Abstract
To overcome egg protective vestments and ensure successful fertilization, mammalian spermatozoa switch symmetrical progressive motility to a powerful, whip-like flagellar motion, known as hyperactivation. The latter is triggered by a calcium influx through the sperm-specific, voltage-dependent, and alkalization-activated calcium channel of sperm - CatSper. The channel comprises nine subunits which together form a heteromeric complex. CatSper-deficient male mice and men with mutations in CatSper genes are infertile. This calcium channel is regulated by various endogenous compounds, such as steroids, prostaglandins, endocannabinoids, and intracellular pH. Being a sperm-specific ion channel that is not expressed anywhere else in the body, CatSper represents an ideal target for the development of female and even male contraceptives. In this review, we discuss the recent advances in studying CatSper functional properties and discuss future steps that are required to take in order to achieve a deep understanding of the molecular basis of CatSper function.
Keywords: Spermatozoa, sperm ion channels, flagellum, CatSper
1. Introduction
Reproduction is the key step to species survival. Sexual reproduction begins with fertilization - the fusion of the male (the spermatozoon) and the female (the ovum) gametes. In placental viviparous animals, fertilization takes place in the ampulla region of the oviduct - a place where the ovulated egg is stored for a short period of time and where it must be found and fertilized by a spermatozoon [1]. To ensure both arrival at the ampulla and fertilization, spermatozoa are dependent on rapid intraflagellar ion changes mediated by a select set of sperm ion channels that are diverse among species both in their molecular identity, as well as in their mode of regulation [2,3]. One of those ion channels is CatSper (CATion channel of SPERm) – a calcium channel of mammalian spermatozoa. CatSper allows calcium influx into the sperm tail that is needed to switch the flagellar waveform from a symmetrical, snake-like flagellar movement, toward a powerful whip-like motion called hyperactivation. This high-amplitude asymmetric flagellar bending together is initiated by: 1) intraflagellar alkalization [4]; 2) membrane depolarization [5], and by 3) an increase in the intracellular calcium concentration [6]. All these changes are under tight control of sperm ion channels and transporters. The hyperactivation is essential for sperm fertility since it enables them to de-attached from the ciliary oviductal epithelium, arrive to the ampulla in time, and later helps to overcome the protective vestments of the egg [7,8]. If sperm cannot hyperactivate, they are unable to fertilize an egg.
The human egg survival time upon ovulation is limited to 12 hours, after which it disintegrates, if not fertilized in time. It takes several hours for human spermatozoa to arrive at the site of fertilization and achieve full fertilizing capacity - a process known as capacitation [9,10]. The latter process comprises a number of physiological changes within the spermatozoon including intracellular alkalization, cholesterol removal from the sperm plasma membrane, and protein tyrosine phosphorylation. An enzyme responsible for the capacitation-associated tyrosine phosphorylation in murine spermatozoa - the tyrosine kinase FER-has been recently identified [11]. To ensure a timely arrival at the ampulla, spermatozoa develop robust motility and are able to overcome numerous obstacles presented by the female reproductive tract, and eventually by the egg's protective vestments. Sophisticated molecular navigation mechanisms that allow sperm cells to progress in the female reproductive tract include ATP-powered flagellar movement and rapid intraflagellar ion changes.
The sperm tail is subdivided into three functional parts: 1) the midpiece, 2) the principal piece, and 3) an endpiece. The majority of sperm ion channels that are functionally characterized to date are located along the principal piece ([12] and Figure 1), with an exception of the purinergic receptor/ion channel P2X2 that is localized in the midpiece of mouse sperm [13]. The progress made in super resolution imaging microscopy opened the door to a detailed characterization of flagellar ion channel distribution. For example, CatSper seems to be tethered to the underlying scaffold structure - the fibrous sheath - to ensure its orderly arrangement, and forms a quadrilateral structure along the sperm tail [14,15]. The CatSper channel is evolutionarily conserved in species from mammals to invertebrates [16], such as sea urchins and sea squirts [17,18], and even found in gametes of the basal fungus Allomyces macrogynus [17,18]. However, all nine CatSper genes are independently lost in several animal lineages, such as fish, amphibians, and birds [2]. So far, the only species with electrophysiologically confirmed CatSper currents are human [3,19], macaque [20], and mouse [15].
