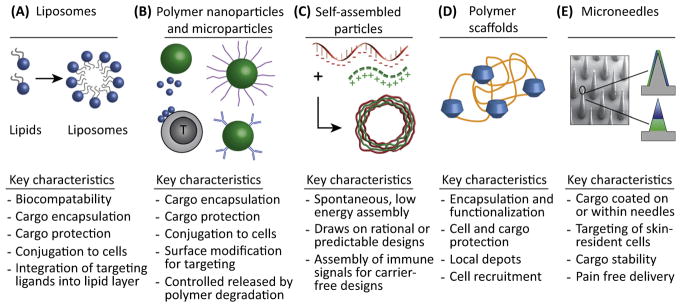
Polymers, lipids, scaffolds, microneedles, and other biomaterials are rapidly emerging as technologies to improve the efficacy of vaccines against infectious disease and immunotherapies for cancer, autoimmunity, and transplantation. New studies are also providing insight into the interactions between these materials and the immune system. This insight can be exploited for more efficient design of vaccines and immunotherapies. Here, we describe recent advances made possible through the unique features of biomaterials, as well as the important questions for further study.
Biomaterials Offer Features That Improve Control of Responses to Vaccines and Immunotherapy
Despite the past advances of vaccines and immunotherapies, there is an increasing need for greater control over the types of immune responses generated to combat infection, cancer, and autoimmunity. Biomaterials – a term encompassing synthetic and natural polymers, lipids, self-assembled nanostructures, and engineered artificial cells – offer unique features to enable this control [1,2]. Some of the broad classes of biomaterials include: (i) nano-particles (NPs) and microparticles (MPs) formed from polymers or lipids that can be conjugated or delivered to immune cells [3,4]; (ii) stable or degradable scaffolds for implantation [5]; and (iii) devices such as microneedle arrays that target immune cells in the skin [6,7]. Biomaterials are already used extensively in humans for prosthetics and implants, but to date, there are few clinical examples in the drug delivery field, and fewer still within vaccines and immunotherapy. Biomaterials offer key design benefits such as control over the loading and release kinetics of multiple immune cargos, and protection from enzymatic degradation and extreme pH. Furthermore, biomaterials can be conjugated with antibodies or receptor ligands to provide molecularly specific targeting to immune cells or tissues; this feature can be exploited to reduce systemic and local toxicity. The main classes of materials and unique features are summarized in Figure 1, while Table 1 highlights the key examples we discuss below.
Figure 1. Key Classes of Biomaterials Being Used to Study and Control Immune Function.
These efforts involve (A) liposomes, (B) polymer nanoparticles and microparticles, (C) self-assembled materials, (D) polymer scaffolds, and (E) microneedles and other macroscopic devices.
Table 1.
Key Examples of Materials for Immune Engineering
| Approach | Biomaterial | Notes | Refs | |
|---|---|---|---|---|
| Intrinsic immunogenicity | Polymer particles | Poly(D,L-lactic-co-glycolic acid) | T cell stimulation is shape dependent | [16,18] |
| Gold NPs | Particle shape and hydrophobicity alter cytokine secretion profiles | [23,99] | ||
| Polystyrene | Ellipsoidal particles improve pharmacokinetics by enhancing circulation time, while smaller particles are taken up more efficiently by pulmonary APCs | [17,21,22] | ||
| Poly(β-amino esters) | The immunogenicity of polymers changes during degradation | [27,28] | ||
| Liposomes | Ellipsoid liposomes exhibit improved pharmacokinetics compared with spheres | [17] | ||
| Self-assembly | Poly(methacrylic acid) | Different immune cell types preferentially interact with distinctly shaped particles | [19] | |
| Peptide nanofibers | Negatively charged surfaces prevent uptake by APCs to limit adaptive immune response | [25] | ||
| Porous particles | Silicon | Surface chemistry of particles changes immunostimulatory effects | [24] | |
| Infectious disease | Polymer particles | Poly(D,L-lactic-co-glycolic acid) | PLGA encapsulation increases uptake of cargo by APCs and allows for controlled release | [29,35,41,8] |
| Poly(D,L-lactic-co-glycolic acid)-b- poly(L-histidine)-b-poly(ethylene glycol) | Particulate delivery of antigen can change the immune response from tolerogenic to long-lived protection | [49] | ||
| Liposomes | Encapsulating cargo into liposomes increases retention time in the draining LN for increased interaction with T cells, yields control over protein density, and can be attached to T cells for targeted delivery | [30,31,39,40] | ||
| Porous particles | Poly(vinyl pyrrolidone) | Porous particles increase the diffusion of intracellular proteases leading to faster and more efficient processing and increase the surface area allowing for increased cargo loading density | [32] | |
| Silicon | Co-delivering signals in porous particles synergistically increases cytokine secretion | [33] | ||
| Self-assembly | Peptide and TLRa | Self-assembling immune signals eliminates carrier effects and provides a platform to control the absolute and relative loading of cargo | [34] | |
| Recombinant protein | Self-assembled proteins can be used to change how an antigen is displayed, thus controlling the desired immune response | [36,42] | ||
| Modified dendrimers | Biomaterials can be used as a tool to efficiently screen vaccine candidates | [50] | ||
| Scaffolds | N-(2-hydroxypropyl)methacrylamide | Density of TLRa on a polymer backbone changes innate immune activation, kinetics, and uptake by APCs | [37] | |
| Mesoporous silica rods | Porous scaffolds loaded with immune signals recruit and program DCs to home to the LN | [38] | ||
| Microneedles | poly(o-nitrobenzyl-methacrylate-co- methyl-methacrylate-co-poly (ethylene-glycol)-methacrylate) | Microneedles can be used to co-deliver immune signals to the APC rich dermal layer for a simple, pain free vaccine design | [46] | |
| Poly(L-lactic acid) | Microneedle delivery can improve patient compliance and elicit antigen specific responses | [47] | ||
| Polyvinyl alcohol | Dissolvable microneedle patches are stable and able to produce comparable antibody response to fresh liquid vaccines in humans | [43–45] | ||
| Hybrid biological and biomaterial particle | Poly(β-amino esters) | Hybrid vaccines can be used to engage APC receptors and enhance uptake | [51] | |
| Cancer | Liposomes | Particle encapsulation can be used to target the delivery of chemotherapeutics to decrease systemic effects and safety concerns, increase uptake and processing by APCs, and enhance tumor-specific T cell function. In addition, liposomes can be modified to exploit naturally occurring immune pathways to direct the type of response | [54,63–65, 69,70] | |
| Polymer Particles | Poly(β-amino esters) | Polymer condensation of DNA cargo can be used to induce the expression of CAR genes in situ eliminating the need for ex vivo expansion | [55] | |
| Polyanhydride | Different polymer chemistries elicit different levels of response when used to encapsulate model tumor antigen | [52] | ||
