Abstract
The skin is the largest organ in the body and plays multiple essential roles ranging from regulating temperature, preventing infection and ultimately defining who we are physically. It is a highly dynamic organ that constantly replaces the outermost cells throughout life. However, when faced with a major injury, human skin cannot restore a significant lesion to its original functionality, instead a reparative scar is formed. In contrast to this, many other species have the unique ability to regenerate full thickness skin without formation of scar tissue. Here we review recent advances in the field that shed light on how the skin cells in regenerative species react to injury to prevent scar formation versus scar forming humans.
Keywords: Skin, Scar, Regeneration, Collagen
1. Introduction
Open wounds or scar tissue caused due to genetic skin diseases or as a result of traumatic burn or blast injuries have a devastating effect on people's lives and pose a huge financial burden. Serious skin disorders count among the most devastating health conditions and most difficult treatment challenges. These arise both from genetic diseases and from injuries, such as the often debilitating long-term suffering of the > 500,000 serious burn victims who are hospitalized every year in the US alone. Today's top treatments, including major skin grafts, still struggle to overcome the key natural limitations of human skin's relatively basic repair processes. While these generally restore structural integrity, they often result in extensive scarring that not only causes disfigurement and accompanying psychological trauma, but also significant loss of functionality, including impaired sensitivity and increased susceptibility to infection.
The development of more effective skin therapies may in the future benefit greatly from emerging new understanding of the remarkable healing abilities exhibited by species that naturally regenerate scar-free.
In recent years regeneration research organisms that are able to regenerate skin without the formation of permanent scar tissue have provided interesting new insights into the mechanism of scar-free wound healing. These research organisms include classic developmental organisms such as Danio rerio, Xenopus genus, and urodele amphibians such as Ambystoma mexicanum. Additionally, newly emerging research organisms including the African spiny mouse, Acomys spp., and the crustacean Paryhale add to the repertoire of research organisms that expand our understanding of the conserved and divergent mechanisms different species employ to functionally regenerate complex tissue.
This review will discuss mammalian skin development and it's response to injury. We will review recent findings in skin regeneration from research organisms and commonalities of the scar-free wound healing process will be discussed.
2. Mammalian skin development
2.1. Epidermis formation and homeostatic replacement
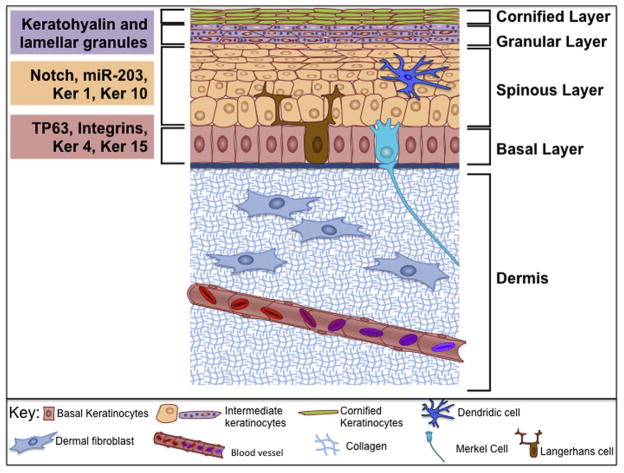
Embryos develop three distinct lineages, the endoderm, ectoderm, and mesoderm. During embryonic development, the skin arises from two different germ layers. The epidermal cells (those lying above the basal lamina); develop from non-neural ectoderm (Nassar and Blanpain, 2012; Sotiropoulou and Blanpain, 2012). In contrast, dermal cells (those lying below the basal lamina), develop from the mesoderm (Fuchs, 2016). Cells of the ectoderm lineage have the potential to become either the nervous system, tooth enamel, or epithelium (Fuchs, 2016). Wnt signaling during the early stages of development plays an important role in determining cell fate (Grigoryan et al., 2008; Nassar and Blanpain, 2012; Sotiropoulou and Blanpain, 2012). Cells that receive Wnt signals will not respond to fibroblast growth factors (FGFs) (Fuchs, 2016; Grigoryan et al., 2008). In the absence of FGF signaling, these ectoderm lineage cells produce bone morphogenic proteins (BMPs), and the BMP signaling cascade ultimately results in these cells becoming epidermis (Clayton et al., 2007; Fuchs, 2016; Grigoryan et al., 2008; Jensen et al., 1999; Jones et al., 1995; Kurata et al., 2004; Lechler and Fuchs, 2005; Poulson and Lechler, 2010; Sotiropoulou and Blanpain, 2012; Strachan and Ghadially, 2008). Eventually, the epidermis will be comprised of four different cell types: the most predominant epidermal cell type is the keratinocyte, which constitutes 80–90% of the cellular epidermis (Fig. 1; Nassar and Blanpain, 2012). The main function of keratinocytes is to form a barrier against the external environment. The mature skin contains, melanocytes, Langerhans cells and Merkel cells (Fig. 1). Each of these cell types has an important function within the skin. For example, the neural crest derived melanocytes produce melanin that affords protection from UV damage (Bin et al., 2016). Langerhans cells are a type of dendritic cell immune cell that protects against infection (Berberich et al., 2003). Lastly, the Merkel cells, are involved in the sense of touch by making contacts with sensory nerve endings (Fig. 1) (Sorenson and Clark Brelje, 2014).
Fig. 1.
Schematic diagram of mature mammalian skin. Skin consists of several layers of keratinocytes (indicated on the right) that differentiate as cells proceed from the basal layer to the cornified layer. As keratinocytes move through these layers of skin, their morphology and gene expression profiles also change (indicated on the left). Basal keratinocytes are the least differentiated cells and will express, TP63, several integrins, Keratin 4 and Keratin 15. Once keratinocytes are in the spinous layer, expression of Notch, miR-203, Keratin 1 and Keratin 10 will occur. After keratinocytes proceed through the granular layer, marked by keratohyalin and lamellar granules, keratinocytes will extrude all organelles and become cornified skeletons that form a ridged keratin network to form a barrier.
During embryonic development the epidermis forms as a single cell layer of multipotent cells that express Tumor protein p63 (TP63 or p63), a gene necessary to maintain the epidermal progenitor population (Koster et al., 2004; Mikkola, 2007; Pozzi et al., 2009; Testoni et al., 2006; Westfall and Pietenpol, 2004). As development progresses, the p63+ epidermal basal progenitors undergo a spindle shift to create one daughter in the basal layer and one daughter in the suprabasal, differentiating layer (Candi et al., 2006; Koster et al., 2004; Mikkola, 2007; Pozzi et al., 2009). The basal daughter continues to express p63, while the suprabasal daughter loses p63 expression in coordination with Notch signaling-dependent differentiation (Okuyama et al., 2007; Tadeu and Horsley, 2013; Truong and Khavari, 2007). Recent research has shown that p63 is post-transcriptionally regulated by miR-203 and that this leads to reduced p63 expression during differentiation (Lena et al., 2008). MiR-203 expression increases in the suprabasal daughter cell, leading to a decreasing abundance of p63; thereby promoting differentiation (Truong and Khavari, 2007).
p63 plays a pivotal role in epidermal development but also has a key role in regulating keratinocyte homeostasis in mature skin (Borrelli et al., 2010; Kouwenhoven et al., 2015; McDade and McCance, 2010; Pozzi et al., 2009; Testoni et al., 2006; Truong et al., 2006). Depletion of p63 in keratinocytes results in decreased proliferation and a subsequent loss of stratified epithelium (Truong et al., 2006). As proliferative progenitors differentiate and migrate away from the basal lamina to become spinous suprabasal cells, a number of transcriptional changes occur which ultimately lead to stratified epithelium. For example, expression of Notch receptors, Keratin 1, and Keratin 10 are increased during this process, with a concordant loss of expression of Keratin 5 and Keratin 14 (Fig. 1). Once cells reach the granular layer, keratinocytes start producing both keratohyalin and lamellar granules (Fuchs, 2016). The final step of terminal differentiation is the extrusion of cellular organelles (including the nucleus) to leave a cornified framework that provides structure and protection (Fuchs, 2016).
In humans, the process outlined in the preceding paragraph takes approximately four weeks (Schoenwolf et al., 2014). This developmental cycle (termed homeostatic replacement) is not restricted to embryonic development and occurs throughout life. In mouse studies of skin development there are currently two models proposed for how homeostatic maintenance of the skin occurs: the hierarchical model and the stochastic model (Alcolea and Jones, 2014; Jensen et al., 1999; Yan and Owens, 2008; Yang et al., 2015). The hierarchical model suggest that divisions by basal progenitor cells will generate rapidly dividing transity amplfligying cells, which then give rise to differentiated cells. While the stochastic model suggest that all basal cells have equal proliferative capacity, and proliferation can yield three different outcomes: 1. basal cell self renewal and one differentiated daughter cell, 2. two basal progenitors, 3. two differentiated daughter cells. The combination of genetic tools and in vivo imaging is currently helping to delineate between the two proposed models (Clayton et al., 2007; Doupe and Jones, 2013; Mascre et al., 2012; Rompolas et al., 2016).