Figure 1. Ion channels of the human sperm flagellum.
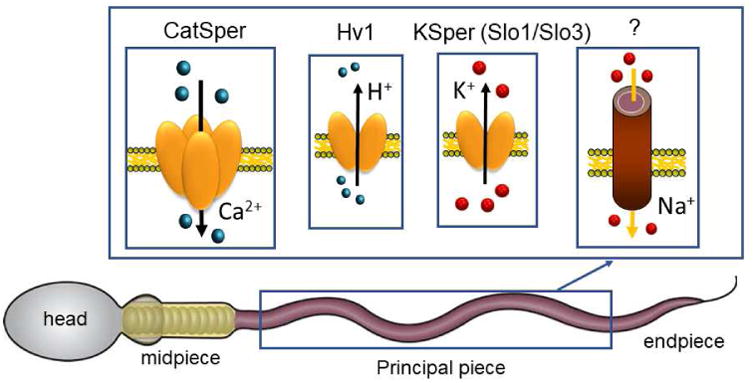
Schematic representation of a human spermatozoon with cellular compartments and distribution of ion channels found along the principal piece: CatSper, calcium channel; Hv1, voltage-gated proton channel; Slo1/Slo3, potassium channels; the identity of a Na+ channel or transporter is yet to be characterized.
2. CatSper: a principal calcium channel of sperm
The first CatSper gene - CatSper1- was cloned in 2001 as a result of the bioinformatics search for the gene sequences that resemble the pore of voltage-gated calcium channels [21]. CatSper is distantly related to the family of transient receptor potential (TRP) channels, and similarly contains six transmembrane (TM) segments with the ion selectivity pore that determines voltage-gated calcium channels. The CatSper channel is a sophisticated complex of nine different subunits: four alpha pore-forming subunits CatSper1 to 4 and five auxiliary subunits CatSper β, γ, and δ, epsilon and zeta [21-26]. The last two have been recently identified and have been shown to co-localize with the rest of the CatSper complex [15]. CatSper1, CatSper2, CatSper3, and CatSper4, are likely assembled as a heterotetramer [27]. Interestingly, each CatSper alpha subunit has different numbers of arginine (R)/lysine (K) residues in the S4 TM domain, with human CatSper1 displaying the strongest voltage sensor: xRxxKxxKxxRxxRxxRxxRx. Human CatSper2 has a slightly weaker one: xQxxRxxRxxRxxKxx, while human CatSper3 and 4 possess particularly weak voltage sensor domains: xxxRxxKxxx and xxxRxxRxxx, respectively. Such a diversity in the strength of the voltage sensors results in the channel retaining an ability to be voltage-dependent, but its voltage-gating abilities are nonexistent. Importantly, male mice [21,27] and humans deficient in Catsper exhibited infertility with no other phenotypical abnormalities, while female Catsper1-deficient mice are healthy and fertile [28,29]. The reports of a patient cohort with a deafness-infertility syndrome (DIS) described the small population of men who have a microdeletion in the 15q15.3 chromosomal region that removes CatSper2 (and causes infertility), as well as removes neighboring STRC gene [28,29]. The latter gene encodes for stereocilin- the protein that is associated with the bundle of the sensory hair cells in the inner ear. Deletion of STRC causes non-syndromic deafness. Therefore, additional deafness phenotype of DIS patients is not caused by a CatSper2 deletion, but stems rather from the absence of STRC. Interestingly, some DIS patients are also diagnosed with severe oligoasthenoteratozoospermia: few sperm cells, abnormal morphology and impaired motility, with 90% of their spermatozoa lacking the tail- a compartment where CatSper is expressed [30].
