Abstract
Dysfunction of the meibomian glands alters secreted meibum quantitatively and qualitatively that can lead to damage to the ocular surface epithelium. In response to an unstable tear film cause by meibomian gland dysfunction, ocular surface epithelium is damaged and expresses inflammatory cytokines leading to secondary ocular inflammation. In turn, inflammatory disorders of the palpebral conjunctiva and lid margin may affect the structure and function of meibomian gland. The disorders include allergic conjunctivitis, long-term usage of contact lenses, dermatological diseases that affect conjunctival homeostasis, Stevens-Johnson’s syndrome or chemical burning of the ocular surface and lid margin.
Keywords: Meibomian gland, Ocular surface inflammation, Contact lens, Conjunctivitis, Rosacea, Chemical burn
1. Introduction
Meibomian glands locate to the tarsal plates of the upper and lower eyelids and the orifice of the gland is opened at the lid margin. The glands play a critical role in ocular surface homeostasis. Lipid components secreted by the gland function as a barrier to prevent excess evaporation of the water component of the tear fluid. Therefore, dysfunction of the meibomian glands (MGD) leads to an impairment of ocular surface lubrication and wetness (dry eye syndrome) as well as induction of local inflammatory responses in the ocular surface. In turn, Suzuki et al. (2015) proposed a concept of one unit, i.e., the “meibomian gland and ocular surface” by encountering patients with meibomitis (an inflammatory form of MGD) of which successful management is essential to the treatment of inflammation in the ocular surface.
The etiology of MGD includes terminal duct obstruction and/or qualitative or quantitative changes in the glandular lipid secretion, impairing ocular surface homeostasis. In turn, we have noticed that primary ocular surface inflammation may also effect the function of the meibomian glands. In the present article we review the abnormalities of the meibomian glands in association with primary ocular surface inflammatory diseases.
2. Conjunctivitis and meibomian gland
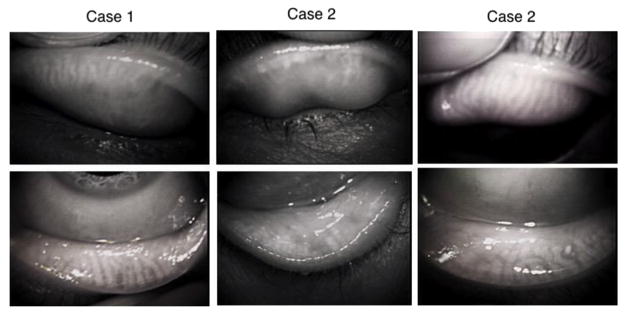
It was reported that conjunctivitis affects the structure of meibomian glands as revealed by non-invasive meibography. Distortion of meibomian gland ducts were found to be significantly greater in patients with allergic conjunctivitis (Fig. 1, case 1) or atopic-keratoconjunctivitis (Ibrahim et al., 2012). Conjunctival inflammation could be associated with the meibomian gland abnormalities in eyelids of the patients with vernal keratoconjunctivitis (Wei et al., 2015). Continuous mechanical stress to the tarsal tissue by eye rubbing might be one of the causes (Arita et al., 2010; Ibrahim et al., 2012). Rynerson and Perry proposed a clinical entity named as DEBS (dry eye and blepharitis). Blepharitis caused by bacterial colonization in the lid margin could cause follicular inflammation, then MGD (Rynerson and Perry, 2016). Blepharokeratoconjunctivitis also causes loss of the gland as evaluated by non-invasive meibography (Yin and Gong, 2016). It was reported that treatment of posterior blepharitis and conjunctival inflammation due to MGD were treated with topical 1% azithromycin suppressed expression levels of IL-1β, IL-8, and MMP-9 mRNA. On the other hand, expression of TGFβ1 increased during treatment and does not decline after drug withdrawal, suggesting this growth factor contribution to the anti-inflammatory activity of azithromycin in MGD (Zhang et al., 2015). Because in other tissue TGFβ1 could be pro-inflammatory, it is to be further investigated if cytokines produced by inflammatory cells in the conjunctiva/sub-conjunctiva directly damage the acini via palpebral conjunctiva.
Fig. 1.
