Abstract
Background and Purpose
Although diabetes mellitus is an established independent risk factor for ischemic stroke, the association between fasting blood glucose and intracerebral hemorrhage is limited and inconsistent. The objectives of the current study was to examine the potential impact of long-term fasting blood glucose concentration on subsequent risk of intracerebral hemorrhage.
Methods
This prospective study included 96,110 participants of the Kailuan study, living in Kailuan community, Tangshan city, China, who were free of cardiovascular diseases and cancer at baseline(2006).Fasting blood glucose concentration was measured in 2006, 2008, 2010, and 2012. Updated cumulative average fasting blood glucose concentration was used as primary exposure of the current study. Incident intracerebral hemorrhage from 2006 to 2015 was confirmed by review of medical records.
Results
During 817,531 person-years of follow-up, we identified 755 incident intracerebral hemorrhage cases. The nadir risk of intracerebral hemorrhage was observed at fasting blood glucose concentration of 5.3 mmol/L. The adjusted hazard ratios and their 95% confidence intervals (CIs) of intracerebral hemorrhage were 1.59(95% CI, 1.26–2.02) for diabetes or fasting blood glucose ≥7.00 mmol/L, 1.31(95%CI, 1.02–1.69) for impaired fasting glucose (fasting blood glucose 6.10–6.99 mmol/L), 0.98(95% CI: 0.78–1.22) for fasting blood glucose 5.60–6.09 mmol/L, and 2.04 (95%CI, 1.23–3.38) for hypoglycemia (fasting blood glucose <4.00 mmol/L), comparing with normal fasting blood glucose 4.00–5.59 mmol/L. The results persisted after excluding individuals who used hypoglycemic, aspirin, antihypertensive agents, or anticoagulants, and those with intracerebral hemorrhagic cases occurred in the first two years of follow-up.
Conclusions
In this large community-based cohort, low (<4.0 mmol/L) and high (≥6.1 mmol/L) fasting blood glucose concentrations were associated with higher risk of incident intracerebral hemorrhage, relative to fasting blood glucose concentrations of 4.00–6.09 mmol/L.
Introduction
Intracerebral hemorrhage (ICH) accounts for approximately 10–15% of all strokes1, 2 and is disproportionately associated with higher mortality and worse functional outcomes than other forms of stroke3. There are no proven interventions to improve the clinical course when ICH occurs and mortality rate due to ICH has remained unchanged in recent decades4, with an even increasing trend reported in some Asian, African, and South Americans Countries5. Therefore, prevention of ICH through understanding and reduction of risk factors has significant implications for public health and clinical practice. This is particularly important for populations where prevalence of vascular risk factors and incidence of ICH are higher, such as eastern Asia as well as African-Americans and Hispanics in the US1, 5, 6.
Although diabetes mellitus is an established independent risk factor for ischemic stroke7, evidence regarding diabetes and ICH risk remains limited. Diabetes could be a potential risk factor for ICH because it leads to atherosclerotic aneurysm and small vessel disease 8–11 and coexists with hypertension, a well-known risk factor for ICH. However previous epidemiological studies 7, 12–15, including 2 meta-analyses 7, 12, have generated mixed results regarding the associations between diabetes and ICH. These studies were limited by small numbers of incident ICH cases in each individual study7, 12, retrospective study design15, and failure to confirm the ICH cases by review of medical records13, 14. Further, low glucose concentrations could be also associated with higher ICH risk because it may be a marker of disease burden or frailty and because it may provoke a surge of sympatho-adrenergic activity and subsequently increase risk for ICH. This notion is supported by the observations that individuals with hypoglycemia have a higher risk for cardiovascular events and death, relative to those with normal glucose status 12, 16, 17. However, the potential impact of hypoglycemia on ICH risk remains unknown.
We, therefore, prospectively examined the potential impact of FBG levels on ICH risk in over 96,000 adults. FBG concentration were measured every two years starting in 2006 (i.e., baseline) through 2012. To reduce within-subject variation and better represent long-term FBG status, we used the updated cumulative average FBG concentration as the primary exposure. Because the etiology and risk factors of ICH may vary based on their location18, in secondary analyses, we examined the relationship between FBG and the ICH location. We also examined whether baseline and the most recent glucose concentration, respectively, were associated with risk of incident ICH.
Methods
Study design and population
The Kailuan study is a prospective study including 101,510 participants (81,110 men and 20,400 women, aged 18–98 years old), started in2006. 19, 20,21. All participants were followed biennially to update information on potential risk factor and newly diagnosed disease. In the current analyses, we excluded 1,239 participants who missed baseline FBG data. We further excluded 4,161 participants with a history of stroke, myocardial infarction, or cancer prior to baseline, because a prior diagnosis of, or active treatment for, cancer and cardiovascular disease could influence current glycemic control, leaving 96,110 participants in the current analyses. The detailed information regarding study design and participants can be found in the Supplemental Text.