| Poly(D,L-lactic-co-glycolic acid) | PLGA NPs co-encapsulating different cargos enhance targeting, uptake, and homing and can change the way a small molecule drug is processed. Polymers can also be used to synthesize artificial APCs to deliver signals in a controlled context. PLGA particles are also being used in conjunction with photothermal therapy to generate tumor associated and deliver the context cues to direct the immune response against them | [53,62,66,72] | ||
| Chitosan | Biomaterials enable formulation of effective vaccines containing whole tumor lysates that actively target DCs where the tumor associated antigens can be processed and presented | [67] | ||
| Poly(lactide-co-glycolide) | [65] | |||
| Synthetic block copolymers | Polymer architecture and pH responsiveness can be optimized for maximum cytosolic delivery of antigen in APCs to maximize activation of the STING pathway | [61] | ||
| Polystyrene | Bispecific nanobioconjugates are useful to induce selective immune-mediated eradication of breast cancer by bringing the appropriate cell types together | [71] | ||
| Microneedles | Hyaluronic acid | Microneedles can be exploited to target the delivery of checkpoint blockade therapies to appropriate cell types, reducing adverse systemic effects | [68] | |
| Poly(L-lactide) | Co-delivering a tumor antigen and TLRa via microneedles elicits antigen specific T cell expansion in a painless approach | [60] | ||
| Self-assembled particles | Polyethylenimine and DNA | NPs can protect DNA cargo and co-deliver enhancing immune cues in an oral vaccine | [59] | |
| Scaffolds | Cryogel | Scaffolds can be used to co-deliver signals to increase DC infiltration at the site of infection | [57] | |
| Alginate | Implantable biopolymers can be used to target delivery of CAR T cells directly to the site of solid tumors. Alginate scaffolds can also deliver immune signals to increase T cell proliferation with memory phenotypes at a resection site | [58,73] | ||
| Tolerance | Polymer particles | Poly(D,L-lactic-co-glycolic acid) | Regulatory small molecules and immune signals can be delivered more safely and effectively using particle encapsulation. Particles can be used to co-deliver signals in a controlled release manner to change the immune response against an self-antigen | [75–78,84, 88–90,97,98] |
| Polystyrene | Negatively charged MPs are taken up by inflammatory monocytes and sequestered in the spleen decreasing systemic inflammation | [76,77] | ||
| Iron oxide | Artificial APCs can be used to expand self-antigen specific regulatory T cell populations and as tools to probe the key design features | [82,83] | ||
| QDs | Allows for precise control over self-antigen display on surfaces to drive tolerance | [79] | ||
| Self-assembled immune signals | Immune signals | Self-assembled immune signals protect cargo from degradation and reduce antigen specific disease in mouse models of MS | [86,87] | |
| Scaffolds | Poly(D,L-lactic-co-glycolic acid) | Encapsulation of insulin and immune signals in a scaffold alter the response to the antigen | [91] | |
| Alginate | Scaffolds can be used to target the delivery of regulatory immune signals and drugs | [94] | ||
| Triglycerol monostearate | Immunosuppressive drug loaded into a hydrogel allows for controllable release in response to proteolytic enzyme overexpressed during inflammation | [95] | ||
| Acellular dermal matrix | [96] | |||
| Engineered erythrocytes | Red blood cells can be exploited for their non-inflammatory clearance to promote tolerance against conjugated antigens | [80,81] |
MPs and NPs can be synthesized from either organic or inorganic materials. Liposomes, for example, are NPs composed of amphipathic lipid molecules surrounding an inner aqueous core (Figure 1A). They have thus far resulted in the most clinically approved drug delivery applications incorporating biomaterials, including chemotherapeutics such as Doxil/Myocet (liposomal doxorubicin), and liposomal vaccines such as Epaxal (hepatitis A) and Inflexal V (influenza). Liposomes are useful owing to biocompatibility, the ability to incorporate hydrophobic and hydrophilic drugs, and robust options for functionalization [8]. Degradable synthetic polymers such as poly (lactide-co-glycolide) (PLGA) are also widely used in MPs and NPs because the chemistry of these polymers allows flexible control of the degradation profiles and resulting release of immune cargos (Figure 1B). Lipid and polymer particles can also be modified with reactive groups to trigger release in response to light, pH, or other cues. Interestingly, recent research reveals that PLGA and many other biomaterials exhibit physico-chemical features (e.g., charge, and repetitive chemical motifs) that can directly activate immune pathways [9,10]. These findings of intrinsic immunogenicity have catalyzed research to elucidate how material properties activate, modulate, or suppress immune pathways, for example, through interaction with Toll-like receptors (TLRs), inflammasomes, or other pathogen sensors. This concept could enable next generation materials that are not just carriers, but also actively direct the responses to the other components (e.g., antigens) of a vaccine or immunotherapy.
From another perspective, the intrinsic immunogenic features of biomaterials can also complicate design because the carriers may adversely alter the response of the immune system to other components. Thus, an emerging area of research focuses on self-assembly of proteins, nucleic acid ligands, or other immune signals into structures that mimic attractive features of biomaterials (e.g., co-delivery), but that are comprised entirely from immune signals [11– 13] (Figure 1C). Self-assembly can also be applied to the synthesis of scaffolds for targeted delivery of immune signals and drugs, or for integration of synthetic materials that create microenvironments to recruit immune cells to target sites (Figure 1D). Lastly, new studies are exploiting delivery devices such as microneedles, microscale metal or polymer needles that target skin-resident immune cells without penetrating the skin deeply enough to cause pain (Figure 1E). Microneedles also eliminate medical sharps and provide increased thermal stability, a feature crucial for effective distribution of vaccines in developing regions with limited refrigeration.
In this review, we highlight ways in which biomaterials can be leveraged to improve vaccines and immunotherapies. We begin with a discussion of the intrinsic immunogenicity of biomaterials in relation to their shape, size, and chemistry. Next, we describe how biomaterials are being tested as vaccines and immunotherapies in infection, cancer, autoimmunity, allergies, and transplantation. We also frame the central concepts biomaterials are being used for in the context of the respective tissues and immunological sites they are applied. Interested readers are directed to several texts for a more comprehensive background on biomaterials [14] and immunology [15].