Morphologically abnormal configuration of meibomian glands in a 35-year-old male patient with chronic allergic conjunctivitis (case 1), a long-term (approximately 20 years) female user (39-year-old) of a soft contact lens (case2) and a 74-year-old male patient with a long-term application of antiglaucoma medications (details unknown) (case 3).
3. Contact lens wearing
Contact lens wearing impairs the function of meibomian gland. Mechanical stress and/or chronic, sub-clinical inflammation could be proposed as underlying mechanism, although it is to be further investigated detailed pathobiology of conjunctival inflammation associated with long-term contact lens wearing. One of the commonly observed characteristics in contact lens-related meibomian gland pathology was orifice pouting/plugging (Cox et al., 2016). Inflammation in the meibomian glands can be detected as punctate hyperreflective components by using in vivo laser scanning confocal microscopy (Fasanella et al., 2016). This technique of observation showed the presence of gland dropout, duct obstruction, and glandular inflammation in meibomian glands of contact lens wearers (Villani et al., 2011).
Soft contact lens could induce allergic reaction in the ocular surface epithelium or palpebral conjunctiva. This might also be the case in contact lens-related allergic conjunctivitis; the condition is associated with an increase in meibomian gland distortion (Fig. 1, case 2). The authors conclude that the allergic reaction, rather than contact lens wear, appears to be responsible for abnormal configuration of meibomian gland in patients with contact lens-related allergic conjunctivitis (Arita et al., 2012).
4. Medication-related meibomian gland diseases
Topical instillation of drugs might also cause ocular surface inflammation, leading secondarily to the damage of meibomian gland. For example, obstructive type of meibomian gland disorders were observed in patients treated with topical anti-glaucoma prostaglandin derivatives (Fig. 1, case 3) (Mocan et al., 2016). Although the exact mechanism underlying the condition is to be investigated, it is known that prostaglandin derivatives could damage the ocular surface epithelium resulting in the induction of local inflammation.
Currently, there is no evidence showing systemic medication induced MGD is due to inflammation. However, it was suggested that systemic medication also could damage the gland or impair its secretory function. We reported patients with loss of meibomian glands with superficial punctate epitheliopathy during treatment of cancer with an anticancer TS-1® combination capsules of tegafur, gimeracil, and oteracil potassium (Taiho Pharmaceutical Co. Ltd, Japan) (Mizoguchi et al., 2015) (Fig. 2). The possible mechanism underlie TS-1®-related MGD might be similar to the obstruction of drainage system or corneal epithelial disorders associated with systemic TS-1, but not via direct toxicity of the drug to the acinar cells. 5-FU leaked to tear fluid might cause local inflammation and obstruction of the orifice of the gland. On the other hand, the influence of inflammatory fibrotic diseases to the structure or function of meibomian glands is to be further examined. Another case is long-standing consequences of isotretinoin use. Without a significant finding in either gland or eyelid margin the secretory function of these glands is impaired, causing dry eye symptoms (Moy et al., 2015).
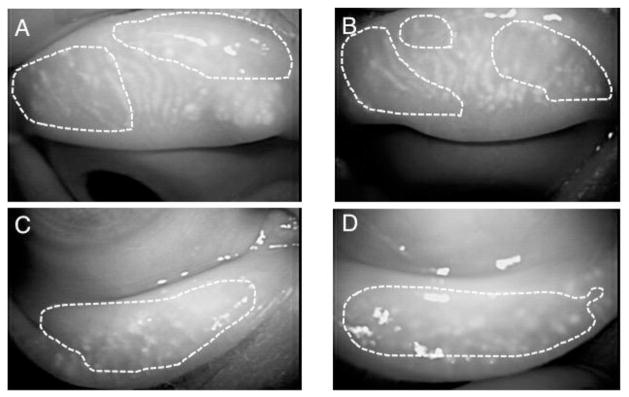
Fig. 2.
Infra-red noninvasive meibography finding of a 59-year-old patient with systemic TS-1 anti-cancer treatment for gallbladder cancer. The meibo-score (Arita et al., 2008) are 3 in all of upper and lower eyelids of both eyes. A, B: right; C, D: left; A, C: upper eyelids; B, D: lower eyelids (reproduced from Mizoguchi et al., 2015).