Assessment of incident ICH
The outcome was the first occurrence of ICH, either nonfatal or ICH death. We linked all participants to the Municipal Social Insurance Institution and/or all of the Discharge Register of the 11 Hospitals to retrieve incidence of ICH, according to the nineth and tenth version of International Classification of Diseases (ICD-9 Code #: 431 and ICD-10 code #: I61). We further searched the death certificates from provincial vital statistics offices in order to add the fatal ICHs out of the hospitals. Additionally, information regarding past medical history of stroke was collected via questionnaire biennially since 2006 in order to identify the ICH cases which did not admit to the local hospitals. In the current analysis, we did not include traumatic intracranial hemorrhages and epidural, subdural, subarachnoid hematomas.
For all potential ICH cases identified by the ICD code, death certificates, and/or questionnaire, two physicians including experienced neurologists reviewed the medical records and judged the case annually, blinded to the FBG concentration. In case of disagreement, the third physicians had been consulted and made the final consensus. Fatal ICH cases were confirmed by medical records, autopsy reports or death certificates with ICH listed as the primary cause. A nonfatal ICH was defined as the sudden onset of a focal neurological deficit and demonstration of acute primary intraparenchymal hemorrhage on either brain computed tomography or magnetic resonance imaging, which was available for all suspected nonfatal ICH cases. ICH was diagnosed according to the World Health Organization criteria.
In 2015, an experienced radiologist examined available brain scan imagines and recorded the locations. ICH location was ascertained only when the report or review of the imaging clearly confirmed the location of ICH. As an example if the radiologist reported only mentioned a left frontal hemorrhage and we did not have images to review, we did not include such a patient in the secondary analyses by hemorrhage location, as this hemorrhage might have been in a deep or non-deep region of the frontal lobe. We included 425 out of 755 incident ICH patients with clear information on parenchymal location of hematomas in our secondary analysis. The hematomas were divided into two categories based on location: deep (i.e. basal, ganglia, thalami, cerebellar, and brainstem) or non-deep hematomas (i.e. lobar).
Assessment of FBG and Covariates
Fasting blood samples were collected in the morning after an 8- to 12-hour overnight fast and transfused into vacuum tubes containing EDTA (Ethylene Diamine Tetra Acetic acid). An auto analyzer (Hitachi 747; Hitachi, Tokyo) was used to measure FBG with the hexokinase/ glucose-6-phosphate dehydrogenase method in 2006, 2008, 2010, and 201221. The coefficient of variation using blind quality control specimens was <2.0%.
To represent long-term glucose patterns of individuals, we calculated updated cumulative average FBG concentration using all available FBG measurements from 2006 to the end of follow up. For example, the incident ICH from 2008 to 2010 was related to the average concentration of FBG in 2006 and 2008. We divided the cohort into five categories using four FBG cutoff points, which were based on current definitions of hypoglycemia (<4.00 mmol/L 22), normal FBG(4.00–5.59 mmol/L23), upper range of normal (5.60–6.09 mmol/L), impaired fasting glucose (6.10–6.99 mmol/L24), and diabetes(≥7.0 mmol/L). Participants with physician-diagnosed diabetes or use of hypoglycemic medications were assigned to the ≥7.00 mmol/L group. We also used baseline and the most recent FBG concentration, respectively, as secondary exposures.
Information on potential covariates was collected in 2006 and updated every two years thereafter 19, 21 and can be found in the Supplemental Text.
Statistical Analysis
All analyses were conducted using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). Two-sided P<0.05 was considered statistically significant. We selected potential confounders a priori based on available literature and assessment of a causal diagram. The Cox proportional hazards model was used to investigate the association between FBG and ICH risk, after adjustment for potential confounders, including baseline age, sex, smoking, alcohol intake, education, physical activity, sodium intake, and family income; updated information on use of antihypertensive, aspirin and lipid-lowering medications during following up; and updated cumulative average of systolic blood pressure, diastolic blood pressure, body mass index(BMI), estimated glomerular filtration rate, and blood concentration of high-density lipoprotein cholesterol(HDL-C), low-density lipoprotein cholesterol(LDL-C), triglycerides, and high sensitive C-reactive protein(hs-CRP) from baseline to the end of follow up. The proportional assumption was satisfied. Continuous variables were divided into categories based on related clinical cut points or quintile. Missing data of every these covariates were coded as extra category.