Biomaterials Exhibit Physicochemical Properties That Can Trigger or Block Immune Pathways
Shape Effects
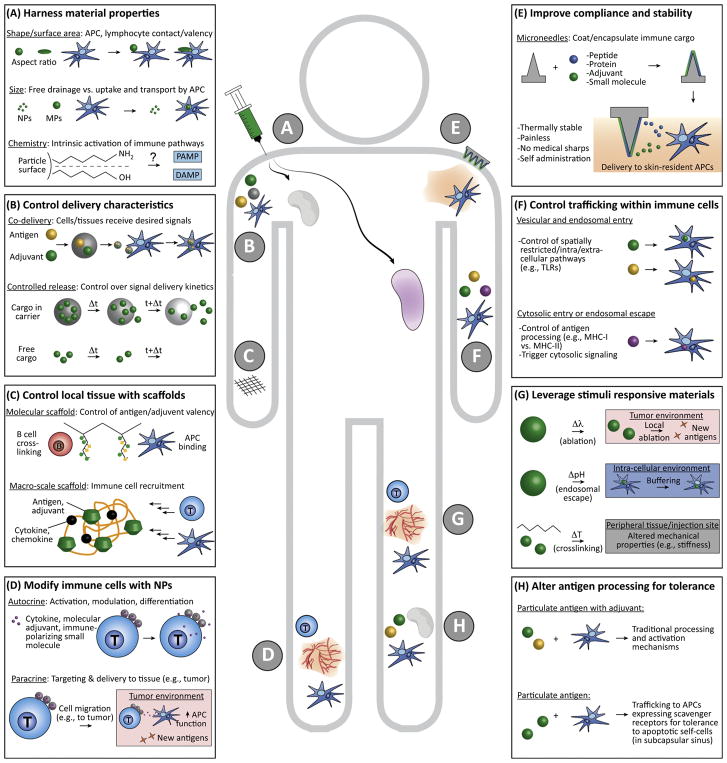
As alluded to above, shape can alter interaction with antigen-presenting cells (APCs) or other immune cells, even in the absence of other immune signals. Several studies reveal ellipsoidal particles improve pharmacokinetics compared to spherical particles, enhancing circulation time to promote immunity [16– 18]. For example, ellipsoidal PLGA particles functionalized with peptide– MHC complex and anti-CD28 on the surface can mimic antigen presentation by APCs to stimulate T cells more effectively than spherical particles owing to increased interaction with T cells (Figure 2A, top). However, these interactions may vary across cell types; NP uptake by macrophages appears biased toward spherical particles, whereas ellipsoidal particles resist uptake [16,19].
Figure 2. Strategies Involving Biomaterials for Engineering Immune Function.
(A) Material shape, size, chemistry, and other physicochemical properties impact drainage through lymphatics, interactions with APCs, and the intrinsic immunogenicity of many common polymers. (B) Biomaterials can be used to control the combinations and relative concentrations of immune cargos reaching APCs and lymphocytes, or, by designing polymers with a desired degradation rate, the cargo delivery kinetics. (C) Scaffolds can be used to control the context or density in which antigens and adjuvants are displayed, and as local environments to recruit APCs or lymphocytes (e.g., by incorporation of chemokines). (D) T cells, APCs, and other immune cells can be modified with nanoparticles incorporating immune signals to exert autocrine effects on the modified cells, or to exert paracrine effects on target cells and tissue to which the modified cell will migrate (e.g., a tumor). (E) Microneedles coated with or incorporating immune cues increase safety and patient compliance by efficiently targeting skin-resident immune cells without pain, generation of medical sharps, or need for refrigeration. (F) Biomaterial carriers can be engineered with specific moieties to control cellular entry and intracellular trafficking for directing spatially restricted immune processes (e.g., TLR signaling and antigen processing). (G) Stimuli responsive materials can exploit physiological (e.g., changes in pH or temperature) or external cues (e.g., UV light) to provide environment-specific control within cells, target tissues, or tumors (e.g., access to neoantigens during NP-enabled local ablation via photothermal exposure). (H) NPs and MPs can alter how antigens are processed to modulate responses away from proimmune and toward regulation. Abbreviations: APC, antigen-presenting cell; MP, microparticle; NP, nanoparticle; TLR, Toll-like receptor.
Size Effects
Biomaterial particles can vary in size on the order of nanometers to microns or larger. While NPs up to several 100 nm, particularly those ≥25 nm, traffic rapidly to draining lymph nodes (LNs), larger particles are retained at the site of injection and are internalized and transported to LNs by APCs [9,20]. Recent work reveals pulmonary APCs [macrophages and dendritic cells (DCs)] take up 50 nm polystyrene particles more efficiently and rapidly compared to 500 nm particles [21]. Notably, APCs that take up 50 nm polystyrene particles also had greater expression of co-stimulatory markers compared to 500 nm particles, due to preferential uptake by several DC subsets that facilitates greater cell-mediated transport to draining LNs. However, 500 nm particles resulted in a nearly twofold increase in uptake by alveolar macrophages, suggesting the ability of larger particles to persist at a site and accumulate in cells. In other studies using antigen-coated polystyrene rods and spheres of different sizes, smaller sizes promoted T helper (Th)1 biased response in the case of the spherical geometries. Conversely, for rod-shaped particles, increasing length, but not width, correlated with a Th2 biased response [22]; possibly because longer particles presented a greater antigen density and surface area for attachment or internalization. Together, these results highlight the importance of particle size in trafficking, uptake by APCs, activation, and subsequent biasing of adaptive response (Figure 2A, center). This interplay between size, shape, and chemical features (discussed below) also underscores a key outstanding question: how best to isolate the role of specific material properties (e.g., shape vs. surface area) to enable more rational design of smart vaccine and immunotherapy carriers.
Chemistry Effects
The chemistry of a material impacts intrinsic immunogenic activity, thus, chemistry can be used as a lever to control this parameter [23,24]. For example, the hydrophobicity of gold NPs designed with specific functional groups alters the expression of genes for interleukin (IL)-2, -6, -10, tumor necrosis factor-α and interferon-γ in vitro and in mice [23]. Surface charge can also influence APC function, for example, negatively charged particles can reduce uptake by APCs, and as a consequence, restrain adaptive immune responses [25]. Although in this work negative surface charge was insufficient to induce tolerance, negatively charged materials may be useful in designing nonimmunogenic carrier platforms. Another consideration is the stability of a particle, as many nanomaterials exist as suspensions that can aggregate with time. In one example, iron NPs formed aggregates that were internalized by peripheral blood mononuclear cells in vitro at greater rates compared to stable suspensions; this change increased TLR signaling, complement activation, and IL-1β production [26]. Additionally, while degradable materials are often used to control the release rates of antigens or other vaccine components, recent studies show the intrinsic immunogenicity of polymers can change over time as polymers degrade and the physicochemical properties change. In one example using a common class of degradable polymers, poly (β-amino esters), particle size and charge changed as molecular weight decreased. Regardless of the starting polymer structure, the material-driven immunogenicity peaked at a characteristic molecular weight range [27,28]. Thus, surface chemistry is important in rational design of biomaterials and presents new opportunities to tailor the types of responses elicited (Figure 2A, bottom).