5. Dermatological disorders and meibomian gland pathology
Rosacea is a skin disorder of which etiology has not yet been uncovered. Abnormality in the innate immune system, dysregulated growth of commensal skin organisms might have roles in the pathogenesis of rosacea (Two et al., 2015). Microbial agents including commensal Demodex mites, Demodex-associated bacteria, Staphylococcus epidermidis, etc, are suggested to be involved in the pathological mechanism of rosacea. Acne rosacea could cause inflammation in the eyelid and ocular surface. It was reported that rosacea is accompanied with significant loss of meibomian glands highly associated with eyelid margin inflammation. (Palamar et al., 2015; Machalińska et al., 2016; Liang et al., 2016). Two Demodex species (folliculorum and brevis) have been identified as causative mites of rosacea. The former one is primarily found in clusters around the root of lashes and lash follicles, whereas the latter is detected in the meibomian (and sebaceous) glands and is much closely related to recurrent chalazia, refractory keratoconjunctivitis or MGD (Cheng et al., 2015; Zhao et al., 2012; Liang et al., 2014). Possible mechanisms of induction of blepharitis and obstruction of meibomian glands include irritation of local tissue inflammation by mite wastes and debris as well as their eggs. However, detailed mechanism of the onset of blepharitis upon Demodex infection is still to be investigated (Czepita et al., 2007). The notion of involvement of rosacea in the health of Meibomian gland is further supported by the fact that rosacea is often associated with chalazion (Chamaillard et al., 2008). We also reported a case of multiple chalazion in a rosacea patient, suggesting the presence of involvement of meibomian gland (Shirai et al., 2015) (Fig. 3).
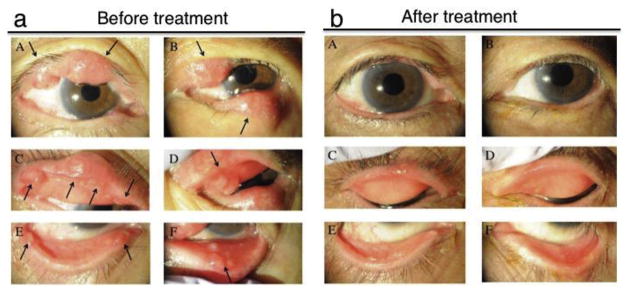
Fig. 3.
Multiple chalazion in a patient with acne rosacea. Chalazion are observed in upper and lower eyelids of both eyes of a 79-year-old woman suffering from acne rosacea (a). Black arrows indicate the chalazion. The patient was treated with ophthalmic ointment of erythromycin lactobionate, colistin sodium methanesulfonate and systemic administration of minocycline hydrochloride (b). A, C and E: right eye; B, D and F: left eye (reproduced from Shirai et al., 2015).
Psoriasis vulgaris is a skin disease characterized by chronic inflammation and keratinocyte hyperproliferation (hyperkeratinization) presumed to be associated with dysregulation of immune system. Jester et al. suggested that hyperkeratinization of the duct epithelium causes plugging, resultant dilation of the ducts and disturbance of the secretory function of the glands (Jester et al., 1989). Thus, hyperkeratinization in eyelid margin could affect the structure and function of meibomian glands by plugging the duct and impairing meibum secretion in patients with Psoriasis vulgaris (Zengin et al., 1996).
6. Stevens-Johnson syndrome and related diseases
Stevens-Johnson syndrome and its more severe form, toxic epidermal necrolysis are a set of diseases characterized by severe immune-based inflammation in mucosal tissue including conjunctiva. The onset of the disease is triggered by systemic medications or kinds of infection. Ocular surface is severely damaged first by acute inflammation, being followed by a chronic, longstanding complication of conjunctival scarring or conjunctivalization of the corneal surface with severe neovascularization. Detailed examination of the meibomian glands in patients of acute phase of Stevens-Johnson syndrome is not easy due to severe discharge and ocular surface pain. A similar damage to the ocular surface was recognized in patients with chronic graft-versus-host disease (Engel et al., 2015). In the later phase of the chronic stage of the disease, the ocular surface homeostasis is impaired with deficiency of limbal stem cells and dry eye caused by the loss of conjunctival goblet cells (Sotozono et al., 2007). It was reported that lid margin keratinization could lead to lipid tear deficiency (Di Pascuale et al., 2005). Meibography showed the total loss of meibomian glands in chronic, long-standing, cases of Stevens-Johnson syndrome. (Alsuhaibani et al., 2011). However, acute influence of severe conjunctival inflammation on the structure of meibomian glands has not been reported.