2.2. Dermis formation
The dermis is the connective tissue layer, separated from the epidermal layer by the basal lamina. Dermal development is often studied as four unique phases of cellular differentiation: Phase 1 is when the layer that will become the dermis is made of undifferentiated mesenchyme; Phase 2 is the initiation of cell differentiation from the undifferentiated mesenchymal cells; Phase 3 is referred to as the dynamic transition, and Phase 4 is final matrix differentiation. In the laboratory mouse, Phase 1 begins at embryonic day (E) 12 and is characterized by unique cellular structure (Breathnach, 1978; Van Exan and Hardy, 1984). For example, the most distinguishing structures of undifferentiated mesenchymal cells are their cilia and complex cell junctions; these structures are abundant in Phase 1 of dermal formation. Additionally, at this phase, the undifferentiated mesenchymal cells disperse in the matrix, which is primarily composed of hyaluronic acid, chondroitin sulfate, and fibronectin (Van Exan and Hardy, 1984). By the beginning of mouse E14, Phase 2 begins. Mesenchymal cells lose their ciliated features, thus functionally transitioning into immature fibroblasts. These immature fibro-blasts are also characterized by a more pronounced Golgi network and rough endoplasmic reticulum (Van Exan and Hardy, 1984). Differentiation of other mesoderm lineage cell types, such as myoblasts and mast cells, also occurs, in correlation with the appearance of diffuse collagen fibrils within the dermal extracellular matrix (Van Exan and Hardy, 1984). Phase 3 begins at mouse E16 and is marked by the presence of more dense fibrils in the extracellular matrix (ECM) and the maturation of fibroblasts. Changes within the ECM include both the aggregation of individual fibrils into bundles as well as a shift to collagen production. In adult humans, collagen I is the most common collagen within the extracellular matrix of the dermis (Kirchhofer et al., 1986; Merlino et al., 1983; Montes and Junqueira, 1982; Uitto et al., 1980).
As Phase 3 is concluding, the structural features of ECM components, such as collagen, become important for classifying tissue differentiation. Collagen production is a multistep process, beginning after translation of the collagen mRNA. Before collagen's quaternary structure can form, multiple post-translational modifications, such as hydroxylation of specific lysine and proline residues, which help stabilize the triple helical structure of collagen (referred to as procollagen) (Montes and Junqueira, 1982; Uitto et al., 1980). The formation of the triple helix occurs within the endoplasmic reticulum (Montes and Junqueira, 1982; Uitto et al., 1980). Fibroblasts secrete procollagen into the extracellular space. Procollagen is then further processed by proteases that cleave both the N and C-terminus ends of the protein, yielding mature collagen (Montes and Junqueira, 1982; Uitto et al., 1980). Mature collagen is then organized into fibrils, where each collagen triple helix will then be both intramolecularly and intermolecularly cross-linked by the enzyme lysyl oxidase (Montes and Junqueira, 1982; Uitto et al., 1980) The product of the intracellular and extracellular processing steps is the final mature fibril collagen (Montes and Junqueira, 1982; Uitto et al., 1980).
In Phase 4, the final phase of dermal development, the dermal matrix continues to mature. This terminal maturation process includes further fibril thickening and the appearance of elastin, which interconnects the collagen fibers (Breathnach, 1978; Van Exan and Hardy, 1984). It remains unclear what transcriptional changes are occurring to direct fibroblasts maturation through each phase of dermal development. Further, defining the molecular circuitry that guides dermal fibroblast maturation, collagen production and modeling could be a crucial step in designing strategies to heal chronic wounds or reduce scarring. To date, there has been little progress made to determine the molecular mechanism of fibroblast and ECM maturation. With recent leaps in genetic manipulation capabilities, research to answer these questions may be possible.
3. The mammalian repair and scar formation process
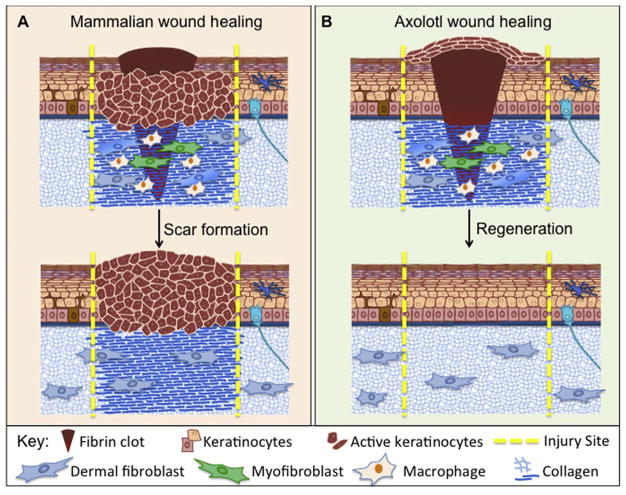
When there is a physical insult to the skin, homeostatic turnover of the skin is temporarily halted and switches instead to a wound healing and reparative response (Ferguson and O'Kane, 2004; Hantash et al., 2008; Kawasumi et al., 2013; Reinke and Sorg, 2012; Takeo et al., 2015; Yates et al., 2012). The extent of the wound will determine the ultimate outcome; simple scratches can be effectively repaired but full-thickness skin wounds most commonly result in scar formation (Fig. 2; Ferguson and O'Kane, 2004; Hantash et al., 2008; Kawasumi et al., 2013; Reinke and Sorg, 2012; Takeo et al., 2015; Yates et al., 2012). Indeed, wound healing in humans and most mammals results in a fibrotic response. The mammalian wound healing process is composed of three overlapping phases: Inflammation, proliferation, and maturation/remodeling (Ferguson and O'Kane, 2004; Hantash et al., 2008; Kawasumi et al., 2013; Reinke and Sorg, 2012; Takeo et al., 2015; Yates et al., 2012).
Fig. 2.
A summary comparison of skin injury response in mammals versus axolotls. (A). In mammalian wound healing, a fibrin clot is formed, and keratinocytes will proliferate and migrate under the clot. As keratinocyte migration is occurring, fibroblasts will enter into the wound bed and proliferate, and will start to express extracellular matrix (ECM) proteins such as collagen. Once there is a provisional matrix, keratinocytes can migrate over to close the wound. Some fibroblasts will differentiate into myofibroblasts and will contract the wound. Scar formation is the result of mammalian wound healing, marked by a thickened epidermis, as well as excessive and un-remodeled collagen deposition. (B). In axolotl wound healing, keratinocytes will migrate over the fibrin clot, closing the wound within 24 h. Once the wound is closed, the keratinocytes will proliferate, and create a wound epidermis. Fibroblasts will enter the wound bed, proliferate, and secrete ECM. Again, some of the fibroblasts will differentiate into myofibroblasts and contract the wound. The cells within the dermis will then continue to remodel the ECM that was deposited until regeneration has been achieved.
The inflammation phase initiates the wound healing process and begins when blood vessel disruption occurs (Eming et al., 2007, 2014, 2017; LeBert and Huttenlocher, 2014; Rosique et al., 2015). Broken blood vessels lead to the release of clotting factors that cause the blood to coagulate and form a fibrin clot (LeBert and Huttenlocher, 2014; Rosique et al., 2015). The fibrin clot serves many purposes, including cessation of bleeding and provision of a temporary matrix for cellular migration (Eming et al., 2007, 2014, 2017). Damage to the tissue releases mitogens such as Tissue Factor and Tumor Necrosis Factor-alpha (TNF-α) which help to recruit immune cells to the injury site (Eming et al., 2007, 2014, 2017). Additionally, platelets are recruited into the fibrin clot from the vasculature, which then secrete growth factors and chemokines to attract immune cells to the wound bed (Eming et al., 2007, 2014, 2017; LeBert and Huttenlocher, 2014; Mescher et al., 2017; Rosique et al., 2015). Neutrophils arrive to the wound and eliminate microorganisms within the wound bed by phagocytosis and the release of antimicrobial granules (MacLeod and Mansbridge, 2016; Willenborg and Eming, 2014). Neutrophils also release chemokines that attract more leukocytes to amplify the immune response (MacLeod and Mansbridge, 2016; Willenborg and Eming, 2014). Macrophages respond to chemokine signals and migrate to the wound site where they release pro-inflammatory factors and phagocytose debris within the wound (Dreymueller et al., 2013; Jetten et al., 2014; MacLeod and Mansbridge, 2016; Sindrilaru and Scharffetter-Kochanek, 2013; Willenborg and Eming, 2014). After the wound is clear of debris, macrophages will start to release growth factors that initiate the formation of a mass of new connective tissue termed granulation tissue (Dreymueller et al., 2013; Jetten et al., 2014; MacLeod and Mansbridge, 2016; Sindrilaru and Scharffetter-Kochanek, 2013; Willenborg and Eming, 2014). Granulation tissue is made up of new blood vessels, macrophages, fibroblasts and loose connective tissue and forms approximately 4 days after injury (Dreymueller et al., 2013; Jetten et al., 2014; MacLeod and Mansbridge, 2016; Sindrilaru and Scharffetter-Kochanek, 2013; Willenborg and Eming, 2014). Within hours of the injury, keratinocytes at the leading edge of the wound start to lose their apical-basal polarity and begin migrating into the wound (Balaji et al., 2015; Kawasumi et al., 2013; Leavitt et al., 2016; Penn et al., 2012; Romano and Sinha, 2011; Takeo et al., 2015; Walmsley et al., 2015).