The dose-response relationship between FBG and ICH were also evaluated by restricted cubic spline25, with 5 knots defined at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of the FBG distribution.
We examined whether the potential impact of FBG on ICH varied according the hematoma location (deep vs non-deep). As the small number of incident ICH cases with non-deep hematomas (n=61), we grouped participants into three FBG categories (hypoglycemic <4.00, normal glycemic 4.00–6.09, and hyperglycemic ≥6.10 mmol/L) for this secondary analysis.
We used likelihood ratio tests to examine potential interactions between FBG concentration and the sex, age(<60 vs. ≥60 y), hypertension(yes/no), and BMI (<25, 25–29, or ≥30 kg/m2) in relation to ICH risk, adjusting for aforementioned covariates.
Because altered fasting glucose concentration might be the consequence of impending ICH (i.e., reverse causality), we conducted a 2-year lag analysis by excluding incident ICH cases from the first 2 years of follow-up. To explore whether the potential association between FBG and ICH risk was confounded by medications, we conducted sensitivity analyses by excluding the participants who used aspirin, antihypertensive, or hypoglycemic medications, separately. Because anticoagulants could increase the risk of ICH, we excluded participants with atrial fibrillation or flutter, deep venous thrombosis, pulmonary infarction, or heart valve disease, which are major indications for use of anticoagulants. We also restricted the analyses to 73,687 participants with complete data on all covariates, excluded 7 cases without autopsy or CT/MRI imaging, and further included 1536 participants with history of MI or cancer at baseline in sensitivity analyses.
Results
During 817,531 person-years (median 9.0 years and interquartile range from 8.7 to 9.2 years) of follow-up, we identified 755 incident ICH cases. Higher FBG concentration was associated with higher prevalence of use of antihypertensive, aspirin, or lipid lowering medications, and higher levels of BMI, blood pressure and lipids. Individuals with FBG <4.0mmol/L (n=687) were more likely to be older, have lower education, BMI and LDL-C, and higher hs-CRP concentration, relative to those with normal FBG (Table 1).
Table 1.
Baseline (2006) characteristics according to updated cumulative average fasting blood glucose concentration, among 96,110 Kailuan participants
| <4.00 mmol/L | 4.0–5.59 mmol/L | 5.60–6.09 mmol/L | 6.10–6.99 mmol/L | ≥7.00 mmol/L | |
|---|---|---|---|---|---|
| n | 687 | 65412 | 12622 | 6331 | 11058 |
| FBG*, mmol/L | 3.75 | 5.01 | 5.80 | 6.43 | 8.72 |
| Age#, year | 60.4 | 54.3 | 56.6 | 57.9 | 60.0 |
| Women#, % | 18.6 | 22.8 | 14.8 | 12.8 | 19.2 |
| Smoking status#, % | |||||
| Current | 33.6 | 32.6 | 37.3 | 37.3 | 30.7 |
| Past | 7.3 | 4.7 | 6.1 | 5.8 | 6.8 |
| Never | 52.3 | 60.0 | 54.6 | 54.6 | 59.1 |
| Alcohol intake#, % | |||||
| Never | 56.0 | 58.4 | 51.5 | 53.2 | 58.6 |
| Past | 6.3 | 2.9 | 3.3 | 3.6 | 4.9 |
| Light† | 3.8 | 5.7 | 5.6 | 4.1 | 3.4 |
| Moderate† | 6.1 | 3.9 | 4.2 | 3.8 | 3.7 |
| Heavy† | 12.1 | 15.3 | 21.4 | 21.4 | 16.1 |
| Physical activity#, % | |||||
| Never | 6.4 | 8.1 | 10.7 | 10.2 | 6.9 |
| 1–2 times/week | 70.3 | 75.0 | 70.2 | 70.4 | 70.0 |
| 3+ times/week | 15.4 | 13.4 | 16.2 | 16.0 | 18.2 |
| Sodium intake#, % | |||||
| ≥10 gram/day | 7.4 | 9.6 | 11.9 | 11.8 | 11.0 |
| 6–9 gram/day | 76.9 | 78.3 | 76.1 | 75.8 | 75.1 |