As mentioned above, examination of pathology of meibomian glands in patients of acute phase of Stevens- Johnson syndrome seems rare in daily medical service. On the other hand, an animal model of Stevens- Johnson syndrome has not been established, although animal experiments might be helpful in identifying alterations of the glands in the earlier phase of the disease after its onset. Chemical burn in the total ocular surface could mimic the situation of severe inflammation in the both cornea and conjunctiva of Stevens-Johnson syndrome, although the underlying mechanism of the inflammation differs from each other. We therefore employ this model of alkali burn of the ocular surface to investigate the acute influence of the conjunctival inflammation to the meibomian gland structure, although alkali might also directly damage the tissue.
7. Chemical burn in the ocular surface and meibomian gland
Chemical burn in the ocular surface often results in the formation of conjunctivalization of the corneal surface and neovascularization with severe dry eye symptoms (Saika et al., 2008; Bunker et al., 2014; Baradaran-Rafii et al., 2017; Saika et al., 2016). The loss of goblet cells and limbal stem cells are believed to largely contribute to these ocular surface changes. However, chemical burn in the area including eyelid margin could damage the orifice of the meibomian gland leading to obstruction of the orifice and impaired secretion of the meibum from the gland. Moreover, because the gland acini are located just beneath the conjunctiva, alkali exposure may also directly damage the gland or indirectly affect the tissue via induction of local inflammation. Fig. 4 shows a case of alkali burn in the eyelid margin. In this case the glands within the burned area were depleted. However, detailed evaluation of the changes in the meibomian gland following chemical injury needs further investigation.
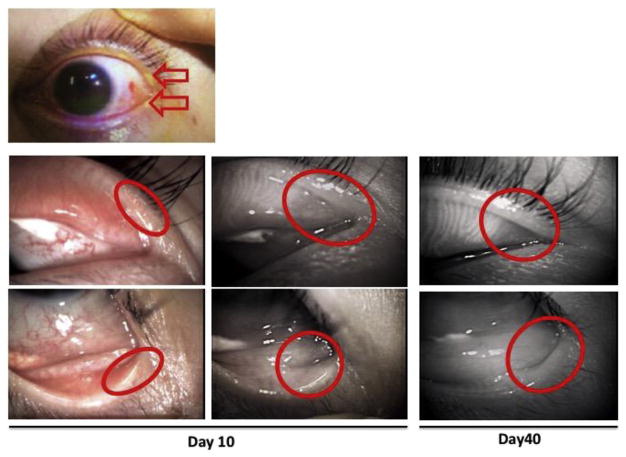
Fig. 4.
Alkali burn with an exposure to slaked lime (Calcium hydroxide) of the eyelid of a 33-year-old man. Meibomian glands of which orifices locate to the burned area (red circles) gradually disappeared at day 10 and 40 (reproduced from Mizoguchi, 2015).
To address this issue, we developed a model of ocular surface alkali burn in C57BL/6 mice (Mizoguchi, 2015). Severe inflammation in ocular surface including lid margin epithelium is induced by an exposure to alkali. The direct effects of alkali to the tissue and infiltration of inflammatory cells are considered to damage the tissue surrounding the orifice, resulting in the retention of the meibum in the duct. Scanning electron microscopy shows that the orifice appeared plugged with debris-like material (Fig. 5). The dilation of the ducts and partial loss of acini were observed in the upper eyelid at day 20 (Fig. 6A). On the other hand whole gland structure including ducts and acini were lost in the lower eyelid (Fig. 6B). Histology in the samples shown in Fig. 6A and B shows that the ducts were extensively dilated and the acinar cells seemed to be scattered among the dilated ducts (Fig. 6C and D). However, melanin pigments in the tissue in C57BL/6 mice disturbed the histological or immunohistochemical analysis of detailed mechanism of the tissue damage in the eyelid and meibomian glands, and an investigation in Balb c mice was performed with similar results (Mizoguchi et al., 2017). In this study we showed clearly the dilation of the ducts was occupied meibum accumulation. In the lower eyelid apoptosis-related acini destruction predominated with neutrophils infiltration (Mizoguchi et al., 2017). Three-dimensional reconstruction image shows that the unaffected meibomian gland consists of a duct and many acinar portion, and shows the loss of acini and dilation of ductal portion in day 20 specimen following an alkali exposure (Fig. 7) as compared with an uninjured gland (Parfitt et al., 2012).