The proliferative phase begins one to two days after injury and is focused on the large amount of proliferation of cells within the wound bed. The proliferative phase is initiated when keratinocytes begin hyperproliferating and migrating into the wound bed. In mice, studies suggest that during injury, keratinocytes proliferate at a rate of approximately 1.8 times per day (Aragona, et al., 2017) In contrast to homeostatic keratinocyte proliferation which occurs at a rate of 1.1 times per week (Clayton et al., 2007), thus, keratinocytes proliferate substantially faster during wound healing. Many of the same players that are important in epidermal development become reactivated during wound healing. The transcription factor p63 that is required for proper development of the epidermis will also regulate the hyperproliferation of keratinocytes after injury (Strachan and Ghadially, 2008; Truong and Khavari, 2007; Truong et al., 2006). In mouse, p63 regulates keratinocyte proliferation such that cells with the highest abundance of p63 marked for degradation will proliferate the most (Suzuki and Senoo, 2013). Interestingly, in other mouse wound healing experiments, it was found that miR-203 expression was also decreased during hyperproliferation. Thus indicating a potential need for dynamic regulation of p63 at a post translational level (Viticchié et al., 2012).
The proliferative phase also includes the process of keratinocytes migrating into the wound bed. Keratinocyte migration is critical for the wound healing process, thus cell-cell and cell-matrix adhesions need to be altered to allow keratinocytes to migrate. Keratinocytes actively remodel the matrix by producing matrix metalloproteinase 1(MMP1) to disrupt cellular contacts (Pilcher et al., 1997, Saarialho-Kere et al., 1993a, 1993b).
In addition to keratinocytes, fibroblasts also propagate during the proliferative phase. Fibroblasts begin to proliferate and initiate ECM production after receiving cytokines from neutrophils and macrophages (Balaji et al., 2015; Zgheib et al., 2014). Indeed, fibroblasts are the main cell type responsible for producing ECM proteins within the wound bed to reconstitute the dermal matrix. The first the major ECM component produced by fibroblasts is fibronectin, but once fibroblasts slow their migration through the wound, protein production shifts towards collagen III and I. In humans, elastin fibers are not deposited until at least 3 months after wounding (Almine et al., 2012; Roten et al., 1996). While ECM production has been regarded as a universal fibroblasts trait, recent findings show that there are at least two types of fibroblasts present in early wound healing. Interestingly, while both fibroblast types ultimately arise from the mesoderm, subtle differences in signaling and protein expression result in fibroblasts stationed to two geographically distinct areas of the steady state dermis. The “upper lineage”, forms the upper dermis, including the dermal papilla that regulates hair growth. These “upper lineage” fibroblasts are recruited during re-epithelialization and are activated by epidermal beta-catenin. The “lower lineage” is the initial fibroblasts to enter the wound bed and deposit ECM, thus mediating dermal repair (Driskell et al., 2013). However, the lower lineage does not support hair follicle formation, and thus explains why scar tissue lacks hair follicles (Driskell et al., 2013; Driskell and Watt, 2015). However, it is still unknown if upper versus lower lineage produce distinct ECM components or mitogenic factors (Driskell and Watt, 2015; Sorrell and Caplan, 2004; Woodley, 2017). Much more research needs to be performed to discern the functional outputs of each fibroblast lineage.
The final phase of wound healing in mammals is comprised of maturation and remodeling of the wound bed. During the maturation/remodeling phase, fibroblasts differentiate into myofibroblasts in a transforming growth factor beta (TGF-β)-dependent process (Penn et al., 2012). Similar to their fibroblasts cousins, myofibroblasts produce abundant quantities of ECM. However, myofibroblasts also bind to collagen fibers and lead to wound contraction. After mature collagen is deposited in the wound bed it is remodeled by MMPs and collagenases secreted by fibroblasts, neutrophils and macrophages within the wound bed. The collagenases cleave through the triple helix at specific sites allowing collagen to be further degraded by additional secreted proteases. Continued collagen deposition and catabolism will slowly replace granulation tissue with a fibrotic scar. Previous studies suggest that hypertrophic scars have decreased amounts of MMP1 and that increasing the amount of MMP1 in scars leads to decreased fibrosis (Bellemare et al., 2005; Eto et al., 2012; Lee et al., 2011; Stuart et al., 2011). However, the collagen deposited within the wound bed will not return to pre-injury condition. During development, collagen is processed into a basket weave pattern, which allows elasticity in the tissue (Fig. 1; LeBert et al., 2015; Montes and Junqueira, 1982; Uitto et al., 1980). After injury, collagen is deposited in a linear fashion but never regains the original basket weave morphology, elasticity or tensile strength (LeBert et al., 2015; Martin and Nunan, 2015; Montes and Junqueira, 1982; Sindrilaru and Scharffetter-Kochanek, 2013; Takeo et al., 2015; Uitto et al., 1980; Walmsley et al., 2015; Xue and Jackson, 2015). In the final remodeling process the tensile strength of the wound will slowly start to increase (van Zuijlen et al., 2003). At the end of the remodeling process the tensile strength of the scar is only up to 70% of intact skin and thus results in tough inflexible scar tissue (Fig. 2) (Finsterbush et al., 1982; Julia et al., 1993; Morin et al., 1989; van Zuijlen et al., 2003)
3.1. Fetal wound healing
One goal in the regenerative medicine community is to determine the cellular and molecular differences between organisms with the ability to functionally regenerate scar-free and those like humans that cannot. Across taxa, multiple species (discussed below) can heal wounds without scar formation and no doubt many more, undescribed to date may possess this remarkable ability. However, another useful insight may come from studying fetal wounds in mammals, which can heal without scar formation. Currently, three differences in fetal and adult wounds have been elucidated. First, there is a different ratio of collagen I to collagen III that are deposited in fetal wounds versus adult wounds (Buchanan et al., 2009; Hantash et al., 2008; Kishi et al., 2012; Namazi et al., 2011). Second, the growth factor profile in fetal versus adults wounds is quite different. For example, the prevalence of different TGFβ isoforms is very different in adult compared to fetal wound beds. TGFβ1 and 2 are absent from fetal wound sites but TGFβ1 is very abundant in adult wounds, specifically in immune cells (Penn et al., 2012; Walraven et al., 2014). Additionally, TGFβ3 is highly expressed in fetal keratinocytes and fibroblasts but minimally expressed or absent in adult wound beds (Penn et al., 2012; Walraven et al., 2014). Furthermore, adult wounds express high levels of platelet-derived growth factor (PDGF) but this is absent from fetal wounds (Buchanan et al., 2009; Hantash et al., 2008; Helmo et al., 2013; Julia et al., 1993; Kishi et al., 2012; Wilgus, 2007). Interestingly, some success has been seen with treating wounds in rat with ectopic TGFβ3 resulting in less scar tissue formation (O'Kane and Ferguson, 1997; Shah et al., 1995).
Finally, a major difference between embryonic and adult wounds is the immune response. In embryos the immune response consists of fewer numbers of less mature immune cells that stay at the wound site for a much shorter period of time (Buchanan et al., 2009; Hantash et al., 2008; Kishi et al., 2012; Leavitt et al., 2016). This is interesting as it may be similar to what is observed in animals with the natural ability to regenerate, where although an immune response is necessary the length of time the immune cells stay at the site of injury is significantly shorter (Kawasumi et al., 2013; Reinke and Sorg, 2012; Seifert et al., 2012b; Takeo et al., 2015; Walmsley et al., 2015; Wilgus, 2007; Godwin et al., 2013; Godwin and Rosenthal, 2014). While humans and other mammals do not retain the ability to heal scar-free into adulthood, several species are able to regenerate skin throughout their entire life.
4. Scar-free regeneration: learning from animals with the natural ability to regenerate
To date the most common models for studying the process of wound healing in mammals are the laboratory mouse, Mus musculus or in vitro cultures of human skin cells. These studies have shed light some light on the overlapping steps of wound healing and have also described roles for several different proteins required along the way. However these studies have not provided enough insights to enable the prevention of scar tissue in humans. Remarkably, many models systems that have the ability to regenerate complex tissues do so without the formation of scar tissue. Species from fish to salamanders and spiny mice can all heal their wounds without scarring and can, in some instances, regenerate significant pieces of complex tissue without scarring. How this unique feat is orchestrated remains a mystery but recent advances in the field have begun to shed light on the cross-species differences and similarities that drive cells towards forming reparative scar tissue versus functional scar-free regeneration. Drosophila and zebrafish have long been key models for studying cellular response to injury. These systems have given key insights into fundamental cell biology processes that drive epithelial wound closure and in addition have highlighted the differences between embryonic and adult wound healing, especially the involvement of immune cells in adult wounding (reviewed in Fuchs (2016), Kawasumi et al. (2013), LeBert and Huttenlocher (2014), and Martin and Nunan (2015).
The crustacean genus Paryhale spp. has recently emerged as an important new species for studying limb regeneration. The short regeneration time, genetic tools and optical properties have enabled interesting new insights into the conservation of aspects of regeneration cross-species. In vivo imaging in the Parhyale spp. has shown how it rapidly closes wounds using haemolymph and then the epidermal cells migrate underneath the fibrin clot to form a wound epidermis that is very reminiscent of the process of wound healing in vertebrate species that regenerate (Alwes et al., 2016).