| <6 gram/day | 7.7 | 8.6 | 9.1 | 9.0 | 9.1 |
| Education#, % | |||||
| Illiteracy or elementary school | 23.3 | 8.6 | 11.6 | 12.7 | 12.7 |
| Middle school | 64.2 | 80.2 | 80.7 | 80.0 | 78.7 |
| College /university | 5.1 | 7.9 | 4.9 | 4.0 | 4.0 |
| Average income#, % | |||||
| <500¥/month | 23.7 | 26.4 | 32.0 | 31.9 | 27.0 |
| 500–2999¥/ month | 60.8 | 63.6 | 58.9 | 59.6 | 61.9 |
| ≥3000¥/ month | 8.0 | 6.6 | 6.1 | 5.1 | 6.4 |
| Deep venous thrombosis or pulmonary infarction‡ | 0.00 | 0.18 | 0.17 | 0.09 | 0.26 |
| Heart valve disease‡ | 0.15 | 0.21 | 0.15 | 0.16 | 0.35 |
| Atrial fibrillation or flutter§‡ | 2.04 | 0.64 | 0.77 | 0.82 | 1.08 |
| Use of antihypertensive agent‡, % | 14.6 | 15.3 | 21.5 | 23.7 | 36.2 |
| Use of lipid-lowering medications‡, % | 0.58 | 1.49 | 2.34 | 1.86 | 5.58 |
| Use of hypoglycemic medications‡, % | -- | -- | -- | -- | 46.9 |
| Use of aspirin‡ | 1.02 | 0.90 | 1.20 | 1.03 | 2.18 |
| hs-CRP*, mg/mL | 1.45 | 0.99 | 1.12 | 1.22 | 1.46 |
| BMI*, Kg/m2 | 23.7 | 24.6 | 25.5 | 25.8 | 26.1 |
| LDL-C*, mmol/L | 1.98 | 2.46 | 2.63 | 2.67 | 2.60 |
| HDL-C*, mmol/L | 1.58 | 1.52 | 1.52 | 1.52 | 1.48 |
| TC*, mmol/L | 4.65 | 4.92 | 5.14 | 5.25 | 5.24 |
| TG*, mmol/L | 1.39 | 1.54 | 1.79 | 2.00 | 2.15 |
| SBP*, mmHg | 132 | 129 | 136 | 139 | 140 |
| DBP*, mmHg | 82 | 83 | 86 | 88 | 87 |
| eGFR*, mL/min/1.73m2 | 78.1 | 85.4 | 84.9 | 83.2 | 81.7 |
Updated cumulative average from baseline to the end of follow up
Measured at baseline
Light drinker: 0.1–0.4 servings/day for women and 0.1–0.9 serving/day for men; moderate: 0.5–1.5 servings/day for women and 1–2 serving/day for men; heavy: >1.5 servings/day for women and >2 serving/day for men; based on 15g of alcohol per day.
Updated duing the follow-up.
Diagnosis of atrial fibrillation or flutter was based on 12-lead electrocardiogram and self-reported physician-diagnosis history and was updated by every survey from 2006.
Abbreviations: hs-CRP, high sensitive C-reactive protein; BMI, body mass index; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
We observed a U-shaped relation between FBG concentration and ICH risk, with the lowest risk at a FBG of 5.3 mmol/L (Figure 1 & Table 2). Similar results were observed in the sensitivity analysis by excluding those who used hypoglycemic, aspirin, or antihypertensive medications at the baseline or during follow-up, excluding ICH onset during the first 2 years of follow-up, excluding participants who may use anticoagulants (Table 2), excluding participants with any missing data on the covariates, excluding cases without autopsy or CT/MRI imaging, or further including participants with history of MI or cancer at baseline ( Supplemental Table I).
Figure 1.
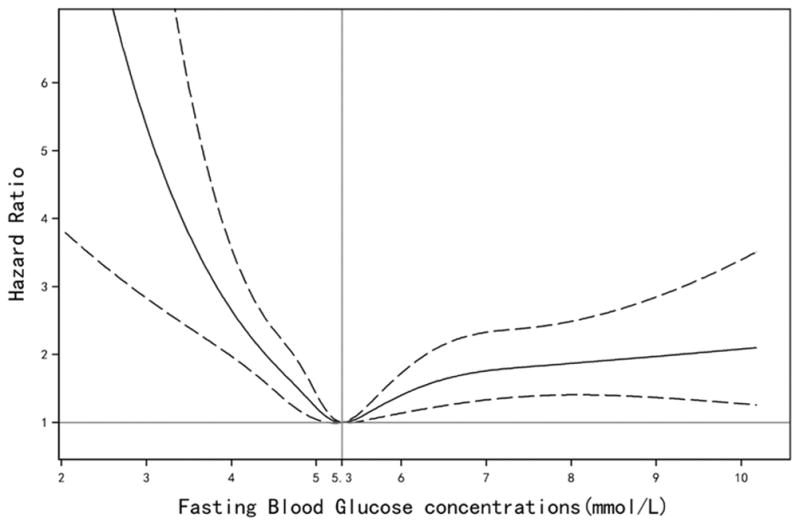
Adjusted hazard ratios of intracerebral hemorrhage according to updated cumulative average fasting blood glucose concentration. Model adjusted for age, sex, smoking, alcohol intake, education, physical activity, average monthly income of each family member, sodium intake, updated use of antihypertensive, aspirin, hypoglycemic, and lipid-lowering medications, and updated cumulative average body mass index, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high sensitive C-reactive protein, systolic blood pressure, diastolic blood pressure, and estimated glomerular filtration rate. Data were fitted by a restricted cubic spline Cox proportional hazards model. The 95% confidence intervals are indicated by the dashed lines.