Being the principal calcium channel of sperm, CatSper is the main mechanism to bring extracellular calcium into the sperm tail, and is therefore vital for sperm fertility. CatSper-deficient sperm cells are unable to hyperactivate, and therefore fail to penetrate the egg's protective vestments [27]. All mammalian eggs are protected by a zona pellucida (ZP) whose thickness varies greatly among species. For example, in humans, the ZP thickness ranges between 10 and 31 μm with an average of 17.5 μm, while the mouse egg ZP is only 6.2 μm thick [31]. Since human sperm cells are the smallest of all species, the ratio of ZP thickness to sperm head size is therefore greater in humans than in mouse. The size of the head of human sperm is 5 μm, while the head of murine sperm is twice that size. Therefore, the [ZP thickness/sperm head size] ratio is 3.5 in human vs. 0.62 in mice. One can appreciate that such an arrangement creates a more difficult condition for human sperm cells to penetrate the ZP that can be overcome by an exceptional forceful asymmetrical bending of the sperm tail.
3. CatSper activators and inhibitors
Both primate and murine CatSper channels are activated by alkalization[5], but murine CatSper is insensitive to other activators of primate CatSper: progesterone and prostaglandins [12]. This may indicate species-specific adaptations of spermatozoa to adjust to a specific activator(s) within the female reproductive tract. The steroid hormone progesterone that is produced and released by cumulus cells surrounding the egg, is known to stimulate an immediate increase in intraflagellar Ca + in primate sperm cells that coincides with the onset of hyperactivated motility [20].
Human CatSper is particularly sensitive to progesterone and pregnenolone sulfate (PS), with an EC50 of 7 nM and 16 nM, respectively [32-34]. This fast activation happens in the absence of classical intracellular soluble secondary messengers, such as Ca2+, ATP, GTP, or cyclic nucleotides, suggesting that CatSper activation is initiated via a receptor directly associated with the CatSper channel and not through G-proteins or protein kinases. The progesterone receptor of human sperm cells has been recently identified as the α/β hydrolase domain-containing protein 2 (ABHD2; [19]). Binding of progesterone or PS to ABHD2 results in the activation of its enzymatic function and leads to the degradation of 2-arachidonoylglycerol (2-AG) that serves as a CatSper inhibitor [19,32]. Interestingly, apart from progesterone, other yet unidentified components of human follicular fluid have been shown to contribute to the rise of intracellular calcium in human sperm cells, probably via activation of CatSper [35].
During sperm maturation in the male reproductive tract, and their follow-up journey in the female genital tract, spermatozoa are exposed to different steroid hormones: from androgens, such as testosterone, to female steroids: estrogen and progesterone. In addition, stress experienced by an individual can result in elevated concentrations of the steroid stress hormone cortisol - and high levels of cortisol have been associated with fertility problems [36]. Testosterone, estrogen and cortisol were recently shown to influence CatSper function [32]. While testosterone, estrogen and cortisol do not directly activate CatSper currents, they likely compete with progesterone and PS for the ABHD2-binding site thus preventing CatSper activation [32]. The effect was especially noticeable for testosterone and cortisol, while effect of estrogen was only observed at supraphysiological concentrations [32]. It is possible that high concentrations of testosterone in the male genital tract prevent premature CatSper activation, and act as an anti-capacitation factor by preventing CatSper activation until testosterone is removed in the female reproductive tract during capacitation. High levels of testosterone in women are associated with an endocrine system disorder known as polycystic ovary syndrome (PCOS), which affects between 5% and 8% of women of reproductive age. While the exact cause of PCOS is unknown, many women with PCOS display hormonal imbalances, such as overproduction of androgens and cortisol, which prevail over the female sex hormones: estrogen and progesterone [37]. This hormonal imbalance leads to impaired ovulation and infertility in about 50% of PCOS patients. It is possible that elevated levels of androgens and cortisol in the female reproductive tract can additionally impact fertilization by affecting sperm progression in the fallopian tubes, and impairing CatSper ability to trigger hyperactivation.