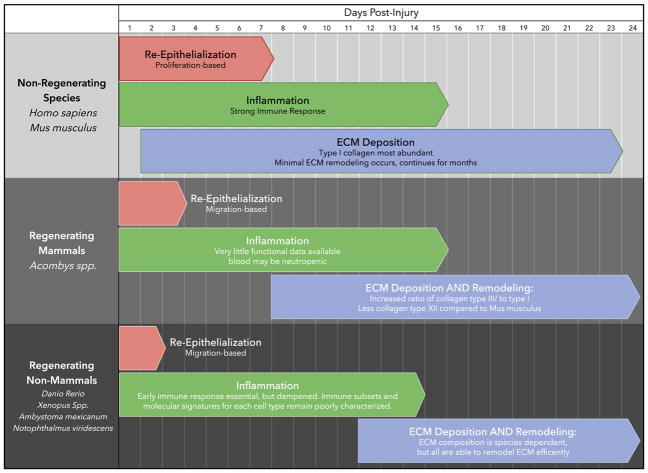
We will discuss several different regeneration research organisms that can regenerate skin, and review the processes that each share (Table 1). Focusing on these shared commonalities of a scar-free wound healing process may lead to treatments for human wounds and scars.
Table 1.
A schematic representative of known cross- species differences in response to injury.
4.1. Danio rerio
Danio rerio, commonly known as zebrafish, have several advantages as a model to study scar-free wound healing such as their genetic tractability and fast generation time. Another advantage of using zebrafish is that while they have the same three phases of wound repair that mammals do, the phases have minimal overlap in zebrafish (Rakers et al., 2010). This allows researchers to study the intricacies of each phase of wound healing separately. This is an advantage because certain proteins may play different roles in each phase. For example, Fibroblasts Growth Factors are not required for re-epithelialization, but are required for later epidermal remodeling and granulation tissue formation (Richardson et al., 2016). Generation of a “skinbow” zebrafish in the Poss lab, has allowed large-scale tracking of skin cells response to injury and has enabled the identification of differences in epithelial cellular responses during normal homeostatic turnover of the skin versus minor exfoliation injuries versus regeneration of major portions of the skin. Indeed the combination of transgenic zebrafish and in vivo imaging has also facilitated the identification of key roles for β-catenin/integrin signaling in re-epithelialization of full-thickness skin wounds in adult zebrafish (Richardson et al., 2016).
4.2. Amphibians
Several different amphibians heal wounds and regenerate scar free. Adult frogs from the Xenpous genus also harbor the ability to heal wounds scar-free. A benefit to using Xenopus as a model is that they are able to regenerate skin appendages, such as exocrine glands. Additionally, Xenopus have a multi-layered epidermis, similar to that of mammals (Bertolotti et al., 2013; Caddy et al., 2010; Franchini and Bertolotti, 2014; Franchini et al., 2016; Otsuka-Yamaguchi et al., 2017; Yoshii et al., 2005). Just like frogs, urodele amphibians, such as newts and axolotls, also have the ability to regenerate skin appendages scar-free (Fig. 2; Levesque et al., 2010; Murawala et al., 2012; Seifert et al., 2012b). Additionally, newts and axolotls are still able to heal their wounds scar-free even after metamorphosis, and do not lose their regenerative ability with age (Godwin and Rosenthal, 2014; Seifert et al., 2012b).
4.3. Acomys spp
A relatively new addition in the repertoire of research organisms with the ability to regenerate is the African spiny mouse from the Acomys genus. Very few mammals are documented as having the ability to regenerate tissue, the best known example is the regeneration of deer antlers each year, which is a form of epimorphic regeneration, whereby a regeneration blastema is formed composed mainly of mesenchymal cells derived from the pedicle periosteum and these cells then give rise to the new antlers which are composed of various cell types including skin, nerves, bone and blood vessels (Li et al., 2014). A lesser known example of mammalian regeneration, is the dolphin which has been documented to regenerate large lesions in the skin without scar formation (Bloom and M, 1994; Bruce-Allen and JR, 1985). More recently a more laboratory-friendly; and potentially genetically tractable mammalian example of regeneration has been identified, the African spiny mouse (Acomys Spp.) (Seifert et al., 2012a). Acomys have skin that tears and releases easily, this feature is postulated to be a mechanism to escape predators. After injury, the spiny mouse will quickly close the wound and will regenerate the missing tissue, including hair follicles and associated structures. Collagen is deposited in the wound bed during the regeneration process, however this is remodeled and the skin returns to a state very similar to that prior to injury (Gawriluk et al., 2016; Seifert et al., 2012a).
5. Common traits of scar-free wound healing
The wound healing process for each of these research organisms has been characterized and there are several similarities that each scar-free wound healing process seems to share. These traits are rapid re-epithelializtion, a dampened immune system, and delayed collagen deposition paired with the ability to remodel the ECM.
5.1. Rapid re-epithelialization
Zebrafish will close full thickness wounds at a rate of 250 μm/hour, and frogs and salamanders will have re-epithelialized these wounds within 24 h (Levesque et al., 2010; Richardson and Hammerschmidt, 2016; Richardson et al., 2016; Seifert et al., 2012b). Each of these studies also show that the re-epithelialization process relies on migration of the keratinocytes at the leading edge of the wound, and the original wound epithelium is only 1–2 cells thick. This is in contrast to human wound closure, which is a proliferative process that occurs at a rate of 0.001 μm2/h (Rezvani et al., 2009). The process of keratinocytes using active migration to close a wound is similar to how adult mammals will also close wounds, while fetal wounds will use an actin-purse string mechanism of closure, similar to that which is seen in Drosophila embryos wound healing (Razzell et al., 2014).
Immediately after wounding, several signals are required for efficient wound closure. One of the first steps required to close the wound is to reorganize the cytoskeleton to allow for migration. At least three different mechanisms can facilitate cytoskeleton rearrangement: ROS (e.g·H2O2), calcium signaling and membrane depolarization. Both calcium and ROS levels are elevated minutes after wounding (Razzell et al., 2013; Stramer et al., 2005). Calcium is able to activate calpain, and once calpain is activated it will cleave spectrin, which anchors the cortical actin network to the plasma membrane. Once this network is detached from the plasma membrane, the cytoskeleton can remodel to allow migration. ROS is able to inhibit Rho-GTPase activity by the ROH-1 redox-sensitive motif, which will promote wound healing (Xu and Chisholm, 2014). Another mechanism for cytoskeletal rearrangement immediately after wounding is depolarization of the plasma membrane on epithelial cells, which occurs after wounding and will gradually extend away from the wound to neighboring cells (Chifflet et al., 2005).
Besides cytoskeletal rearrangement, it has been shown in several species including zebrafish and xenopus that both ROS and calcium are also required for attracting leukocytes to the wound site, which is critical for healing (Niethammer et al., 2009; Razzell et al., 2013). ROS is able to regulate the activity of several proteins in signaling cascades that aid in the wound healing process. For example, hydrogen peroxide is able to destabilize the phosphatase Dusp6 that allows for increased phosphorylation of Erk1/2, allowing for the activation of several downstream proteins and genes. Whilst in human keratinocytes H2O2 has been shown to play an essential role in activating signaling factors like IKKα, that promotes migration through dynamic interactions with the EGF promoter depending on the redox state within cells (Lisse and Rieger, 2017)
After the wound has been covered, the keratinocytes proliferate to create a thickened epidermis. This contrasts with both human and mouse wound healing, where keratinocytes at the leading edge of the wound hyperproliferate to re-epithelialize. However recent studies with cultured human keratinocytes show that in response to a scratch injury human keratinocytes also produce hydrogen peroxide in cells within 2–3 cells adjacent to the scratch, within 30 mins of injury. Interestingly, this study shows that addition of exogenous hydrogen peroxide results in detachment and migration of the keratinocytes adjacent to the scratch (Lisse et al., 2016; Lisse and Rieger, 2017). This might suggest that although this pathway is conserved cross-species that the amount produced in mammalian cells may not suffice to activate the necessary pro-regenerative down-stream pathways. Another factor that may contribute to the differences observed in keratinocyte migration is the composition of matrix metalloproteinase (MMP's) that are secreted after wounding to degrade the extracellular matrix. Variations in the composition of MMPs between humans, mouse and salamanders have been described, however the functional consequences of this have not yet been rigorously tested (Bonnans et al., 2014; Denis et al., 2013; Godwin et al., 2014; Hynes, 2009; LeBert et al., 2015; Mercer et al., 2012; Miyazaki et al., 1996; Onda et al., 1990; Santosh et al., 2011; Seifert and Maden, 2014; Sorokin, 2010; Stuart et al., 2011; Xue and Jackson, 2015; Yang and Bryant, 1994; Yang et al., 1999).
Acomys spp. are able to also fully close a 4 mm wound within 3 days of injury, which will take 7–9 days in Mus musculus. While it has not been studied if the wound closure process in Acomys spp. is migratory or proliferative, it has been shown that contraction of the wound accounts for more than 50% of the wound closure at 24 h post wounding (Seifert et al., 2012b). In the future it will be interesting to determine if the spiny mouse uses a re-epithelialization process that mimics other regenerative animals, or if the process requires proliferation in combination with contraction.