Table 2.
Adjusted hazard ratio of intracerebral hemorrhage, according to updated cumulative average fasting blood glucose concentration
| <4.00 mmol/L | 4.00–5.59 mmol/L | 5.60–6.09 mmol/L | 6.10–6.99 mmol/L | ≥7.00 mmol/L | |
|---|---|---|---|---|---|
| # of Case / population | 16/687 | 437/65412 | 94/12622 | 72/6331 | 136/11058 |
| Incidence rate, per 1000 person-year | 3.04 | 0.78 | 0.88 | 1.35 | 1.47 |
| Age- and sex-adjusted hazard ratio | 2.95(1.78–4.86) | 1.00 | 1.05(0.84–1.31) | 1.55(1.20–1.99) | 1.69(1.39–2.05) |
| Multivariate-adjusted hazard ratio 1* | 2.81(1.70–4.65) | 1.00 | 1.07(0.85–1.33) | 1.56(1.21–2.00) | 1.68(1.38–2.03) |
| Multivariate-adjusted hazard ratio 2† | 2.04(1.23–3.38) | 1.00 | 0.98(0.78–1.22) | 1.31(1.02–1.69) | 1.59(1.26–2.02) |
| Sensitivity analyses | |||||
| 2-year lag analysis†‡ | 1.70(0.84–3.46) | 1.00 | 1.15(0.88–1.49) | 1.29(0.94–1.78) | 1.96(1.49–2.58) |
| Excluding hypoglycemic-users† | 2.00(1.20–3.31) | 1.00 | 0.97(0.77–1.21) | 1.29(1.00–1.66) | 1.56(1.23–1.98) |
| Excluding antihypertensive-users† | 2.32(1.32–4.07) | 1.00 | 1.16(0.89–1.50) | 1.47(1.09–1.99) | 1.77(1.34–2.33) |
| Excluding aspirin-users† | 1.26(0.76–2.10) | 1.00 | 0.96(0.77–1.21) | 1.21(0.94–1.56) | 1.47(1.16–1.86) |
| Excludingpotential† anticoagulant-users§† | 1.19(0.70–2.00) | 1.00 | 0.93(0.74–1.18) | 1.20(0.93–1.55) | 1.44(1.13–1.83) |
Multivariate-adjusted hazard ratio 1* adjusted for age (year), sex, smoking (current, past, or never), alcohol intake (never, past, light, moderate, or heavy), education (illiteracy/elementary school, middle school, or college/university), physical activity (never, sometimes, or active), average monthly income of each family member (<500, 500–2999, or ≥3000¥), and sodium intake (≥10.0, 6.0–9.9, or <6.0 gram/day).
Multivariate-adjusted hazard ratio 2† included variables in model 1 and further adjusted for updated use of antihypertensive, aspirin, hypoglycemic, and lipid-lowering medications (yes/no for each), and updated cumulative average body mass index (≥30.0, 25.0–29.9, or <25.0 kg/m2), triglycerides(quintile), high-density lipoprotein cholesterol(quintile), low-density lipoprotein cholesterol(quintile), high sensitive C-reactive protein (<1.00, 1.00–2.99, or≥3.00mg/ml), systolic blood pressure(quintile), diastolic blood pressure(quintile), and estimated glomerular filtration rate(quintile).
excluded person time and ICH events from the first 2 years of follow-up
excluded participants with deep venous thrombosis, pulmonary infarction, heart valve disease, or atrial fibrillation or flutter
In a secondary analysis, we found that FBG < 4.0 mmol/L tended to have higher risk of both deep and non-deep location of ICH. Interestingly, hyperglycemia was associated with higher risk of ICH with deep hematoma (adjusted HR=1.43; 95%CI: 1.08–1.88) but not with non-deep ICH (adjusted HR=0.99; 95%CI: 0.58–1.68) (Supplemental Table II).
We observed that relationships between FBG and ICH were modified by sex, and hypertension (P-interaction<0.05 for both), but not by age and BMI. The association between hyperglycemia and ICH risk was more pronounced in women, relative to men(Figure 2). Participants without hypertension and FBG <4.0 mmol/L had a more than 2-fold increased risk of ICH, and a strong gradient across elevated FBG categories, whereas the relationship was less convincing for those with hypertension(Figure 2).