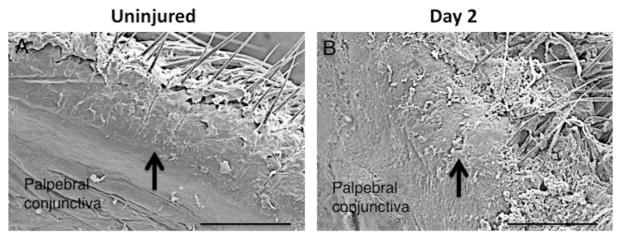
Fig. 5.
Scanning electron microscopy shows the orifice of the meibomian gland in the mouse upper eyelid (a). Two days after the alkali burn in ocular surface the orifice is found to be plugged with debris-like material (b).
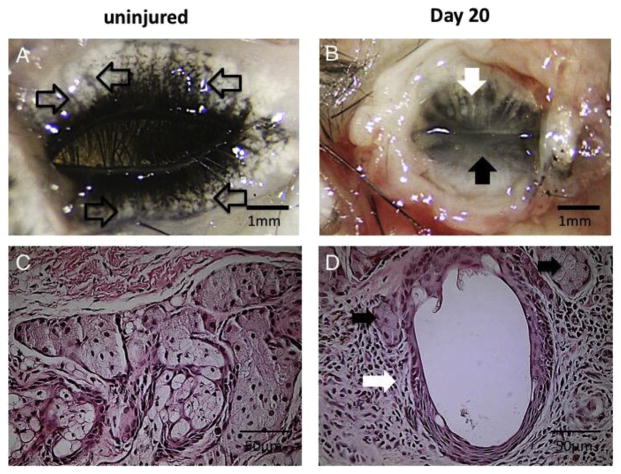
Fig. 6.
A. Eyelids of a C57BL/6 mouse. Eyelids were excised and observed from the behind. Acini were observed (open arrows). B. Meibomian glands of a C57BL/6 mouse 20 day s after ocular surface alkali burn. Loss of the acini and dilated ducts were observed in the upper eyelid (white arrow). In the lower eyelid meibomian gland was not observed (black arrow). C. Histology of meibomian glands of a C57BL/6 mouse. D. Histopathology of meibomian glands in the upper eyelid of a C57BL/6 mouse 20 days after ocular surface alkali burn. Largely dilated duct (open arrow) of the meibomian glands is observed, acinar cells (black arrows) are also seen among hypercellular mesenchyme (reproduced from Mizoguchi, 2015).
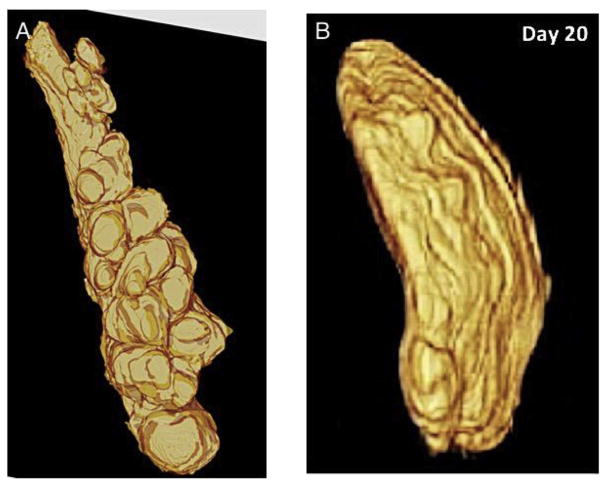
Fig. 7.