5.2. The role of the Immune system
Several recent studies have shown commonalities in the cell types present in the mammalian immune system and the immune system of species that regenerate scar free. Previously it had been suggested that animals that maintain the capacity to regenerate have a different immune response than non-regenerating animals. Several studies across regenerating species show that ablating the immune system impairs regeneration. In zebrafish, when the early immune response is inhibited, such as the recruitment of macrophages, regenerative capability is diminished. In frogs and axolotls, treatment with the inflammatory agonist Beryllium causes a failure of regeneration in tissues normally capable of regeneration (Mescher et al., 2013; Thornton, 1947, 1949). Further, macrophages have also been shown to be essential for limb regeneration in the axolotl (Godwin et al., 2013). During axolotl limb regeneration macrophages phagocytose cellular debris, release growth factors and cytokines to attract other immune and non-immune cells and remodeling the extracellular matrix. In mammals M1 and M2 macrophages have been defined based on the timing of their appearance after injury they appear and the cytokines they present (Eming et al., 2014; Martin and Nunan, 2015). Less is known about the molecular nature of macrophages within species that regenerate scar-free, one could speculate that there are inherent differences in the timing and molecules that are secreted in species that scar versus those that regenerate. In the future, it will be important to molecularly define the sub-types of immune cells present in regenerative species.
While it is clear that having an immune response is critical for full regeneration, there is also substantial evidence that suggests that having an “immature” immune system could be beneficial when it comes to regeneration. During Xenopus spp. development, there is a refractory period between stages 45 and 47 where regeneration does not occur (Beck et al., 2003). This refractory period ends after the development of T regulatory cells whose role is to dampen the immune response (Beck et al., 2003). Additionally, different immune responses are evoked when injury occurs either in the regeneration period versus the refractory period (Fukazawa et al., 2009). Further, if Xenopus spp. is immunosuppressed during the refractory period, the regenerative ability is restored (Fukazawa et al., 2009). During the refractory period, it has also been shown that there is an increase in the amount of immune cells that traffic to the wound area, suggesting that there may be an increased immune response during this time (Franchini and Bertolotti, 2014). Several studies in Xenopus spp. now confirm a connection between the development of the immune system and the loss of regenerative ability (King et al., 2012; Mescher et al., 2013; Mescher et al., 2017; Paredes et al., 2015).
Axolotls are able to regenerate throughout their life, even after metamorphosis. Even though axolotls have both T and B cells, they are still considered “immune deficient” since tissue transplantation can occur without acute rejection and axolotls cannot easily clear viral infections (Cotter et al., 2008). While axolotl T and B cells have reasonable cell receptor repertoires (Andre et al., 2007, 2011; Charlemagne, 1979; de Guerra et al., 1995; Fellah et al., 2002a, 2002b; Kaufman et al., 1990; Volk et al., 1998), they have limited MHC class II repertoires (Laurens et al., 2001; Tournefier et al., 1988). With a limited MHC class II repertoire, immunodeficiency could be explained by limited presentation of class II peptides, however in inbred laboratory mice, where MHC-II repertoires are often restricted to a single MHC-II, mice still have the ability to mount robust immune responses to foreign tissue engraftments and acute viral infections (Choi et al., 2017). Other studies comparing the immune system of axolotls and mammals have identified a difference in the neutrophil profile cross species. While both axolotls and mammals recruit neutrophils to the injury site, the proportional amounts of total immune cells at the injury site are quite different. Axolotls recruit only a low number of neutrophils to an injury site and additionally were found to have comparatively low numbers circulating in the blood (Seifert et al., 2012b). Mice in comparisons have much higher levels of circulating neutrophils, many of which arrive at an injury site. Neutrophils function to kill bacteria and also release signaling factors to recruit other inflammatory cells. This relative difference between species could conceivably influence the outcome of the wound healing response (Denis et al., 2013; Godwin and Brockes, 2006; Godwin and Rosenthal, 2014; Levesque et al., 2010; Seifert et al., 2012b). Thus, a combination of these factors in the axolotl may allow for the “immune deficient” phenotype seen. These studies clearly show that at the basic cellular level species that can regenerate do in fact have a similar immune cell repertoire to humans. More recent work in the African spiny mouse has begun to shed more light on the molecular responses of immune cells after injury.
A microarray comparing expression profiles during wounding of Mus musculus and Acomys spp. was recently performed by Maden and colleagues (Brant et al., 2015). This study found that during the wound healing process, there was a strong immune response elicited from Mus musculus, but there was little to no increase in the expression of most cytokines or chemokines assayed when it came to Acomys spp. expression profile (Brant et al., 2015). Additionally, further analysis of Acomys spp. blood revealed it was neutropenic with more mast cells than neutrophils present. This is in stark contrast to Mus musculus (and Homo sapiens) where neutrophils are far more abundant than mast cells in the peripheral blood. Surprisingly, there were no F4/80 positive macrophages found within the Acomys spp. wound (Brant et al., 2017). This is unusual as macrophages are critical in most regenerative species. However, it is possible that macrophages in the spiny mouse do not express the typical markers found in other species and thus are more difficult to identify. As this field matures it will be important to further explore the role of different immune sub-sets and define molecular signatures for each cell type during wound healing in both regeneration competent and incompetent animals.
5.3. Extracellular matrix deposition and remodeling
Another major difference between species that can or cannot heal scar free is in the deposition and remodeling of the extracellular matrix (ECM). In addition, the proteins that comprise the ECM seem to be tied to the ability of the animal to remodel the ECM to pre-injury architecture. Animals that heal by scarring will undergo very early ECM deposition (Denis et al., 2013; Levesque et al., 2010; Seifert et al., 2012b). In Mus musculus, collagen can be detected as early as 3 days after injury, prior to re-epithelialization (Chmielowiec et al., 2007). This is in contrast to regenerating animals, such as zebrafish and axolotls, where collagen levels are decreased and first a provisional matrix made of hyaluronic acid, tenascin C, and fibronectin is first synthesized and deposited (Govindan and Iovine, 2015). In axolotl ECM very little fibronectin is found and the main component of the initial ECM is tenascin C (Seifert et al., 2012b). In axolotls collagen can be first detected around 14 days after injury (Erickson et al., 2016; Seifert et al., 2012a). Similarly, collagen deposition seems to be delayed in the spiny mouse, where collagen deposition occurs by 8 days after injury (Brant et al., 2015). In addition to the timing of the deposition, it also appears that axolotls and spiny mice deposit different collagens as compared to Mus musculus.
In axolotls the use of the histological stain picrosirius red has indicated that collagen type III is the initial collagen to be deposited, and is later replaced by collagen type I (Seifert et al., 2012a). The same picrosirius red staining was also performed on spiny mice, and similar results to the axolotl were seen. In the spiny mouse, more collagen III staining was observed than collagen I during wound healing. This differed drastically from the laboratory mouse, which showed a propensity for collagen type I fibers (Seifert et al., 2012a, 2012b). More recently, a proteomics approach was taken to determine the collagens present within the wounds of both Mus musculus and Acomys spp. In this study, the abundance of many collagens were found to be increased during the wound healing process, the most abundant in Mus musculus being collagen type XII. Interestingly collagen XII was not detected at elevated levels in Acomys spp. In fact very few collagens appeared to be up-regulated during regeneration in the Acomys spp. mice during wound healing (Brant et al., 2015). However, more recent work focusing on the ear punch model of regeneration, which includes full regeneration of scar-free skin the spiny mouse has shown that indeed collagen I and III are up-regulated early in Mus musculus but that one of the main differences in spiny mouse is the amount of collagen I versus ECM components like Tenascin C which are found in the wound bed (Gawriluk et al., 2016). These findings illustrate again the importance of the composition of the extracellular matrix in scar-free epimorphic regeneration.
To date little is known about how the amount and timing of collagen and other extracellular components is controlled during regeneration. Recent work in axolotl has identified a novel role for SALL4 in scar-free wound healing (Erickson et al., 2016). SALL4 was found to actively regulate the transcription of collagen I and collagen XII during skin regeneration. Depletion of SALL4 in the wound bed leads to earlier deposition of both collagens and a lack of eventual remodeling of the collagen; resulting in a scar-like phenotype (Erickson et al., 2016). This work would suggest that the timing and amount of collagen deposited that is important during skin regeneration.
Intriguingly, it is apparent that the composition and structure of the ECM can control both function and migration of immune cells. For example, studies in mice have revealed that during an influenza infection of the lungs, CD8+ T cells will begin to express α1β1 integrin that will allow CD8+ T cells to enter the lung by binding to collagen I and IV (Ray et al., 2004). Further, many ECM molecules, such as proteoglycans, are negatively charged allowing the ECM to interact with other charged molecules, such as growth factors and chemokines. Thus, the structure and the composition of the ECM can affect the local concentration of factors that can regulate the behavior of leukocytes (Hynes, 2009; Sorokin, 2010), Lastly, the density and morphology of the ECM can affect leukocyte migration. Dense ECM can impede leukocyte migration where loose ECM can in fact encourage leukocyte motility (Bonnans et al., 2014). While it is apparent that the ECM can regulate immune function and migration, the relationship of the ECM and immune cells has yet to be explored within regenerating species.
At present we have a many observations about which collagens and other ECM components are present in the wound bed of species that can regenerate as compared to those that cannot regenerate. It is known that elastin is produced during regeneration in pro-regenerative species but that in non-regenerative mammals very little elastin is present in the scar tissue; resulting in its non-flexible texture (Bhangoo et al., 1976). In part this information is largely descriptive, what we are lacking is an understanding of how pro-regenerative species regulate and remodel their ECM to a pre-injury state on a molecular and cellular level.