Figure 2.
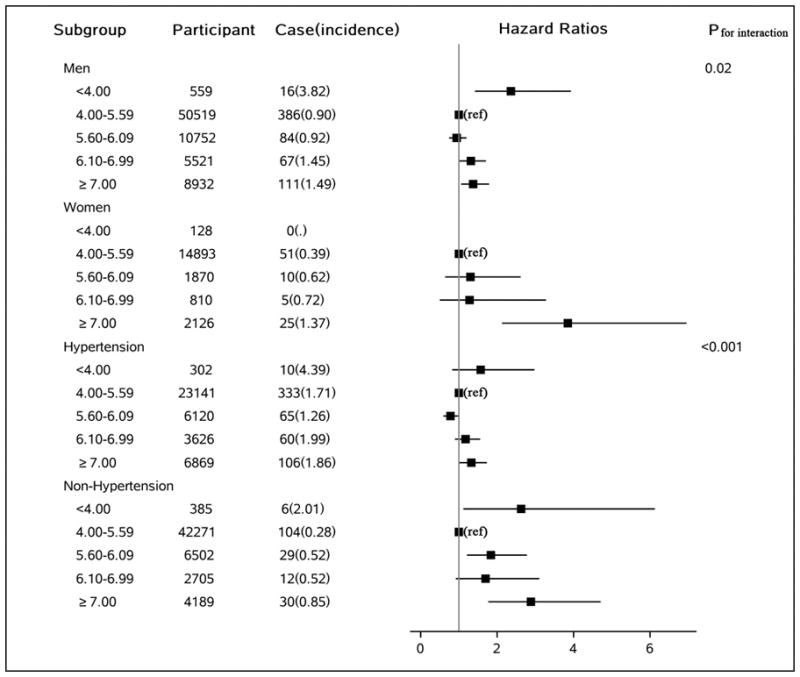
Adjusted hazard ratio of intracerebral hemorrhage by sex or hypertension, according to updated cumulative average fasting blood glucose concentration(mmol/L). p value is for interaction test. Model adjusted for age, sex, smoking, alcohol intake, education, physical activity, average monthly income of each family member, sodium intake, updated use of antihypertensive, aspirin, hypoglycemic, and lipid-lowering medications, and updated cumulative average body mass index, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high sensitive C-reactive protein, systolic blood pressure, diastolic blood pressure, and estimated glomerular filtration rate. Hypertension was defined as a systolic equal or higher than 140mmHg or a diastolic blood pressure equal or higher than 90mmHg or use antihypertensive agents.
When we used the baseline (2006) FBG as exposure, we found that FBG ≥5.60 mmol/L, but not FBG <4.00 mmol/L, was associated with increased ICH risk (Supplemental Table III), relative to FBG of 4.00–5.59 mmol/L. However, the most recent FBG concentration was not significantly associated with altered short-term (0.1–1.9 year) ICH risk.
Discussion
In this large prospective study including 96,110 participants, we observed that FBG levels both in the hypoglycemic and hyperglycemic range are associated with increasd risk of incident ICH, after adjustment for potential confounders. These results obtained from a prospective observational study of an unprecedentedly large community dwelling cohort have important implications for prevention of ICH.
We found that the risk for ICH increased in participants with FBG of 6.1mmol/L or higher. Our observations are consistent with a prior meta-analysis of diabetes and ICH12 (pooled HR=1.56). However, only 2 out of 79 included studies in this meta-analysis had more than 100 ICH cases, and the small number of ICH patients in these individual studies may preclude the accurate estimate of the association between diabetes and ICH. Nevertheless, this meta-analysis clearly reported the need for more powerful analyses to reliably characterize the strength of associations in ICH.
Our new finding that FBG of 6.1–6.9 mmol/L, which falls in the range of impaired fasting glucose26 was associated with higher ICH risk is consistent with previous observations regarding prediabetes and total stroke. A meta-analysis27 of 5 prospective cohort studies found that individuals with FBG of 6.1–6.9 mmol/L had a 21% increased risk of total stroke; however, ICH was not separately analyzed in this study. Consistently, in a recent study conducted among Chinese adults without known diabetes, per 1-mmol/L higher usual random plasma glucose level was associated with 5% increased risk of ICH.14 However, random plasma glucose is limited by its great intra-individual variation and might not accurately reflect an individual’s longer-term status.