Biomaterials Can Improve Immunogenicity and Durability of Vaccines against Infectious Disease
While vaccines for infectious disease have already been transformative, biomaterials are being investigated to improve immunogenicity and durability, and to make design and delivery of antigens and adjuvants more efficient. Biomaterials can also increase stability to enable the use of antigens that are difficult to work with, serve as delivery technologies that increase shelf-life for distribution in developing regions, and offer improved compliance through simpler, less painful delivery routes.
Biomaterial Vaccines against Model Antigens
Many material platforms are initially investigated with model antigens to establish concepts that can be extended to disease-relevant antigens. Encapsulation of vaccine cargo into polymeric or lipid particles is a continuing theme in the vaccine field to improve co-delivery of antigen and adjuvant, or achieve desirable delivery kinetics through controlled cargo release (Figure 2B). The particulate nature of material platforms also generally enhances uptake by APCs [29– 31]. One recent example used crosslinked lipid NPs encapsulating model antigen (ovalbumin; Ova) combined with a TLR agonist (TLRa) to increase antigen-specific T cells 13-fold by increasing retention in draining LNs [31]. Particles can also be engineered to be porous, increasing cargo loading density compared to traditional solid particles, while maintaining attractive features such as uniform particles with tunable sizes [32]. Porosity can also increase the diffusion of intracellular proteases, resulting in faster processing and presentation of antigen. This idea has been used to co-deliver two types of pattern recognition receptors, TLRas and NOD-like receptor agonists (NLRa), along with Ova, in porous silica particles [33]. In mice, these coloaded particles polarized responses toward Th1 function without CD8+ T cell activation, in comparison to delivery of the individual agonists.
Another new approach involves electrostatic assembly of cationic and anionic immune signals onto a template that can later be removed to create carrier free vaccine capsules. These self-assembled materials, termed immune polyelectrolyte multilayers (iPEMs), are composed entirely of antigens, nucleic-acid based adjuvants, or other charged immune signals [13]. Coating a sacrificial core with Ova peptide appended with cationic amino acid and RNA-based TLRas created an approach to control the absolute and relative loadings of each component. In mice, iPEMs improved expansion of antigen-specific T cells and provided protection during a melanoma model expressing Ova [34]. This carrier-free approach also eliminates any complicating factors arising from the intrinsic immunogenicity of biomaterials discussed above.
The studies above and others highlight the benefits of using biomaterials to co-deliver signals [35], but there are many open questions as to the commonality of design needs across vaccines and immunological targets. For example, another recent study showed delivering signals in separate NPs generates efficacious responses compared to co-delivery of antigen and adjuvant in the same polymer particle [36]. This dichotomy might result from a competing effects between potency resulting from coactivation of multiple pathways in an individual cell (i. e., cargo in the same particle), compared with activating fewer pathways in a greater number of cells (i.e., cargo in different particles).
In addition to co-delivery, biomaterials can be used to create scaffolds that control the context and valency in which immune signals are presented (Figure 2C). In one approach, a TLRa was conjugated to a polymer scaffold to improve the unfavorable pharmacokinetic properties associated with delivery of soluble TLRas in clinical applications [37]. Depending on the density of TLRa on the polymer backbone and temperature (i.e., physiological and ambient), adjuvant distribution and retention time in draining LNs could be controlled. This control enhanced uptake and innate immune activation. Scaffolds are also a useful tool to modulate host cell populations because they provide spatiotemporal control of chemical and mechanical signals. This idea has been used to control DC recruitment and subsequent homing to draining LNs by loading scaffolds built from porous silica rods with Ova, granulocyte– macrophage colony-stimulating factor (GM-CSF), and TLRa [38]. These materials provide a large pore volume and large surface area for controlled cargo release, while creating a 3D microenvironment to recruit and program DCs that then traffic to the draining LN. Compared to nonporous and soluble controls, this approach increased systemic antibody and cytotoxic T cell levels, delayed tumor growth, and increased survival in a mouse model of EG7.OVA lymphoma. The concepts illustrated in this section using model antigen are readily adaptable to the more clinically relevant models below.
Biomaterial Vaccines for HIV
Biomaterials have recently been investigated as options to increase the efficacy of candidate HIV therapies in mice and rhesus macaques. Liposomes, for example, have been used to deliver a HIV viral envelope protein to increase lymphatic uptake and retention, and increase antigen capture by APCs. Liposomal delivery enabled co-delivery of TLR agonists and T cell helper epitopes with tunable protein density to enhance the strength and durability of antibody responses in mice [39]. The tunability was used to promote B cell receptor aggregation, thus enhancing cross presentation. Another emerging approach is attachment of NPs loaded with modulatory immune cues to T cells or APCs to alter their function during vaccination or immunotherapy (Figure 2D). This idea has been used to improve cytotoxic T cell recognition of infected target cells in a mouse model of HIV by chemically conjugating antigen-specific cytotoxic T cell lymphocytes (CTLs) with NPs loaded with IL-15Sa; a dimer of IL-15 and IL-15Ra [40].
Biomaterial Vaccines against Influenza
Influenza has a well-established vaccine, but biomaterials are being investigated for next generation versions that protect against evolving strains, eliminate the need for annual redesign, or improve patient compliance. PLGA NPs, for example, are being tested in pigs to induce cross-protection against evolving swine flu virus because they can encapsulate and protect inactivated viral antigens and controllably release cargo [41]. Additionally, PLGA NPs co-delivering whole virus antigen and multiple TLRas (TLR4a and TLR7a) have been used to elicit robust immunity against H1N1 influenza in rhesus macaques [35]. NPs built from influenza antigens through self-assembly have also been used to ensure display in the native conformations to drive cross-protection [42].
Another promising strategy recently entering the clinic is delivery of vaccine components using microneedles (Figure 2E). A new first-in-human trial using dissolvable microneedle patches for influenza vaccination revealed the vaccines were stable for more than 1 year and generated titers similar to those of existing injectable vaccines even when self-administered by recipients [43– 45]. Such advances could transform the way vaccines are delivered, as well as the accessibility of effective formulations in developing regions. Not surprising, microneedles are also being explored as vaccines for HIV [46,47].
