assembled a 3-center retrospective cohort of patients with hip monoarticular arthritis who had a synovial fluid culture obtained. Only a minority had septic arthritis, although 40% underwent hip drainage in the operating room. Patients with septic arthritis of the hip had a higher median synovial fluid WBC count than those with Lyme disease or other inflammatory arthritis. However, commonly used synovial fluid WBC count cut-points resulted in the misclassification of a number of patients with Lyme arthritis while missing a few with septic arthritis. We suggest that clinicians should use more than just synovial fluid cell counts in isolation to decide whether to perform operative joint washout for patients with hip monoarthritis.
The challenge for clinicians is to distinguish among arthritis types before bacterial culture or serology test results are available.2,15 The Kocher criteria were designed to help identify which patients with irritable hip should undergo arthrocentesis to evaluate for septic arthritis and have undergone broad validation.8,12 Because our study population was limited to patients who had arthrocentesis performed, we only evaluated the sensitivity and PPV of the Kocher criteria for those with septic arthritis. Importantly, 3 patients (12% of those with septic arthritis) had a Kocher score of <2, revealing that the Kocher criteria cannot be used in isolation to guide clinical decision-making.12,20,21 Although patients with a positive Gram-stain result for a bacterial pathogen should have emergent arthroscopy, one-third of septic arthritis cases would have been missed by relying on synovial fluid Gram-stain, making it an unreliable screening test. In addition, given the overlap of synovial fluid WBC counts between patients with Lyme and septic arthritis, relying on synovial fluid counts alone to guide initial clinical decision-making can lead to the misclassification of patients with both septic and Lyme arthritis. Thus, no apparent combination of clinical findings and laboratory studies available at the initial encounter can be used to reliably diagnose septic arthritis.
Similar to previous researchers, we also found that Lyme disease was a common cause of hip arthritis in patients from Lyme disease endemic areas.7 However, our comparison of synovial fluid cell counts differs slightly from our previous study of patients with knee monoarticular arthritis from Lyme disease endemic areas.4 In that 2-center study, the synovial fluid WBC counts from the knees of patients with Lyme and septic arthritis were similar. In contrast, patients with septic arthritis of the hip had higher median synovial fluid WBC counts and ANCs than those with Lyme disease or other inflammatory arthritis. However, a substantial portion of patients with Lyme arthritis had elevated synovial fluid WBC counts, and of those with septic arthritis, a number had low synovial fluid WBC counts. Treating clinicians cannot rely on synovial fluid WBC counts alone to identify all patients with septic arthritis of the hip.
Although septic arthritis of the hip is an orthopedic emergency requiring immediate operative intervention as well as parenteral antibiotics,22 patients with other types of hip arthritis, including Lyme arthritis and transient synovitis, do not require these potentially dangerous interventions.8,13 Patients with Lyme arthritis should be treated with a course of appropriate oral antibiotics; those with transient synovitis should receive anti-inflammatory medications. One-third of patients with Lyme disease and other inflammatory arthritis underwent a potentially unnecessary operative procedure. Although the vast majority of study patients were hospitalized, most patients with either Lyme arthritis or other arthritis could be safely treated as outpatients.
In Lyme disease endemic areas, a rapid and accurate diagnostic test for Lyme disease would reduce diagnostic confusion for clinicians who care for patients with hip effusions. In a previous prospective study of >1000 patients presenting to the ED for evaluation of Lyme disease, clinician suspicion had only minimal accuracy for the diagnosis of Lyme disease.23 This work reveals the need for rapid and accurate laboratory diagnostics to avoid both under- and overdiagnosis. Lyme arthritis is a late manifestation of Borrelia burgdorferi infection, so a robust immune response (as evidenced by positive serology results) should be present. In our study, the first-tier tests had only modest specificity for Lyme arthritis, and the supplemental immunoblot takes several days to return results, leaving clinicians with the challenge of providing treatment without definitive serology results. C6 is a newer first-tier Lyme disease test and has a higher specificity than other first-tier tests.24,25 None of the 3 participating clinical centers used the C6 assay during the study period. Although the current C6 assay still takes several hours to obtain results, point-of-care diagnostics are in development.26 Rapid availability of accurate Lyme disease tests could assist clinical decision-making and allow clinicians to avoid unnecessary invasive procedures and promptly initiate appropriate therapy for patients with Lyme disease arthritis.