Three-dimensional reconstruction images of an uninjured meibomian gland (a) and the dilated duct of an eyelid specimen at 20 days after ocular surface alkali burn (b). Marked dilation with decreased number of acini are observed in a day 20 sample (a, reproduced from Parfitt et al., 2012).
The reason underlying the difference in damage to the meibomian gland between upper and lower eyelids is not known. One explanation may include differences in the degree of inflammation within the conjunctival/subconjunctival tissue due to the level of exposure to the alkali. Tear meniscus between ocular surface and lower eyelid might retain more alkali solution as compared with the space between upper eyelid and ocular surface. Moreover, tear fluid could wash out the alkali in the upper eyelid. Further study is to be conducted to uncover the reason of the difference of the damage patter between upper and lower eyelids.
8. Concluding remarks
The effect of ocular surface inflammation on meibomian gland function is not well understood. In this review we show that ocular surface and palpebral conjunctival including eyelid margin inflammation is associated with alteration in meibomian gland structure and potentially function. In the clinical setting it is recommended to examine the meibomian glands in patients with ocular surface inflammation and infection during the disease as well as after resolving the condition. Systemic and topical anti-inflammation treatment is to be included in strategies to maintain the function of meibomian glands besides targeting ocular surface inflammation.
Footnotes
This study was supported by a grant of Wakayama Medical Award for Young Researchers (to SM), Grants from the Ministry of Education, Science, Sports and Culture of Japan (C15K10876 to MK, C24791869 to TS, Start-up funding to HI, C25462729 to SS), and NEI Eye Research Grant EY021510 (Jester).
Financial interests
None for all the authors.
References
- Alsuhaibani AH, et al. Utility of meibography in the evaluation of meibomian glands morphology in normal and diseased eyelids. Saudi J Ophthalmol. 2011;25:61–66. doi: 10.1016/j.sjopt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita R, et al. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Arita R, et al. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea. 2010;29:858–860. doi: 10.1097/ICO.0b013e3181ca3668. [DOI] [PubMed] [Google Scholar]
- Arita R, et al. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol. 2012;56:14–19. doi: 10.1007/s10384-011-0103-6. [DOI] [PubMed] [Google Scholar]
- Baradaran-Rafii A, et al. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017;15:48–64. doi: 10.1016/j.jtos.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker DJ, et al. Alkali-related ocular burns: a case series and review. J Burn Care Res. 2014;35:261–268. doi: 10.1097/BCR.0b013e31829b0037. Review. [DOI] [PubMed] [Google Scholar]
- Chamaillard M, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167–171. doi: 10.1001/archdermatol.2007.50. [DOI] [PubMed] [Google Scholar]
- Cheng AM, et al. Recent advances on ocular Demodex infestation. Curr Opin Ophthalmol. 2015;26:295–300. doi: 10.1097/ICU.0000000000000168. Review. [DOI] [PubMed] [Google Scholar]
- Cox SM, et al. Margin and meibomian gland characteristics and symptoms in lens wearers. Optom Vis Sci. 2016;93:901–908. doi: 10.1097/OPX.0000000000000900. [DOI] [PubMed] [Google Scholar]
- Czepita D, et al. Demodex folliculorum and Demodex brevis as a cause of chronic marginal blepharitis. Ann Acad Med Stetin. 2007;53:63–67. Review. [PubMed] [Google Scholar]
- Di Pascuale MA, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912. doi: 10.1016/j.ophtha.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Engel LA, et al. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transpl. 2015;50:961–967. doi: 10.1038/bmt.2015.72. [DOI] [PubMed] [Google Scholar]
- Fasanella V, et al. In vivo laser scanning confocal microscopy of human meibomian glands in aging and ocular surface diseases. J Ophthalmol. 2016 doi: 10.1155/2016/7432131. ID7432131. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim OM, et al. In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology. 2012;119:1961–1968. doi: 10.1016/j.ophtha.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Jester JV, et al. Meibomian gland dysfunction. II The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30:936–945. [PubMed] [Google Scholar]