Biomaterials Targeting Other Infectious Diseases
Other infectious diseases being targeted by biomaterial-based approaches include methicillin-resistant Staphylococcus aureus, where PLGA NPs are being tested to combat antibiotic resistance [48]. One candidate vaccine against Chlamydia trachomatis – a common sexually transmitted disease with no vaccine – involves pH-responsive polymers and TLRas linked to UV-inactivated C. trachomatis [49]. This approach provides control over how antigen and adjuvant is processed during uptake and endosomal acidification (Figure 2F,G, middle), enhancing endosomal TLR signaling and driving long-lived protection in a humanized mouse challenge model. Similarly, responsive cationic polymers are being studied to improve the immunogenicity of candidate vaccines for Zika virus [50] and pneumococcal disease [51]. Together, these examples illustrate another emerging trend: biomaterials can be exploited to control the context in which immune signals are presented, or the tissues and subcellular organelles the signals reach.
Biomaterials Improve Targeting, Selectivity, and Potency of Immunotherapies to Fight Cancer
Many of the capabilities that make biomaterials attractive for vaccines against infectious disease are also being investigated to combat cancer. Some of these include: (i) co-delivery; (ii) targeted delivery; (iii) enhancement of adoptive transfer; and (iv) improved efficacy and safety of existing treatments. Several studies are using OVA-expressing tumor models to understand how biomaterial chemistry and tumor antigen display impact antitumor immunity [52,53], while others are translationally focused, targeting specific cancers.
Biomaterial Therapies for Leukemia and Lymphoma
An exciting strategy arising with biomaterials in cancer is conjugation of T cells with NPs incorporating immune cues to enhance antitumor response. In one example, NPs loaded with chemotherapeutics were conjugated to autologous T cells ex vivo, then adoptively transferred into a mouse with disseminated lymphoma [54]. This strategy reduced tumor burden by targeting the chemotherapy to the lymphoid sites where the T cells and lymphomas home; thus, the NP served as a drug depot for controlled and sustained release to the T cell. Other approaches seek to eliminate adoptive transfer entirely. For example, in a mouse model of leukemia, cationic polymers have been used to condense DNA encoding leukemia-specific chimeric antigen receptor (CAR) [55]. The NPs were then functionalized with anti-CD3 fragments to target T cells, inducing expression of the CAR gene in situ and providing long-term disease remission. Such approaches could lead to efficient and effective routes for analogous outcomes in the clinic.
Biomaterial Vaccines for Melanoma
Owing to the simplicity and accessibility of mouse models, melanoma remains the most common cancer model in which biomaterial vaccines and immunotherapies are tested. Implantable scaffolds are one key example, as scaffolds can create local depots to recruit immune cells, or modulate their function [56]. Tumor cells, TLRas, and recruitment factors (e.g., GM-CSF) can be coencapsulated in porous polymer gels to recruit DCs to mount durable, tumor-specific T cell responses in mice [57]. Alternatively, other scaffolds have been loaded with CAR T cells for direct targeting to solid tumors [58]. In another approach, live attenuated bacteria were modified with self-assembled NPs containing DNA encoding antiangiogenic factors. The bacteria served as an adjuvant to activate T cells, while the DNA restrained angiogenesis [59].
Self-assembly is also another theme being exploited in cancer. For example, iPEMs built on microneedles using melanoma peptides and TLRas have been used for delivery to the immune-rich dermal layer in mice to generate melanoma-specific T cells [60]. In another approach, spontaneous micelle vaccines composed of tumor peptides and synthetic polymers that activate STING (stimulator of interferon gene) signaling generated strong antitumor inhibition in multiple mouse models [61]. In particular, these examples underscore the theme that carriers can be engineered to also actively trigger desirable immune pathways.
A number of co-delivery applications also focus on delivery of immunomodulatory cues during cancer vaccination and immunotherapy to tailor immune response. In one approach, PLGA MPs loaded with rapamycin could be used to control the polarization of T cells between effector or memory phenotypes, depending on the level of drug in the particles during tumor vaccination [62]. In another report, lipoprotein-mimicking NPs coupled with personalized sets of tumor peptides and adjuvants enhanced uptake and processing by APCs, generating broad specificity T cell responses that inhibited tumor growth in mice that was not achieved without co-delivery [63].
As in the STING example above, an emerging theme is to co-opt existing immune pathways to enhance vaccines and immunotherapies. One exciting biomaterials example coupled amphiphilic vaccines comprised of antigen and adjuvant with an albumin-binding domain that efficiently shuttled vaccine components to LNs through natural albumin trafficking mechanisms [64]. Another approach demonstrated effective eradication of large tumors through LN targeting and combinatorial activation of both innate and adaptive immune responses using a four-part vaccine: tumor-antigen targeting antibody, recombinant IL-2, anti-PD-1 (a checkpoint inhibitor), and the vaccine antigen [65]. Lastly, artificial APCs composed of PLGA particles functionalized with anti-CD28 and MHC displaying tumor antigen have been designed to activate T cells [66]. When combined with checkpoint blockade during a metastatic mouse model of melanoma, this therapy synergistically suppressed tumor growth.
Biomaterials can also provide targeting that reduces systemic toxicity and provides dose sparing. This idea has increased the efficacy of candidate immunotherapies involving whole tumor cell lysates through improved targeting to DCs [65,67]. Biomaterials have also been used to increase the efficacy of checkpoint blockade therapies by better selective targeting to specific cell types, decreasing systemic side effects [68,69]. Only now are some of these ideas filtering into clinical trials [70], highlighting the need to use more rigorous preclinical models in testing biomaterials and to push toward clinical studies.
Biomaterial Immunotherapies for Breast Cancer
In breast cancer, one recent approach used NPs as substrates to create bispecific conjugates that exploited epidermal growth factor receptor 2 (HER2) and calreticulin-mediated phagocytosis signaling to bring cancer cells and immune cells together. This approach facilitated phagocytosis and resulted in eradication of HER2-expressing tumors in mice [71]. Another strategy used biomaterials and photothermal therapy to ablate tumors in mice, providing APCs access to tumor associated antigens (Figure 2G). Photothermal therapy works through local absorption of energy from a laser by nanoparticles draining into a tumor to generate local heating. Importantly, encapsulating TLRa in the NPs with a photothermal agent enhanced the tumor specific immune response and generated functional effector memory that protected against tumor rechallenge [72].