Several biological mechanisms could explain the observed association between high FBG and ICH. Abnormal glucose metabolism could impair normal endothelial function9, and subsequently lead to cerebral small vessel disease(SVD)8. Degenerative changes in the walls of cerebral small vessels could cause ischemic lacunar infarcts, fibrinoid necrosis, microaneurysm formation and ICH10. The two most common causes of ICH in elderly can be distinguished by the location of ICH in most cases, i.e. deep ICH mostly caused by hypertensive SVD whereas lobar ICH mostly resulting from cerebral amyloid angiopathy, the latter caused by accumulation of amyloid-beta in cortical and leptomeningeal vessel walls11. An important support to the view that FBG abnormalities can mechanistically contribute to the risk of ICH comes from our secondary analysis of ICH locations. The association between hyperglycemia and increased risk was found only for ICHs in deep locations. SVD driven by classical vascular risk factors, hypertension being the best known, is almost invariably the cause of such deep ICHs and our results suggest that hyperglycemia might be a contributing factor. The number of incident lobar ICH was relatively small and it is impossible to prove or rule out a contribution from FBG disturbances on the risk of lobar ICH at this time.
Significant associations between high FBG and ICH risk were only observed when we used baseline or cumulative average, rather than the most recent FBG concentration (updated every 2 years), as the predictor. This finding suggests that chronic exposure, rather than short-term exposure, to hyperglycemia could result in unfavorable effects on ICH, supporting the potential causal association between high FBG and ICH risk. This finding also shows that any future study to confirm this association should include FBG assessments at multiple time-points rather than relying on a single FBG measurement. Significant sex-differences in the FBG-ICH relation observed in the current study are consistent with a systematic review,7 in which the risk of total stroke associated with diabetes was significantly higher in women than men. However, most of the included studies focused on the total stroke rather than ICH. Further studies are warranted to replicate our observations.
In the current study, low FBG concentration (<4.0 mmol/L) was also associated with higher risk for ICH, consistent with a previous study13. Low FBG could lead to activation of the sympathetic nervous system and adrenergic hormone release which might induce vasoconstriction and hemodynamic changes, resulting in several ICH-related physiological changes. Alternatively hypoglycemia may be a marker of disease burden or frailty. In our study, hypoglycemia was associated with older age and lower levels of BMI and LDL, which were associated with higher ICH risk in previous studies28. Consistently, we found that the association between low FBG and ICH was more pronounced among non-hypertensive participants. However, our results should be interpreted with caution because the significant association was only seen when we used cumulative average FBG as the exposure and only 687 participants (0.45% of the population) with FBG <4.0 mmol/L. Therefore, we cannot exclude the possibility of a chance finding. Another possible interpretation is that the hypoglycemia-ICH relationship could be partially due to use of aspirin29 and anticoagulants30, which could lead to bleeding and hypoglycemia31. This is indicated by our sensitivity analyses in which excluding aspirin-users and those who might use anticoagulants attenuated the association between hypoglycemia and ICH greatly.
Our study has several limitations. Oral glucose tolerance testing, casual random glucose, and HbA1C concentration were not available in the Kailuan study and some cases of diabetes or pre-diabetes cases could therefore have been missed. However, repeated assessment of these indices in a large community-based cohort was unfeasible. Use of cumulative average of FBG in our study would reduce random errors greatly. We did not collect information on use of non-aspirin antithrombotic drugs. However, in the primary analysis we excluded participants with a history of cardiovascular disease at baseline and further excluded the participants with atrial fibrillation or flutter, deep venous thrombosis, pulmonary infarction, or heart valve disease in the sensitivity analysis, which are major indications for anticoagulant use. The current study included only Chinese adults living in the Kailuan community who had higher incidence of ICH and the similar prevalence of diabetes comparing with Caucasians32, thus it may not be generalizable to other populations. However, the biological effects of FBG on ICH in this cohort should be the same as those among men and women in general. Prior studies clearly showed a higher prevalence of ICH in eastern Asian populations including China, it is therefore plausible that our cohort would be representative of a high ICH risk population where knowledge of these findings would be most valuable. Another limitation is that we only included small number of women (n=19,827). However, in the subgroup analysis, we still observed significant association between high FBG and ICH risk. Further, for the ICH location analyses, we did not include ICH cases when specific location of deep or not deep was not clearly confirmed, which could underestimate the prevalence of lobar ICH. Because we started to document the ICH location in 2015 and we did not obtain the brain imagines for some cases. Only one experienced radiologist examined all these imagines, misclassification of outcome is thus inevitable. Due to these limitations, our findings regarding FBG and ICH location should be interpreted with caution.
Conclusions
Both low and high FBG concentrations were associated with higher future risk of ICH in this large community-based cohort. The lowest risk of ICH was observed among those in the normal FBG range (4.00–6.09 mmol/L). Replication of our findings in other ethnic populations with assessment of other markers of glycemic status, such as HbA1C, may strengthen the link between abnormal glycemic control and ICH risk.