5.4. Scar-free regeneration, future perspectives
Regeneration research organisms have to date given us unique insights into the complex cellular and molecular circuitry that must be carefully modulated to direct a pro-regenerative scar free response to injury. The development of more molecular tools in these diverse organisms will be essential for researchers to continue to decipher the exact molecular blueprint that enables these animals to regenerate wounds without scarring. Healing wounds scar-free may seem like an unnecessary, purely cosmetic advantage to some but is known to have a dramatic impact on the quality of life of affected individuals. Although scientists are making advances in biomaterials that mimic aspects of the human skin and in developing tools to 3D print skin, these substitutes still lack essential functions of the skin like sensory input, flexibility and thermoregulation. As humans can repair small wounds scar-free, this might suggest that mammals have the latent ability to heal scar-free. This latent capacity for regeneration may, in turn, be inhibited at a certain organismal size, when reparative response becomes favored.
Studies on these amazing research organisms with the natural ability to regenerate that share many commonalities in cell types and molecular circuitry are beginning to unravel intriguing similarities and differences in the response to injury cross-species. In the future, it will be exciting to determine if we can translate these findings into therapies that can promote more effective regenerative responses in humans.
Acknowledgments
We thank Keith Sabin for feedback on the manuscript and Christophe Echeverri for help with illustrations. This work is supported by NIH NICHD R01 HD092451 to KE.
References
- Alcolea MP, Jones PH. Lineage analysis of epidermal stem cells. Cold Spring Harbor Perspectives in Medicine. 2014;4:a015206. doi: 10.1101/cshperspect.a015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almine JF, Wise SG, Weiss AS. Elastin signaling in wound repair. Birth defects research. Part C. Embryo today : reviews. 2012;96:248–257. doi: 10.1002/bdrc.21016. [DOI] [PubMed] [Google Scholar]
- Alwes F, Enjolras C, Averof M. Live imaging reveals the progenitors and cell dynamics of limb regeneration. eLife. 2016:5. doi: 10.7554/eLife.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre S, Kerfourn F, Affaticati P, Guerci A, Ravassard P, Fellah JS. Highly restricted diversity of TCR delta chains of the amphibian Mexican axolotl (Ambystoma mexicanum) in peripheral tissues. European journal of immunology. 2007;37:1621–1633. doi: 10.1002/eji.200636375. [DOI] [PubMed] [Google Scholar]
- Andre S, Kerfourn F, Fellah JS. Molecular and biochemical characterization of the Mexican axolotl CD3 (CD3epsilon and CD3gamma/delta) Immunogenetics. 2011;63:847–853. doi: 10.1007/s00251-011-0560-6. [DOI] [PubMed] [Google Scholar]
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD, Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nature communications. 2017;8:14684. doi: 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Watson CL, Ranjan R, King A, Bollyky PL, Keswani SG. Chemokine Involvement in Fetal and Adult Wound Healing. Advances in wound care. 2015;4:660–672. doi: 10.1089/wound.2014.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;3:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Bellemare J, Roberge CJ, Bergeron D, Lopez-Valle CA, Roy M, Moulin VJ. Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars. The Journal of pathology. 2005;206:1–8. doi: 10.1002/path.1737. [DOI] [PubMed] [Google Scholar]
- Berberich C, Ramirez-Pineda JR, Hambrecht C, Alber G, Skeiky YA, Moll H. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. Journal of immunology (Baltimore, Md : 1950) 2003;170:3171–3179. doi: 10.4049/jimmunol.170.6.3171. [DOI] [PubMed] [Google Scholar]
- Bertolotti E, Malagoli D, Franchini A. Skin wound healing in different aged Xenopus laevis. Journal of morphology. 2013;274:956–964. doi: 10.1002/jmor.20155. [DOI] [PubMed] [Google Scholar]
- Bhangoo K, Quinlivan J, Connelly J. Elastin fibers in scar tissue. Plastic Reconstr Surg. 1976;57:308–313. doi: 10.1097/00006534-197603000-00005. [DOI] [PubMed] [Google Scholar]
- Bin BH, Kim DK, Kim NH, Choi EJ, Bhin J, Kim ST, Gho YS, Lee AY, Lee TR, Cho EG. Fibronectin-Containing Extracellular Vesicles Protect Melanocytes against Ultraviolet Radiation-Induced Cytotoxicity. The Journal of investigative dermatology. 2016;136:957–966. doi: 10.1016/j.jid.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Bloom P, MY The injury and subsequent healing of a serious propeller strike to a bottle nose dolphin resident in the cold waters off the Northumberland coast of England. Aquat Mamm. 1994;20:59–64. [Google Scholar]
- Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nature reviews Molecular cell biology. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli S, Candi E, Hu B, Dolfini D, Ravo M, Grober OM, Weisz A, Dotto GP, Melino G, Vigano MA, Mantovani R. The p63 target HBP1 is required for skin differentiation and stratification. Cell death and differentiation. 2010;17:1896–1907. doi: 10.1038/cdd.2010.59. [DOI] [PubMed] [Google Scholar]
- Brant JO, Lopez MC, Baker HV, Barbazuk WB, Maden M. A Comparative Analysis of Gene Expression Profiles during Skin Regeneration in Mus and Acomys. PloS one. 2015;10:e0142931. doi: 10.1371/journal.pone.0142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant JO, Yoon JH, Polvadore T, Barbazuk WB, Maden M. Cellular events during scar-free skin regeneration in the spiny mouse, Acomys. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2016;24:75–88. doi: 10.1111/wrr.12385. [DOI] [PubMed] [Google Scholar]
- Breathnach AS. Development and differentiation of dermal cells in man. The Journal of investigative dermatology. 1978;71:2–8. doi: 10.1111/1523-1747.ep12543601. [DOI] [PubMed] [Google Scholar]
- Bruce-Allen LJG., Jr Wound healing in the bottle nose dolphin. Can J Fish Aquat Sci. 1985;42:216–228. [Google Scholar]
- Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Advances in clinical chemistry. 2009;48:137–161. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- Caddy J, Wilanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, Rank G, Auden A, Srivastava S, Papenfuss TA, Murdoch JN, Humbert PO, Parekh V, Boulos N, Weber T, Zuo J, Cunningham JM, Jane SM. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Developmental cell. 2010;19:138–147. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, Knight RA, Melino G. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell death and differentiation. 2006;13:1037–1047. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- Charlemagne J. Thymus independent anti-horse erythrocyte antibody response and suppressor T cells in the Mexican axolotl (Amphibia, Urodela, ambystoma mexicanum) Immunology. 1979;36:643–648. [PMC free article] [PubMed] [Google Scholar]
- Chifflet S, Hernandez JA, Grasso S. A possible role for membrane depolarization in epithelial wound healing. American journal of physiology Cell physiology. 2005;288:C1420–1430. doi: 10.1152/ajpcell.00259.2004. [DOI] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. The Journal of cell biology. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Kim HR, Kim KS, Jung YS, Cho JY, Hwang DY, Song HK. Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains. Laboratory animal research. 2017;33:124–131. doi: 10.5625/lar.2017.33.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Cotter JD, Storfer A, Page RB, Beachy CK, Voss SR. Transcriptional response of Mexican axolotls to Ambystoma tigrinum virus (ATV) infection. BMC genomics. 2008;9:493. doi: 10.1186/1471-2164-9-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guerra A, Guillet F, Charlemagne J, Fellah JS. Identification of cDNA clones encoding HMG 2, a major protein of the mexican axolotl hydrocortisone-sensitive thymocytes. Developmental and comparative immunology. 1995;19:417–423. doi: 10.1016/0145-305x(95)00025-o. [DOI] [PubMed] [Google Scholar]
- Denis JF, Levesque M, Tran SD, Camarda AJ, Roy S. Axolotl as a Model to Study Scarless Wound Healing in Vertebrates: Role of the Transforming Growth Factor Beta Signaling Pathway. Advances in wound care. 2013;2:250–260. doi: 10.1089/wound.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays : news and reviews in molecular, cellular and developmental biology. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- Dreymueller D, Denecke B, Ludwig A, Jahnen-Dechent W. Embryonic stem cell-derived M2-like macrophages delay cutaneous wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:44–54. doi: 10.1111/j.1524-475X.2012.00858.x. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends in cell biology. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. The Journal of investigative dermatology. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science translational medicine. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science (New York, NY ) 2017;356:1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- Erickson JR, Gearhart MD, Honson DD, Reid TA, Gardner MK, Moriarity BS, Echeverri K. A novel role for SALL4 during scar-free wound healing in axolotl. Npj Regenerative Medicine. 2016;1:16016. doi: 10.1038/npjregenmed.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto H, Suga H, Aoi N, Kato H, Doi K, Kuno S, Tabata Y, Yoshimura K. Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Laboratory investigation; a journal of technical methods and pathology. 2012;92:214–223. doi: 10.1038/labinvest.2011.127. [DOI] [PubMed] [Google Scholar]
- Fellah JS, Andre S, Kerfourn F, Guerci A, Durand C, Aubet G, Charlemagne J. Structure, diversity and expression of the TCRdelta chains in the Mexican axolotl. European journal of immunology. 2002a;32:1349–1358. doi: 10.1002/1521-4141(200205)32:5<1349::AID-IMMU1349>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Fellah JS, Tuffery P, Etchebest C, Guillet F, Bleux C, Charlemagne J. Cloning and modeling of CD8 beta in the amphibian ambystoma Mexicanum. Evolutionary conserved structures for interactions with major histocompatibility complex (MHC) class I molecules Gene. 2002b;288:95–102. doi: 10.1016/s0378-1119(02)00437-7. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterbush A, Rousso M, Ashur H. Healing and tensile strength of CO2 laser incisions and scalpel wounds in rabbits. Plastic and reconstructive surgery. 1982;70:360–362. doi: 10.1097/00006534-198209000-00012. [DOI] [PubMed] [Google Scholar]