As with melanoma, scaffold implants are being investigated to increase the efficacy of adoptive T cell therapy in breast cancer. In one design, porous polymer implants integrate a synthetic collagen-mimetic peptide, IL-15 superagonist, and membrane-bound ligands coupling anti-CD3, anti-CD28, and anti-CD137 antibodies. When implanted in a mouse breast cancer resection model, these implants led to increased T cell proliferation, biased toward memory phenotypes, and prevented tumor relapse [73]. Such approaches could support treatment of inoperable and incompletely removed tumors because scaffolds can be placed directly at the resection site to serve as a depot.
Biomaterials Can Promote Tolerance in Autoimmunity, Allergies, and Transplantation
In addition to driving proinflammatory responses as above, biomaterials are also emerging as a powerful tool to promote regulatory responses for tolerance. Dysregulation in tolerance can lead to autoimmune disease – where self-molecules are attacked, allergies, and rejection of transplants. Generally, the treatments for these conditions are immunosuppressants, or slow build-up of tolerance with sensitization (e.g., for allergies). While these approaches are beneficial, they are generally nonspecific, have off target effects, or require life-long treatment. For example, in multiple sclerosis (MS) – a disease in which myelin lining the central nervous system is attacked, even monoclonal antibodies do not distinguish between healthy and self-reactive immune cells. Thus, treatments are not curative and can leave patients immunocompromised. In this final area of review, there are two general approaches: (i) use of biomaterials to change how self-antigens are processed; and (ii) co-delivery of self-antigens with cues that alter the response to self-antigen. Below we discuss how biomaterials are being used in model tolerance systems, and in disease models of MS, diabetes, allergies, and transplantation.
Biomaterials to Promote Tolerance in Model Systems
Model systems of tolerance are being used to generate insights that can then be applied to different diseases [74]. In one example, PLGA NPs were used for intravenous and subcutaneous delivery of rapamycin to induce tolerance against coadministered model antigens [75]. These NPs targeted rapamycin selectively to APCs, in contrast to free rapamycin that was distributed systemically, acting on multiple cell types. Importantly, this work demonstrated an effective and safe way to induce antigen-specific tolerance in mice and nonhuman primates. Knowledge gained using biomaterials in model platforms provides the basis for expansion to disease models.
Biomaterial Immunotherapies in Mouse Models of MS
Experimental autoimmune encephalomyelitis (EAE) and relapsing-remitting (RR)-EAE are common mouse models of progressive- and RR-MS, respectively. In several recent reports, particles are used to deliver self-antigens to promote tolerance by changing how they are processed or the cells internalizing the particles [76– 79]. In particular, self-antigen encapsulated or displayed on NPs can enhance the trafflcking of these antigens to domains in LNs or spleen where APCs expressing scavenger receptors reside (Figure 2H). The hypothesis is that antigens engaging these receptors are processed through tolerogenic pathways associated with clearance of apoptotic host cell debris, against which tolerance is generally desired. In a variety of autoimmune models, this approach reverses or eliminates disease – paralysis, in the case of EAE. Self-antigens have also been conjugated to red blood cells, which are normally recycled at high rates, and thus also prone to clearance through protolerogenic pathways [80,81].
Understanding the specific design features that lead to tolerance is important in moving from empirical methodologies to rational design. Recently, monodispersed quantum dots (QDs) were used to demonstrate that the density at which self-antigen is displayed on NPs correlates with the degree of tolerance, even when the total number of self-peptides is fixed [79]. In particular, mice with EAE exhibit greater therapeutic effects when infused with a higher number of QDs displaying lower densities of self-antigen compared with fewer QDs each displaying a higher density of self-antigen. In a different mechanistic study, NPs coated with self-antigens bound to MHC-II molecules were used to directly engage T cell receptors (i.e., without APC interaction) [82,83]. This approach expanded antigen-specific T regulatory (Treg) cell levels in mouse models of MS, diabetes, and arthritis. Importantly, the work revealed molecular features impacting T cell fate, with the dose of peptide: MHC controlling the degree of T cell expansion, while the density of the complexes on the NP surface dictated the extent of polarization to Treg cells [83]. Thus for both strategies targeting APCs [79] or T cells [83], design features such as display density play a crucial role – parameters difficult or impossible to control with traditional therapies.
Biomaterials can also be exploited to co-deliver cues with self-antigens to alter the antigen-specific immune response [84,85]. Recently, intra-LN injection of polymeric particles encapsulating self-antigen was used to study tolerance with respect to the local structure and function of the LN microenvironment [78]. Treatments with particles coencapsulating self-antigen and rapamycin led to local reorganization of treated nodes, as well as other distant nodes, systemic expansion of Treg cells, and permanent reversal of paralysis in mice with EAE. Thus, local reprogramming of the LN environment led to tolerance that was systemic, but antigen-specific. Self-assembly has been used for co-delivery as well, promoting tolerance with carrier-free vaccines that eliminate the intrinsic immunogenic effects of biomaterials discussed earlier [86,87]. TLR signaling is overactive in many human autoimmune diseases, yet bio-materials can activate TLRs. In one study, iPEM capsules, built from self-antigen and regulatory antagonists of TLR signaling, eradicated paralysis in mice during EAE, and, in samples from human MS patients, reduced myelin-specific inflammatory responses [86].
Biomaterial Immunotherapies for Allergies
Immunotherapies, such as allergy shots, are already established in treating allergies. However, frequent trips to a physician’s office are required, and treatment is slow, sometimes ineffective, and difficult to pinpoint to specific antigens. Particle encapsulation is being investigated because it offers a controlled release platform to reduce adverse reactions to concentrated allergens and allow for coencapsulation of other immune cargos to direct the response away from hypersensitivity [88– 90]. These approaches use many strategies already discussed, with one theme emerging as polarization of T cell response away from Th2 responses to allergens.
Biomaterial Immunotherapies for Diabetes
Clinically, diabetes is often treated with frequent insulin injections, but several biomaterial approaches are being tested, particularly for type 1 diabetes. In one strategy a hybrid scaffold-particle encapsulated denatured insulin antigen and a peptide hydrogel containing GM-CSF [91]. This hybrid system reduced type 1 diabetes in mice compared to controls; possibly through establishment of a microenvironment that recruits and expands immune cells in granulomas observed during treatment. Using platforms discussed earlier, disease-associated antigens linked to red blood cells can maintain normoglycemia in a mouse model of type 1 diabetes [80,81], while NPs displaying peptide: MHC complexes potently expanded antigen specific Treg cells to control diabetes in mice [82].