Supplementary Material
Acknowledgments
Sources of Funding: The study was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by Grant 2015085 from the Doris Duke Charitable Foundation, by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL111771 and by the NINDSational Institute of Neurological Disorders and Stroke (R21NS087235-02).
Footnotes
Disclosures: None
References
- 1.Tsai C-F, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in chinese vs white populations a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks a population-based estimate of temporal trends in stroke incidence from the greater cincinnati/northern kentucky stroke study. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. The Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahuranec DB, Lisabeth LD, Sánchez BN, Smith MA, Brown DL, Garcia NM, et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82:2180–2186. doi: 10.1212/WNL.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Mensah GA, Norrving B, Murray CJ, Roth GA. Atlas of the global burden of stroke (1990–2013): The gbd 2013 study. Neuroepidemiology. 2015;45:230–236. doi: 10.1159/000441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Wu B, Wang W-Z, Lee L-M, Zhang S-H, Kong L-Z. Stroke in china: Epidemiology, prevention, and management strategies. The Lancet Neurology. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 7.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. The Lancet. 2014;383:1973–1980. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 8.Nezu T, Hosomi N, Aoki S, Kubo S, Araki M, Mukai T, et al. Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertension Research. 2015;38:291–297. doi: 10.1038/hr.2015.4. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61:2993–2997. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. New England Journal of Medicine. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 11.Haley KE, Greenberg SM, Gurol ME. Cerebral microbleeds and macrobleeds: Should they influence our recommendations for antithrombotic therapies? Current cardiology reports. 2013;15:1–7. doi: 10.1007/s11886-013-0425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C, Guallar E, Linton JA, Lee D-C, Jang Y, Son DK, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988–1993. doi: 10.2337/dc12-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bragg F, Li L, Bennett D, Guo Y, Lewington S, Bian Z, et al. Association of random plasma glucose levels with the risk for cardiovascular disease among chinese adults without known diabetes. JAMA cardiology. 2016;1:813–823. doi: 10.1001/jamacardio.2016.1702. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (interstroke): A case-control study. The Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 16.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes care. 2011;34:S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. New England Journal of Medicine. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 18.Zebian B, Critchley G. Spontaneous intracranial haemorrhage. Surgery (Oxford) 2015;33:363–368. [Google Scholar]
- 19.Wu Z, Jin C, Vaidya A, Jin W, Huang Z, Wu S, et al. Longitudinal patterns of blood pressure, incident cardiovascular events, and all-cause mortality in normotensive diabetic people. Hypertension. 2016;68:71–77. doi: 10.1161/HYPERTENSIONAHA.116.07381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke; a journal of cerebral circulation. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: The kailuan prospective study. International journal of epidemiology. 2015;44:689–699. doi: 10.1093/ije/dyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: A report of a workgroup of the american diabetes association and the endocrine society. Diabetes care. 2013;36:1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.TAS Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37:S81. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization; Geneva: 2013. 2006. [Google Scholar]
- 25.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The sas lgtphcurv9 macro. Boston, MA: Channing Laboratory; 2011. [Google Scholar]
- 26.Genuth S, Alberti K, Bennett P, Buse J, DeFronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes care. 2003;26:3160–3168. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 27.Lee M, Saver JL, Hong K-S, Song S, Chang K-H, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: Meta-analysis. BMj. 2012;344:e3564. doi: 10.1136/bmj.e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroll ME, Green J, Beral V, Sudlow CL, Brown A, Kirichek O, et al. Adiposity and ischemic and hemorrhagic stroke: Prospective study in women and meta-analysis. Neurology. 2016;87:1473–1481. doi: 10.1212/WNL.0000000000003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: A meta-analysis of randomized controlled trials. JAMA : the journal of the American Medical Association. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 30.García-Rodríguez LA, Gaist D, Morton J, Cookson C, González-Pérez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81:566–574. doi: 10.1212/WNL.0b013e31829e6ffa. [DOI] [PubMed] [Google Scholar]
- 31.Romley JA, Gong C, Jena AB, Goldman DP, Williams B, Peters A. Association between use of warfarin with common sulfonylureas and serious hypoglycemic events: Retrospective cohort analysis. BMJ. 2015;351:h6223. doi: 10.1136/bmj.h6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai C-F, Anderson N, Thomas B, Sudlow CL. Risk factors for ischemic stroke and its subtypes in chinese vs. Caucasians: Systematic review and meta-analysis. International Journal of Stroke. 2015;10:485–493. doi: 10.1111/ijs.12508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