So far, no functional expression of CatSper in any heterologous systems has been achieved, as the channel is resistant to functional expression and the formation of the complex. It is possible that we are still missing the essential CatSper subunits that are required to assemble the full complex, as its precise composition still needs to be elucidated. Just recently, two additional CatSper subunits have been identified [15]. Interestingly, the CatSper channel complex is organized in quadrilateral longitudinal nanodomains along the sperm flagellum [15], forming a precisely ordered structured channel arrays. This makes CatSper a possible candidate for electron cryotomography studies [38] to determine the channel composition in greater depth.
Yet at this moment, the characterization of this channel and its regulation by various stimuli is limited to its native expression system - sperm cells. On the other hand, such an exclusive expression makes CatSper an excellent target for novel contraceptives for both women and men. As this channel seems to be regulated by steroids, it is not surprising that certain plant compounds being similar in structure to steroid hormones have been shown to affect CatSper [32]. Indeed, the triterpenoid pristimerin, which is found in Tripterygium wilfordii (also known as “Thunder God Vine”) and Maytenus ilicifolia, prevents CatSper activation by progesterone and PS and could serve as a potential prototype for future non-hormonal contraceptives [32].
CatSper is also modulated by various exogenous compounds. Chemical UV filters, such as 3-BC, that are frequent components in sunscreens have been recently shown to mimic the effect of progesterone and presumably are able to activate the CatSper channel as shown by calcium imaging experiments [39]. Yet, direct electrophysiological recordings of CatSper currents in the presence of UV filters are needed to assess their specific CatSper-modulation potential.
Future directions in studying this unique calcium channel of sperm involves an understanding of the precise molecular arrangement of the CatSper complex, the stoichiometry of its subunits, and ultimately revealing the detailed anatomy of the channel.
Highlights.
5. In vivo, a successful fertilization is dependent on sperm hyperactivated motility;
6. Hyperactivation is triggered by a calcium influx into flagellum via sperm-specific calcium channel CatSper;
7. CatSper is a sophisticated heteromeric complex of nine different subunits. It is expressed exclusively in the sperm cells and is vital for fertilization. This ion channel represents an attractive target to develop unisex contraceptives.
Acknowledgments
This work was supported by NIH R01GM111802, R21HD081403, Pew Biomedical Scholars Award 00028642, Alfred P. Sloan Award FR-2015-65398, and Packer Wentz Endowment Will to P.V.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coy P, Garcia-Vazquez FA, Visconti PE, Aviles M. Roles of the oviduct in mammalian fertilization. Reproduction. 2012;144:649–660. doi: 10.1530/REP-12-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper JC, Phadnis N. Parallel Evolution of Sperm Hyper-Activation Ca2+ Channels. Genome Biol Evol. 2017;9:1938–1949. doi: 10.1093/gbe/evx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MR, Mansell SA, Meyers SA, Lishko PV. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium. 2015;58:105–113. doi: 10.1016/j.ceca.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod. 2007;76:660–665. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- 5.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 6.Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci U S A. 1993;90:4660–4664. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Hung PH, Suarez SS. Ejaculated mouse sperm enter cumulus-oocyte complexes more efficiently in vitro than epididymal sperm. PLoS One. 2015;10:e0127753. doi: 10.1371/journal.pone.0127753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardon F, Markello RD, Hu L, Deutsch ZI, Tung CK, Wu M, Suarez SS. Dynamics of Bovine Sperm Interaction with Epithelium Differ Between Oviductal Isthmus and Ampulla. Biol Reprod. 2016;95:90. doi: 10.1095/biolreprod.116.140632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- 10.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- *11.Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sanchez-Cardenas C, De la Vega-Beltran JL, Da Ros VG, Greer PA, Darszon A, et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development. 