Biomaterial Immunotherapies for Transplantation
Similar to treatments for autoimmune diseases, transplant rejection is treated with immunosuppressive drugs that cause systemic side effects. Several material platforms involve co-delivering replacement tissue or cells with immunomodulatory factors before or during transplantation, or using materials for immune isolation [92,93]. Recently, scaffolds have been used to target immune signals and drugs [94– 96]. For example, a self-assembled hydrogel loaded with immunosuppressant was used for triggered release in response to proteolytic enzymes overexpressed during inflammation. Relative to soluble drug, injection of the hydrogel sustained drug levels in tissue and increased vascular allograft survival in mice [95]. No evidence of systemic toxicity was observed with either formulation. Other work has loaded peptide antigens in PLG NPs to improve long-term engraftment of bone marrow in mice by changing how the antigen is processed [97], while MPs containing an immunosuppressive drug have been coated with LN-targeting antibodies to prolong survival in a heart allograft model [98].
Concluding Remarks
Biomaterials offer unique opportunities compared with existing vaccines and immunotherapies. However questions remain (see Outstanding Questions): can biomaterials eliminate the need for multiple vaccinations through controlled cargo release? What are the long-term effects of biomaterials on disease development and progression as these materials persist and degrade to specific byproducts, and are there considerations with respect to multiple exposures across a lifetime? Furthermore, it remains unclear how readily biomaterial vaccines and immunotherapies can be extended to humans, from the mouse models used in most studies. The impact of physicochemical properties of materials on specific immune pathways must also be developed. Still, biomaterials allow better control over responses to antigens, adjuvants, or immunomodulators and can be used to target these cues to particular tissues or cell populations, or to modify immune cells or pathogens. Each of these ideas are being explored across infectious disease, cancer, and tolerance, highlighting one final feature of biomaterials – they are platform technologies for extension to a variety of diseases. An important goal is to develop the fundamental understanding needed to create design rules for selecting materials that enable efficient, rational strategies that accelerate translation to the clinic.
Outstanding Questions.
How can the physicochemical properties of biomaterials be effectively decoupled to create design rules for different applications?
Can the need for multiple vaccination and booster injections be eliminated through slowly degrading biomaterials?
How can biomaterials be used to enable more rational approaches to vaccine and immunotherapy design?
How readily can biomaterial platforms be scaled up for use in large animals and humans, and how can the need for this effort be broadly encouraged?
Are there long-term effects of biomaterials on disease development and progression?
Trends.
Biomaterials have intrinsic immunogenic features (size, shape, and chemistry) that can be harnessed to create carriers that actively direct responses to vaccines and immunotherapies, or to modify immune cell function in vivo.
Biomaterials can provide control over the combinations and relative concentrations of ligands to simultaneously target multiple immune populations and pathways, or to target these signals to specific cells, organelles, or tissues.
In addition to immunogenic properties, biomaterials can support decreased systemic effects and pain, improved cargo stability, and enable self-administration in developing geographic regions.
Increased collaboration between material scientists and immunologists may enable the fast integration of emerging understanding in both fields.
Acknowledgments
C.M.J. is a research scientist at the VA Maryland Health Care System, Baltimore, MD, USA. This work was supported in part by NSF CAREER Award Number 1351688, the National Multiple Sclerosis Society Award Number RG-1501-02968, NIH Number 1R01AI062765, NIH Number 1R01AI114496, the Damon Runyon Foundation Number DRR3415, the Juvenile Diabetes Research Foundation Number 2-SRA-2016-319-S-B. M.L.B. is a trainee of the NIH T32 Host-Pathogen Interaction Fellowship (Number AI089621). S.J.T. is a trainee of the NIH/NCI Cancer Research Fellowship (Number 43479). C.M.J. is a Young Investigator of the Alliance for Cancer Gene Therapy (Number 15051543) and the Melanoma Research Alliance (Number 348963).
Footnotes
Disclaimer Statement
The contents of this work do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.Gammon JM, et al. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget. 2016;7:15421–15443. doi: 10.18632/oncotarget.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tostanoski LH, et al. Engineering tolerance using bio-materials to target and control antigen presenting cells. Discov Med. 2016;21:403–410. [PubMed] [Google Scholar]
- 3.Sahdev P, et al. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao K, et al. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Adv Mater. 2014;26:6530–6541. doi: 10.1002/adma.201402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YC, et al. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indermun S, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 8.Bracho-Sanchez E, et al. Micro and nano material carriers for immunomodulation. Am J Transplant. 2016;16:3362–3370. doi: 10.1111/ajt.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andorko JI, et al. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J. 2015;17:323–338. doi: 10.1208/s12248-014-9708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp FA, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudalla GA, et al. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat Mater. 2014;13:829–836. doi: 10.1038/nmat3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, et al. Polyelectrolyte multilayers assembled entirely from immune signals on gold nanoparticle templates promote antigen-specific T cell response. ACS Nano. 2015;9:6465–6477. doi: 10.1021/acsnano.5b02153. [DOI] [PubMed] [Google Scholar]
- 13.Chiu YC, et al. Modular vaccine design using carrier-free capsules assembled from polyionic immune signals. ACS Biomater Sci Eng. 2015;1:1200–1205. doi: 10.1021/acsbiomaterials.5b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratner BD, et al. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press; 2004. [Google Scholar]
- 15.Murphy K, Weaver C. Janeway’s Immunobiology. Garland Science; 2016. [Google Scholar]
- 16.Meyer RA, et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–1525. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wibroe PP, et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol. 2017;12:589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- 18.Sunshine JC, et al. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35:269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, et al. Shape-dependent activation of cytokine secretion by polymer capsules in human monocyte-derived macrophages. Biomacromolecules. 2016;17:1205–1212. doi: 10.1021/acs.biomac.6b00027. [DOI] [PubMed] [Google Scholar]
- 20.Irvine DJ, et al. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy CL, et al. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J Immunol. 2013;191:5278–5290. doi: 10.4049/jimmunol.1203131. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, et al. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220:141–148. doi: 10.1016/j.jconrel.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 23.Moyano DF, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahbazi MA, et al. Surface chemistry dependent immunostimulative potential of porous silicon nanoplatforms. Biomaterials. 2014;35:9224–9235. doi: 10.1016/j.biomaterials.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Wen Y, et al. Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano. 2016;10:9274–9286. doi: 10.1021/acsnano.6b03409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhoef JJ, et al. Iron nanomedicines induce Toll-like receptor activation, cytokine production and complement activation. Biomaterials. 2017;119:68–77. doi: 10.1016/j.biomaterials.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Andorko JI, et al. Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 2016;32:24–34. doi: 10.1016/j.actbio.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andorko JI, et al. Impact of molecular weight on the intrinsic immunogenic activity of poly (beta amino esters) J Biomater Res A. 2016;105:1219–1229. doi: 10.1002/jbm.a.35970. [DOI] [PubMed] [Google Scholar]
- 29.Bailey BA, et al. Toward a single-dose vaccination strategy with self-encapsulating PLGA microspheres. Adv Healthc Mater. 2017;6:1601418. doi: 10.1002/adhm.201601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin JO, et al. Modular delivery of CpG-incorporated lipid-DNA nanoparticles for spleen DC activation. Biomaterials. 2017;115:81–89. doi: 10.1016/j.biomaterials.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Li AV, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dierendonck M, et al. Nanoporous hydrogen bonded polymeric microparticles: facile and economic production of cross presentation promoting vaccine carriers. Adv Funct Mater. 2014;24:4634–4644. [Google Scholar]