Our study has several important limitations. First, we relied on electronic database queries for case identification. Although we may have missed eligible cases, searching by hip synovial fluid cultures rather than diagnosis codes provided the most comprehensive method of case identification available. Second, our study was retrospective, and clinical information was abstracted from each patient’s medical record. Although there could have been recording or reviewer bias, we relied on the results of diagnostic tests, which were accurately recorded in the medical record, for our primary predictors and outcome measures. Third, we did not exclude patients who had received antibiotics before diagnostic arthrocentesis. Although only a minority were pretreated with antibiotics, a patient with septic arthritis might have had a false-negative bacterial culture result, leading to diagnostic misclassification. However, synovial fluid WBC counts were similar after the exclusion of pretreated patients (results not shown). Additionally, some bacterial septic arthritis pathogens (ie, K kingae) are challenging to grow on a culture plate27 and could result in a patient with septic arthritis having a false-negative synovial fluid culture result. However, participating microbiology laboratories used the blood culture technique for synovial fluid, which should have allowed the growth of these more fastidious bacterial organisms. Fourth, a minority (∼15%) of patients were not tested for Lyme disease, potentially leading to patient misclassification. Fifth, patients with a positive 2-tiered Lyme disease serology result may have had a previous infection instead of active Lyme disease,28,29 and several patients had a history of previous Lyme disease. In fact, 1 patient with septic arthritis and a positive Lyme disease 2-tiered serology result was assumed to have previous immunity. Newer approaches to Lyme disease diagnosis are needed to distinguish previous from active infection.30 Sixth, patients with pelvic or femoral osteomyelitis and reactive hip arthritis also require parenteral antibiotics. However, radiologic imaging (eg, MRI or bone scan), not synovial fluid analysis, remains the method of diagnosis, and clinicians must always maintain a high index of suspicion for these clinical conditions.31,32 Finally, our study was conducted at 3 centers, each located in Lyme disease endemic areas, such that the findings are less relevant to patients without potential exposure to Lyme disease. Although our study captured all eligible patients presenting to the EDs of 1 of 3 participating clinical sites over a 5- to 10-year period, both Lyme disease and septic arthritis were relatively uncommon.
Conclusions
Septic arthritis caused a minority of cases of hip monoarticular arthritis in our 3-center cohort of patients undergoing hip arthrocentesis. Synovial fluid cell counts alone did not help to accurately distinguish among arthritis types, and many patients with Lyme arthritis had an unnecessary invasive surgical procedure. Newer-generation Lyme disease diagnostics are needed to avoid the under- and overdiagnosis of Lyme arthritis at the time that initial management decisions must be made.
Glossary
- ANC
absolute neutrophil count
- CI
confidence interval
- ED
emergency department
- ESR
erythrocyte sedimentation rate
- IgG
immunoglobulin G
- IQR
interquartile range
- PPV
positive predictive value
- WBC
white blood cell
Footnotes
Ms Dart helped to design the study, performed data abstraction and the primary data analysis, and drafted the initial manuscript; Dr Michelson designed the electronic case identification tool, contributed to data analysis, and revised the manuscript; Drs Aronson and Garro supervised patient enrollment and data abstraction and revised the manuscript; Dr Lee and Mr Glerum conducted data abstraction and revised the manuscript; Drs P. Nigrovic, Kocher, and Bachur contributed to data interpretation and revised the manuscript; Dr L. Nigrovic conceived and designed the study, supervised and performed data abstraction, supervised the data analysis, and drafted the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Donald M. and Eleanor Meisel Scholar Grant (Ms Dart); Joint Biology Consortium Research-based Center (NIH grant P30 AR070253; Dr P. Nigrovic); and a Clinical and Translational Science Award (grant KL2 TR001862; Dr Aronson) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Welkon CJ, Long SS, Fisher MC, Alburger PD. Pyogenic arthritis in infants and children: a review of 95 cases. Pediatr Infect Dis. 1986;5(6):669–676 [DOI] [PubMed] [Google Scholar]
- 2.Cruz AI Jr, Aversano FJ, Seeley MA, Sankar WN, Baldwin KD. Pediatric Lyme arthritis of the hip: the great imitator? J Pediatr Orthop. 2017;37(5):355–361 [DOI] [PubMed] [Google Scholar]
- 3.Deanehan JK, Kimia AA, Tan Tanny SP, et al. . Distinguishing Lyme from septic knee monoarthritis in Lyme disease-endemic areas. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e695 [DOI] [PubMed] [Google Scholar]