- Franchini A, Bertolotti E. The thymus and skin wound healing in Xenopus laevis adults. Acta histochemica. 2014;116:1141–1147. doi: 10.1016/j.acthis.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Franchini A, Della Rocca A, Bertolotti E. The spleen and skin wound healing in Xenopus adults. Journal of morphology. 2016;277:888–895. doi: 10.1002/jmor.20542. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Current topics in developmental biology. 2016;116:357–374. doi: 10.1016/bs.ctdb.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development (Cambridge, England) 2009;136:2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, Kiama SG, Smith JJ, Ezenwa VO, Seifert AW. Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nature communications. 2016;7:11164. doi: 10.1038/ncomms11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar-free repair and regeneration: insights from regenerative diversity among vertebrates. The international journal of biochemistry & cell biology. 2014;56:47–55. doi: 10.1016/j.biocel.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Godwin JW, Brockes JP. Regeneration, tissue injury and the immune response. Journal of anatomy. 2006;209:423–432. doi: 10.1111/j.1469-7580.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Rosenthal N. Scar-free wound healing and regeneration in amphibians: immunological influences on regenerative success. Differentiation; research in biological diversity. 2014;87:66–75. doi: 10.1016/j.diff.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Govindan J, Iovine MK. Dynamic remodeling of the extra cellular matrix during zebrafish fin regeneration. Gene expression patterns : GEP. 2015;19:21–29. doi: 10.1016/j.gep.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes & development. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Frontiers in bioscience : a journal and virtual library. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- Helmo FR, Machado JR, Guimaraes CS, de Teixeira VP, dos Reis MA, Correa RR. Fetal wound healing biomarkers. Disease markers. 2013;35:939–944. doi: 10.1155/2013/567353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science (New York, NY ) 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development (Cambridge, England) 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MP, van der Hulst RR, Xanthoulea S. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PloS one. 2014;9:e102994. doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Julia MV, Albert A, Morales L, Miro D, Sancho MA, Garcia X. Wound healing in the fetal period: the resistance of the scar to rupture. Journal of pediatric surgery. 1993;28:1458–1462. doi: 10.1016/0022-3468(93)90430-s. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Skjoedt K, Salomonsen J. The MHC molecules of nonmammalian vertebrates. Immunological reviews. 1990;113:83–117. doi: 10.1111/j.1600-065x.1990.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Kawasumi A, Sagawa N, Hayashi S, Yokoyama H, Tamura K. Wound healing in mammals and amphibians: toward limb regeneration in mammals. Current topics in microbiology and immunology. 2013;367:33–49. doi: 10.1007/82_2012_305. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Reinhardt CA, Zbinden G. Collagen synthesis in growing human skin fibroblasts. Experimental cell biology. 1986;54:177–182. doi: 10.1159/000163354. [DOI] [PubMed] [Google Scholar]
- Kishi K, Okabe K, Shimizu R, Kubota Y. Fetal skin possesses the ability to regenerate completely: complete regeneration of skin. The Keio journal of medicine. 2012;61:101–108. doi: 10.2302/kjm.2011-0002-ir. [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes & development. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven EN, Oti M, Niehues H, van Heeringen SJ, Schalkwijk J, Stunnenberg HG, van Bokhoven H, Zhou H. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO reports. 2015;16:863–878. doi: 10.15252/embr.201439941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata S, Okuyama T, Osada M, Watanabe T, Tomimori Y, Sato S, Iwai A, Tsuji T, Ikawa Y, Katoh I. p51/p63 Controls subunit alpha3 of the major epidermis integrin anchoring the stem cells to the niche. The Journal of biological chemistry. 2004;279:50069–50077. doi: 10.1074/jbc.M406322200. [DOI] [PubMed] [Google Scholar]
- Laurens V, Chapusot C, del Rosario Ordonez M, Bentrari F, Padros MR, Tournefier A. Axolotl MHC class II beta chain: predominance of one allele and alternative splicing of the beta1 domain. European journal of immunology. 2001;31:506–515. doi: 10.1002/1521-4141(200102)31:2<506::aid-immu506>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, Longaker MT. Scarless wound healing: finding the right cells and signals. Cell and tissue research. 2016;365:483–493. doi: 10.1007/s00441-016-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBert DC, Huttenlocher A. Inflammation and wound repair. Seminars in immunology. 2014;26:315–320. doi: 10.1016/j.smim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBert DC, Squirrell JM, Rindy J, Broadbridge E, Lui Y, Zakrzewska A, Eliceiri KW, Meijer AH, Huttenlocher A. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development (Cambridge, England) 2015;142:2136–2146. doi: 10.1242/dev.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Park SE, Rah DK. Effects of hepatocyte growth factor on collagen synthesis and matrix metalloproteinase production in keloids. Journal of Korean medical science. 2011;26:1081–1086. doi: 10.3346/jkms.2011.26.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena AM, Shalom-Feuerstein R, Rivetti di Val Cervo P, Aberdam D, Knight RA, Melino G, Candi E. miR-203 represses 'stemness' by repressing DeltaNp63. Cell death and differentiation. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- Levesque M, Villiard E, Roy S. Skin wound healing in axolotls: a scarless process. Journal of experimental zoology Part B, Molecular and developmental evolution. 2010;314:684–697. doi: 10.1002/jez.b.21371. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao H, Liu Z, McMahon C. Deer antler–a novel model for studying organ regeneration in mammals. The international journal of biochemistry & cell biology. 2014;56:111–122. doi: 10.1016/j.biocel.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Lisse TS, King BL, Rieger S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing. Scientific reports. 2016;6:20328. doi: 10.1038/srep20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisse TS, Rieger S. IKKalpha regulates human keratinocyte migration through surveillance of the redox environment. Journal of cell science. 2017;130:975–988. doi: 10.1242/jcs.197343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Advances in wound care. 2016;5:65–78. doi: 10.1089/wound.2014.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. The British journal of dermatology. 2015;173:370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- McDade SS, McCance DJ. The role of p63 in epidermal morphogenesis and neoplasia. Biochemical Society transactions. 2010;38:223–228. doi: 10.1042/BST0380223. [DOI] [PubMed] [Google Scholar]
- Mercer SE, Cheng CH, Atkinson DL, Krcmery J, Guzman CE, Kent DT, Zukor K, Marx KA, Odelberg SJ, Simon HG. Multi-tissue microarray analysis identifies a molecular signature of regeneration. PloS one. 2012;7:e52375. doi: 10.1371/journal.pone.0052375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino GT, McKeon C, de Crombrugghe B, Pastan I. Regulation of the expression of genes encoding types I, II, and III collagen during chick embryonic development. The Journal of biological chemistry. 1983;258:10041–10048. [PubMed] [Google Scholar]
- Mescher AL, Neff AW, King MW. Changes in the inflammatory response to injury and its resolution during the loss of regenerative capacity in developing Xenopus limbs. PloS one. 2013;8:e80477. doi: 10.1371/journal.pone.0080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW, King MW. Inflammation and immunity in organ regeneration. Developmental and comparative immunology. 2017;66:98–110. doi: 10.1016/j.dci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Mikkola ML. p63 in skin appendage development. Cell cycle (Georgetown, Tex ) 2007;6:285–290. doi: 10.4161/cc.6.3.3798. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Uchiyama K, Imokawa Y, Yoshizato K. Cloning and characterization of cDNAs for matrix metalloproteinases of regenerating newt limbs. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6819–6824. doi: 10.1073/pnas.93.13.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes GS, Junqueira LC. Biology of collagen. Revue canadienne de biologie experimentale. 1982;41:143–156. [PubMed] [Google Scholar]
- Morin G, Rand M, Burgess LP, Voussoughi J, Graeber GM. Wound healing: relationship of wound closing tension to tensile strength in rats. The Laryngoscope. 1989;99:783–788. doi: 10.1288/00005537-198908000-00003. [DOI] [PubMed] [Google Scholar]
- Murawala P, Tanaka EM, Currie JD. Regeneration: the ultimate example of wound healing. Seminars in cell & developmental biology. 2012;23:954–962. doi: 10.1016/j.semcdb.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? International journal of dermatology. 2011;50:85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- Nassar D, Blanpain C. Epidermal development and homeostasis. Seminars in cell & developmental biology. 2012;23:883. doi: 10.1016/j.semcdb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. The international journal of biochemistry & cell biology. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Okuyama R, Ogawa E, Nagoshi H, Yabuki M, Kurihara A, Terui T, Aiba S, Obinata M, Tagami H, Ikawa S. p53 homologue, p51/p63, maintains the immaturity of keratinocyte stem cells by inhibiting Notch1 activity. Oncogene. 2007;26:4478–4488. doi: 10.1038/sj.onc.1210235. [DOI] [PubMed] [Google Scholar]