- 33.Gause KT, et al. Codelivery of NOD2 and TLR9 ligands via nanoengineered protein antigen particles for improving and tuning immune responses. Adv Funct Mater. 2016;26:7526–7536. [Google Scholar]
- 34.Chiu YC, et al. Assembly and immunological processing of polyelectrolyte multilayers composed of antigens and adjuvants. ACS Appl Mater Interfaces. 2016;8:18722–18731. doi: 10.1021/acsami.6b06275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohsen MO, et al. Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J Control Release. 2017;251:92–100. doi: 10.1016/j.jconrel.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Lynn GM, et al. In vivo characterization of the physico-chemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson MC, et al. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33:861–868. doi: 10.1016/j.vaccine.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RB, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53. doi: 10.1016/j.biomaterials.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhakal S, et al. Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J Control Release. 2017;247:194–205. doi: 10.1016/j.jconrel.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Karch CP, et al. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomedicine. 2017;13:241–251. doi: 10.1016/j.nano.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Rouphael NG, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by micro-needle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390:649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mistilis MJ, et al. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 2016:1–11. doi: 10.1007/s13346-016-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arya J, et al. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeMuth PC, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013;12:367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeMuth PC, et al. Vaccine delivery with microneedle skin patches in nonhuman primates. Nat Biotechnol. 2013;31:1082–1085. doi: 10.1038/nbt.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, et al. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv Funct Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stary G, et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chahal JS, et al. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 2017;7:252. doi: 10.1038/s41598-017-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, et al. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci Adv. 2016;2:e1600264. doi: 10.1126/sciadv.1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wafa EI, et al. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017;50:417–427. doi: 10.1016/j.actbio.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SY, et al. Immune complexes mimicking synthetic vaccine nanoparticles for enhanced migration and cross-presentation of dendritic cells. Adv Funct Mater. 2016;26:8072–8082. [Google Scholar]
- 54.Huang B, et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7:291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith TT, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali OA, et al. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bencherif SA, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun. 2015;6:7556. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith TT, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 2017;127:2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu Q, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15:2732–2739. doi: 10.1021/acs.nanolett.5b00570. [DOI] [PubMed] [Google Scholar]
- 60.Zeng Q, et al. In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater Sci Eng. 2017;3:195–205. doi: 10.1021/acsbiomaterials.6b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo M, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gammon JM, et al. Low-dose controlled release of mTOR inhibitors maintains T cell plasticity and promotes central memory T cells. J Control Release. 2017;263:151–161. doi: 10.1016/j.jconrel.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuai R, et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moynihan KD, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kosmides A, et al. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials. 2017;118:16–26. doi: 10.1016/j.biomaterials.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi GN, et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, et al. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 69.Oberli MA, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kranz LM, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 71.Yuan H, et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763–769. doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 72.Chen Q, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stephan SB, et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearson RM, et al. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Adv Drug Deliv Rev. 2017;114:240–255. doi: 10.1016/j.addr.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kishimoto TK, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol. 2016;11:890–899. doi: 10.1038/nnano.2016.135. [DOI] [PubMed] [Google Scholar]
- 76.Getts DR, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunter Z, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8:2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tostanoski LH, et al. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 2016;16:2940–2952. doi: 10.1016/j.celrep.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hess KL, et al. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. Adv Funct Mater. 2017;27:1700290. doi: 10.1002/adfm.201700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kontos S, et al. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013;110:E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pishesha N, et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci U S A. 2017;114:3157–3162. doi: 10.1073/pnas.1701746114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clemente-Casares X, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 83.Singha S, et al. Peptide– MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017;12:701–710. doi: 10.1038/nnano.2017.56. [DOI] [PubMed] [Google Scholar]
- 84.Gammon JM, et al. Controlled delivery of a metabolic modulator promotes regulatory T cells and restrains autoimmunity. J Control Release. 2015;210:169–178. doi: 10.1016/j.jconrel.2015.05.277. [DOI] [PubMed] [Google Scholar]
- 85.Northrup L, et al. Combining antigen and immunomodulators: emerging trends in antigen-specific immunotherapy for autoimmunity. Adv Drug Deliv Rev. 2016;98:86–98. doi: 10.1016/j.addr.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Tostanoski LH, et al. Design of polyelectrolyte multilayers to promote immunological tolerance. ACS Nano. 2016;10:9334–9345. doi: 10.1021/acsnano.6b04001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hess KL, et al. Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity. Biomaterials. 2017;118:51–62. doi: 10.1016/j.biomaterials.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smarr CB, et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre-and postsensitization. Proc Natl Acad Sci U S A. 2016;113:5059–5064. doi: 10.1073/pnas.1505782113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srivastava KD, et al. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol. 2016;138:536–543e4. doi: 10.1016/j.jaci.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 90.Maldonado RA, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoon YM, et al. A combination hydrogel microparticle-based vaccine prevents type 1 diabetes in non-obese diabetic mice. Sci Rep. 2015;5:13155. doi: 10.1038/srep13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zakrzewski JL, et al. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32:786–794. doi: 10.1038/nbt.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung L, et al. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Verbeke CS, et al. Multicomponent injectable hydrogels for antigen-specific tolerogenic immune modulation. Adv Healthc Mater. 2017;6:1600773. doi: 10.1002/adhm.201600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gajanayake T, et al. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci Transl Med. 2014;6:249ra110. doi: 10.1126/scitranslmed.3008778. [DOI] [PubMed] [Google Scholar]
- 96.Zhang W, et al. Biopatterned CTLA4/Fc matrices facilitate local immunomodulation, engraftment, and glucose homeostasis after pancreatic islet transplantation. Diabetes. 2016;65:3660–3666. doi: 10.2337/db16-0320. [DOI] [PubMed] [Google Scholar]
- 97.Hlavaty KA, et al. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2016;76:1–10. doi: 10.1016/j.biomaterials.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azzi J, et al. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 2016;15:1202–1213. doi: 10.1016/j.celrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niikura K, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]