- Liang L, et al. High prevalence of demodex brevis infestation in chalazia. Am J Ophthalmol. 2014;157:342–348. doi: 10.1016/j.ajo.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Liang H, et al. In vivo confocal microscopy evaluation of ocular and cutaneous alterations in patients with rosacea. Br J Ophthalmol. 2016;2015:308110. doi: 10.1136/bjophthalmol-2015-308110. [DOI] [PubMed] [Google Scholar]
- Machalińska A, et al. Morphological and functional evaluation of meibomian gland dysfunction in rosacea patients. Curr Eye Res. 2016;41:1029–1034. doi: 10.3109/02713683.2015.1088953. [DOI] [PubMed] [Google Scholar]
- Mizoguchi S. Pathobiology of meibomian gland diseases. Ganka. 2015;57:1425–1430. in Japanese. [Google Scholar]
- Mizoguchi S, et al. Abnormalities in the meibomian glands in patients with oral administration of anticancer combination drug-capsule TS-1(®): a case report. BMC Cancer. 2015;15:796. doi: 10.1186/s12885-015-1781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi S, et al. Ocular surface alkali injury damages meibomian glands in mice. Ocul Surf. 2017 doi: 10.1016/j.jtos.2017.04.003. S1542-0124(16)30131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocan MC, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016;25:770–774. doi: 10.1097/IJG.0000000000000495. [DOI] [PubMed] [Google Scholar]
- Moy A, et al. Effects of isotretinoin on meibomian glands. Optom Vis Sci. 2015;92:925–930. doi: 10.1097/OPX.0000000000000656. [DOI] [PubMed] [Google Scholar]
- Palamar M, et al. Evaluation of dry eye and meibomian gland dysfunction with meibography in patients with rosacea. Cornea. 2015;34:497–499. doi: 10.1097/ICO.0000000000000393. [DOI] [PubMed] [Google Scholar]
- Parfitt GJ, et al. A novel immunofluorescent computed tomography (ICT) method to localise and quantify multiple antigens in large tissue volumes at high resolution. PLoS One. 2012;7:e53245. doi: 10.1371/journal.pone.0053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clin Opththalmol. 2016;10:2455–2467. doi: 10.2147/OPTH.S114674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, et al. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008;27:177–196. doi: 10.1016/j.preteyeres.2007.12.002. Review. [DOI] [PubMed] [Google Scholar]
- Saika S, et al. Modulation of Smad signaling by non-TGFβ components in myofibroblast generation during wound healing in corneal stroma. Exp Eye Res. 2016;142:40–48. doi: 10.1016/j.exer.2014.12.015. Review. [DOI] [PubMed] [Google Scholar]
- Shirai K, et al. A case of ocular rosacea with multiple chalazion. Ganka. 2015;57:1605–1609. in Japanese. [Google Scholar]
- Sotozono C, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114:1294–1302. doi: 10.1016/j.ophtha.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Suzuki T, et al. Meibomian glands and ocular surface inflammation. Ocul Surf. 2015;13:133–149. doi: 10.1016/j.jtos.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Two AM, et al. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72:749–758. doi: 10.1016/j.jaad.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Villani E, et al. In vivo confocal microscopy of meibomian glands in contact lens wearers. Invest Ophthalmol Vis Sci. 2011;52:5215–5219. doi: 10.1167/iovs.11-7427. [DOI] [PubMed] [Google Scholar]
- Wei Q, et al. In vivo confocal microscopy of meibomian glands and palpebral conjunctiva in vernal keratoconjunctivitis. Indian J Ophthalmol. 2015;63:327–330. doi: 10.4103/0301-4738.158073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Gong L. The evaluation of meibomian gland function, morphology and related medical history in Asian adult blepharokeratoconjunctivitis patients. Blepharokeratoconjunctivitis. Acta Ophthalmol. 2016 doi: 10.1111/aos.13136. (in press) [DOI] [PubMed] [Google Scholar]
- Zengin N, et al. Tear film and meibomian gland functions in psoriasis. Acta Ophthalmol Scand. 1996;74:358–360. doi: 10.1111/j.1600-0420.1996.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Effects of azithromycin on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease. JAMA Ophthalmol. 2015;133:1117–1123. doi: 10.1001/jamaophthalmol.2015.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YE, et al. Association of blepharitis with Demodex: a meta-analysis. Ophthalmic Epidemiol. 2012;19:95–102. doi: 10.3109/09286586.2011.642052. [DOI] [PubMed] [Google Scholar]