- 4.Deanehan JK, Nigrovic PA, Milewski MD, et al. . Synovial fluid findings in children with knee monoarthritis in lyme disease endemic areas. Pediatr Emerg Care. 2014;30(1):16–19 [DOI] [PubMed] [Google Scholar]
- 5.Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing Lyme arthritis from other etiologies. Pediatrics. 2009;123(3):959–965 [DOI] [PubMed] [Google Scholar]
- 6.Bachur RG, Adams CM, Monuteaux MC. Evaluating the child with acute hip pain (“irritable hip”) in a Lyme endemic region. J Pediatr. 2015;166(2):407–411.e1 [DOI] [PubMed] [Google Scholar]
- 7.Glotzbecker MP, Kocher MS, Sundel RP, Shore BJ, Spencer SA, Kasser JR. Primary Lyme arthritis of the pediatric hip. J Pediatr Orthop. 2011;31(7):787–790 [DOI] [PubMed] [Google Scholar]
- 8.Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662–1670 [DOI] [PubMed] [Google Scholar]
- 9.Nigrovic LE, Thompson AD, Fine AM, Kimia A. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease-endemic area. Pediatrics. 2008;122(5). Available at: www.pediatrics.org/cgi/content/full/122/5/e1080 [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sultan J, Hughes PJ. Septic arthritis or transient synovitis of the hip in children: the value of clinical prediction algorithms. J Bone Joint Surg Br. 2010;92(9):1289–1293 [DOI] [PubMed] [Google Scholar]
- 12.Kocher MS, Mandiga R, Zurakowski D, Barnewolt C, Kasser JR. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A(8):1629–1635 [DOI] [PubMed] [Google Scholar]
- 13.Heyworth BE, Shore BJ, Donohue KS, Miller PE, Kocher MS, Glotzbecker MP. Management of pediatric patients with synovial fluid white blood-cell counts of 25,000 to 75,000 cells/mm3 after aspiration of the hip. J Bone Joint Surg Am. 2015;97(5):389–395 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz DL, Katzap E, Horowitz S, Barilla-LaBarca ML. Approach to septic arthritis. Am Fam Physician. 2011;84(6):653–660 [PubMed] [Google Scholar]
- 15.McGillicuddy DC, Shah KH, Friedberg RP, Nathanson LA, Edlow JA. How sensitive is the synovial fluid white blood cell count in diagnosing septic arthritis? Am J Emerg Med. 2007;25(7):749–752 [DOI] [PubMed] [Google Scholar]
- 16.Fowler ML, Zhu C, Byrne K, et al. . Pathogen or contaminant? Distinguishing true infection from synovial fluid culture contamination in patients with suspected septic arthritis. Infection. 2017;45(6):825–830 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44(31):590–591 [PubMed] [Google Scholar]
- 18.Sivak SL, Aguero-Rosenfeld ME, Nowakowski J, Nadelman RB, Wormser GP. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch Intern Med. 1996;156(18):2105–2109 [PubMed] [Google Scholar]
- 19.Lantos PM, Lipsett SC, Nigrovic LE. False positive Lyme disease IgM immunoblots in children. J Pediatr. 2016;174:267–269.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956–962 [DOI] [PubMed] [Google Scholar]
- 21.Caird MS, Flynn JM, Leung YL, Millman JE, D’Italia JG, Dormans JP. Factors distinguishing septic arthritis from transient synovitis of the hip in children. A prospective study. J Bone Joint Surg Am. 2006;88(6):1251–1257 [DOI] [PubMed] [Google Scholar]
- 22.Singhal R, Perry DC, Khan FN, et al. . The use of CRP within a clinical prediction algorithm for the differentiation of septic arthritis and transient synovitis in children. J Bone Joint Surg Br. 2011;93(11):1556–1561 [DOI] [PubMed] [Google Scholar]
- 23.Nigrovic LE, Bennett JE, Balamuth F, et al. ; Pedi Lyme Net . Accuracy of clinician suspicion of Lyme disease in the emergency department. Pediatrics. 2017;140(6):e20171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branda JA, Strle K, Nigrovic LE, et al. . Evaluation of modified 2-tiered serodiagnostic testing algorithms for early Lyme disease. Clin Infect Dis. 2017;64(8):1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsett SC, Branda JA, McAdam AJ, et al. . Evaluation of the C6 Lyme enzyme immunoassay for the diagnosis of Lyme disease in children and adolescents. Clin Infect Dis. 2016;63(7):922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Immunetics C6 B. burgdorferi (Lyme) ELISATM kit. 2015. Available at: www.immunetics.com/lyme.html. Accessed March 19, 2018
- 27.Yagupsky P, Porsch E, St Geme JW III. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011;127(3):557–565 [DOI] [PubMed] [Google Scholar]
- 28.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis. 2001;33(6):780–785 [DOI] [PubMed] [Google Scholar]
- 29.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect. 2012;18(12):1236–1240 [DOI] [PubMed] [Google Scholar]
- 30.Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med. 1998;128(5):354–362 [DOI] [PubMed] [Google Scholar]
- 31.Waseem M, Kumari D, Toledano T. Fever and hip pain: not always due to a septic hip [published online ahead of print March 2, 2017]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 32.Kerr DL, Loraas EK, Links AC, Brogan TV, Schmale GA. Toxic shock in children with bone and joint infections: a review of seven years of patients admitted to one intensive care unit. J Child Orthop. 2017;11(5):387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]