2016;143:2325–2333. doi: 10.1242/dev.136499. This paper describes tyrosine kinase FER that is responsible for capacitation-inducing changes in spermatozoa. Kinase-inactivating mutation in FER makes spermatozoa unable to capacitate and reduces their fertility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarro B, Miki K, Clapham DE. ATP-activated P2X2 current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1111695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014;157:808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Chung JJ, Miki K, Kim D, Shim SH, Shi HF, Hwang JY, Cai X, Iseri Y, Zhuang X, Clapham DE. CatSperzeta regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. eLife. 2017;6 doi: 10.7554/eLife.23082. This paper describes two additional members of CatSper complex, and provides a detailed organization of CatSper channel in murine and human spermatozoa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Wang X, Clapham DE. Early evolution of the eukaryotic Ca2+ signaling machinery: conservation of the CatSper channel complex. Mol Biol Evol. 2014;31:2735–2740. doi: 10.1093/molbev/msu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinal-Enriquez J, Priego-Espinosa DA, Darszon A, Beltran C, Martinez-Mekler G. Network model predicts that CatSper is the main Ca(2+) channel in the regulation of sea urchin sperm motility. Sci Rep. 2017;7:4236. doi: 10.1038/s41598-017-03857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert R, Flick M, Bonigk W, Alvarez L, Trotschel C, Poetsch A, Muller A, Goodwin N, Pelzer P, Kashikar ND, et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 2015;34:379–392. doi: 10.15252/embj.201489376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y, Lishko PV. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science. 2016;352:555–559. doi: 10.1126/science.aad6887. This paper describes novel progesterone receptor ABHD2 and describes novel pathway of membrane steroid signaling. It also provides an insignt on human and mouse CatSper regulation by various endogenous lipids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumigama S, Mansell S, Miller M, Lishko PV, Cherr GN, Meyers SA, Tollner T. Progesterone Accelerates the Completion of Sperm Capacitation and Activates CatSper Channel in Spermatozoa from the Rhesus Macaque. Biol Reprod. 2015;93:130. doi: 10.1095/biolreprod.115.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol. 2003;1:53. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF. Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem. 2005;280:32238–32244. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945–18952. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Liu J, Cho KH, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod. 2009;81:539–544. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2011;2:153. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildebrand MS, Avenarius MR, Smith RJH. CATSPER-Related Male Infertility. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, Stephens K, Amemiya A, Ledbetter N, editors. GeneReviews((R)) 1993. [PubMed] [Google Scholar]
- 29.Jaiswal D, Singh V, Dwivedi US, Trivedi S, Singh K. Chromosome microarray analysis: a case report of infertile brothers with CATSPER gene deletion. Gene. 2014;542:263–265. doi: 10.1016/j.gene.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 30.Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci U S A. 2013;110:6823–6828. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedford JM. Singular features of fertilization and their impact on the male reproductive system in eutherian mammals. Reproduction. 2014;147:R43–52. doi: 10.1530/REP-13-0436. [DOI] [PubMed] [Google Scholar]
- 32.Mannowetz N, Miller MR, Lishko PV. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc Natl Acad Sci U S A. 2017;114:5743–5748. doi: 10.1073/pnas.1700367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strünker T, Goodwin N, Brenker C, Kashikar N, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 34.Lishko PV, Botchkina IL, Kirichok Y. Progesterone Activates the Principal Ca2+ Channel of Human Sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 35.Brown SG, Costello S, Kelly MC, Ramalingam M, Drew E, Publicover SJ, Barratt CLR, Da Silva SM. Complex CatSper-dependent and independent [Ca2+]i signalling in human spermatozoa induced by follicular fluid. Hum Reprod. 2017;32:1995–2006. doi: 10.1093/humrep/dex269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- 37.Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22:709–724. doi: 10.1093/humupd/dmw027. [DOI] [PubMed] [Google Scholar]
- 38.Muhleip AW, Joos F, Wigge C, Frangakis AS, Kuhlbrandt W, Davies KM. Helical arrays of U- shaped ATP synthase dimers form tubular cristae in ciliate mitochondria. Proc Natl Acad Sci U S A. 2016;113:8442–8447. doi: 10.1073/pnas.1525430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehfeld A, Dissing S, Skakkebaek NE. Chemical UV Filters Mimic the Effect of Progesterone on Ca2+ Signaling in Human Sperm Cells. Endocrinology. 2016;157:4297–4308. doi: 10.1210/en.2016-1473. [DOI] [PubMed] [Google Scholar]