- Onda H, Goldhamer DJ, Tassava RA. An extracellular matrix molecule of newt and axolotl regenerating limb blastemas and embryonic limb buds: immunological relationship of MT1 antigen with tenascin. Development. 1990;108:657–668. doi: 10.1242/dev.108.4.657. [DOI] [PubMed] [Google Scholar]
- Otsuka-Yamaguchi R, Kawasumi-Kita A, Kudo N, Izutsu Y, Tamura K, Yokoyama H. Cells from subcutaneous tissues contribute to scarless skin regeneration in Xenopus laevis froglets. Developmental dynamics : an official publication of the American Association of Anatomists. 2017;246:585–597. doi: 10.1002/dvdy.24520. [DOI] [PubMed] [Google Scholar]
- Paredes R, Ishibashi S, Borrill R, Robert J, Amaya E. Xenopus: An in vivo model for imaging the inflammatory response following injury and bacterial infection. Developmental biology. 2015;408:213–228. doi: 10.1016/j.ydbio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. International journal of burns and trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. The Journal of cell biology. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. The Journal of cell biology. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi S, Zambelli F, Merico D, Pavesi G, Robert A, Maltere P, Gidrol X, Mantovani R, Vigano MA. Transcriptional network of p63 in human keratinocytes. PloS one. 2009;4:e5008. doi: 10.1371/journal.pone.0005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakers S, Gebert M, Uppalapati S, Meyer W, Maderson P, Sell AF, Kruse C, Paus R. 'Fish matters': the relevance of fish skin biology to investigative dermatology. Experimental dermatology. 2010;19:313–324. doi: 10.1111/j.1600-0625.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Current biology : CB. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzell W, Wood W, Martin P. Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation. Development (Cambridge, England) 2014;141:1814–1820. doi: 10.1242/dev.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke JM, Sorg H. Wound repair and regeneration. European surgical research. Europaische chirurgische Forschung Recherches chirurgicales europeennes. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- Rezvani O, Shabbak E, Aslani A, Bidar R, Jafari M, Safarnezhad S. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy/wound management. 2009;55:22–28. [PubMed] [Google Scholar]
- Richardson R, Hammerschmidt M. The role of Rho kinase (Rock) in re-epithelialization of adult zebrafish skin wounds. Small GTPases. 2016:1–7. doi: 10.1080/21541248.2016.1219208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Metzger M, Knyphausen P, Ramezani T, Slanchev K, Kraus C, Schmelzer E, Hammerschmidt M. Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals. Development (Cambridge, England) 2016;143:2077–2088. doi: 10.1242/dev.130492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano RA, Sinha S. Dynamic life of a skin keratinocyte: an intimate tryst with the master regulator p63. Indian journal of experimental biology. 2011;49:721–731. [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, Boucher J, Klein AM, Greco V. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science (New York, NY) 2016;352:1471–1474. doi: 10.1126/science.aaf7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosique RG, Rosique MJ, Farina JA., Junior Curbing Inflammation in Skin Wound Healing: A Review. International journal of inflammation. 2015;2015:316235. doi: 10.1155/2015/316235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roten SV, Bhat S, Bhawan J. Elastic fibers in scar tissue. Journal of cutaneous pathology. 1996;23:37–42. doi: 10.1111/j.1600-0560.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Chang ES, Welgus HG, Parks WC. Expression of interstitial collagenase, 92-kDa gelatinase, and tissue inhibitor of metalloproteinases-1 in granuloma annulare and necrobiosis lipoidica diabeticorum. The Journal of investigative dermatology. 1993a;100:335–342. doi: 10.1111/1523-1747.ep12470032. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. The Journal of clinical investigation. 1993b;92:2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosh N, Windsor LJ, Mahmoudi BS, Li B, Zhang W, Chernoff EA, Rao N, Stocum DL, Song F. Matrix metalloproteinase expression during blastema formation in regeneration-competent versus regeneration-deficient amphibian limbs. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:1127–1141. doi: 10.1002/dvdy.22503. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH. Larsen's Human Embryology E-Book. Elsevier Health Sciences; 2014. [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012a;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Maden M. New insights into vertebrate skin regeneration. International review of cell and molecular biology. 2014;310:129–169. doi: 10.1016/B978-0-12-800180-6.00004-9. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PloS one. 2012b;7:e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. Journal of cell science. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Advances in wound care. 2013;2:357–368. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson R, Clark Brelje T. Atlas of Human Histology. a guide to microscopic structure of cells, tissues and organs. 3 2014. [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nature reviews Immunology. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. Journal of cell science. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Blanpain C. Development and homeostasis of the skin epidermis. Cold Spring Harbor perspectives in biology. 2012;4:a008383. doi: 10.1101/cshperspect.a008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan LR, Ghadially R. Tiers of clonal organization in the epidermis: the epidermal proliferation unit revisited. Stem cell reviews. 2008;4:149–157. doi: 10.1007/s12015-008-9020-6. [DOI] [PubMed] [Google Scholar]
- Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. The Journal of cell biology. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K, Paderi J, Snyder PW, Freeman L, Panitch A. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PloS one. 2011;6:e22139. doi: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D, Senoo M. Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. The Journal of investigative dermatology. 2012;132:2461–2464. doi: 10.1038/jid.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadeu AM, Horsley V. Notch signaling represses p63 expression in the developing surface ectoderm. Development (Cambridge, England) 2013;140:3777–3786. doi: 10.1242/dev.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harbor perspectives in medicine. 2015;5:a023267. doi: 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testoni B, Borrelli S, Tenedini E, Alotto D, Castagnoli C, Piccolo S, Tagliafico E, Ferrari S, Vigano MA, Mantovani R. Identification of new p63 targets in human keratinocytes. Cell cycle (Georgetown, Tex ) 2006;5:2805–2811. doi: 10.4161/cc.5.23.3525. [DOI] [PubMed] [Google Scholar]
- Thornton CS. Some effects of beryllium nitrate on regeneration. The Anatomical record. 1947;99:631. [PubMed] [Google Scholar]
- Thornton CS. Beryllium inhibition of regeneration; morphological effects of beryllium on amputated fore limbs of larval Amblystoma. Journal of morphology. 1949;84:459–493. doi: 10.1002/jmor.1050840305. [DOI] [PubMed] [Google Scholar]
- Tournefier A, Guillet F, Ardavin C, Charlemagne J. Surface markers of axolotl lymphocytes as defined by monoclonal antibodies. Immunology. 1988;63:269–276. [PMC free article] [PubMed] [Google Scholar]
- Truong AB, Khavari PA. Control of keratinocyte proliferation and differentiation by p63. Cell cycle (Georgetown, Tex ) 2007;6:295–299. doi: 10.4161/cc.6.3.3753. [DOI] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes & development. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Booth BA, Polak KL. Collagen biosynthesis by human skin fibroblasts. II. Isolation and further characterization of type I and type III procollagens synthesized in culture. Biochimica et biophysica acta. 1980;624:545–561. doi: 10.1016/0005-2795(80)90095-1. [DOI] [PubMed] [Google Scholar]
- Van Exan RJ, Hardy MH. The differentiation of the dermis in the laboratory mouse. The American journal of anatomy. 1984;169:149–164. doi: 10.1002/aja.1001690204. [DOI] [PubMed] [Google Scholar]
- van Zuijlen PP, Ruurda JJ, van Veen HA, van Marle J, van Trier AJ, Groenevelt F, Kreis RW, Middelkoop E. Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns : journal of the International Society for Burn Injuries. 2003;29:423–431. doi: 10.1016/s0305-4179(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Viticchie G, Lena AM, Cianfarani F, Odorisio T, Annicchiarico-Petruzzelli M, Melino G, Candi E. MicroRNA-203 contributes to skin re-epithelialization. Cell death & disease. 2012;3:e435. doi: 10.1038/cddis.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H, Charlemagne J, Tournefier A, Ferrone S, Jost R, Parisot R, Kaufman J. Wide tissue distribution of axolotl class II molecules occurs independently of thyroxin. Immunogenetics. 1998;47:339–349. doi: 10.1007/s002510050368. [DOI] [PubMed] [Google Scholar]
- Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, Wearda T, Muhonen E, McArdle A, Tevlin R, Atashroo DA, Senarath-Yapa K, Lorenz HP, Gurtner GC, Longaker MT. Scarless wound healing: chasing the holy grail. Plastic and reconstructive surgery. 2015;135:907–917. doi: 10.1097/PRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM. Altered TGF-beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:3–13. doi: 10.1111/wrr.12098. [DOI] [PubMed] [Google Scholar]
- Westfall MD, Pietenpol JA. p63: Molecular complexity in development and cancer. Carcinogenesis. 2004;25:857–864. doi: 10.1093/carcin/bgh148. [DOI] [PubMed] [Google Scholar]
- Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy/wound management. 2007;53:16–31. quiz 32-13. [PubMed] [Google Scholar]
- Willenborg S, Eming SA. Macrophages - sensors and effectors coordinating skin damage and repair. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2014;12(214-221):214–223. doi: 10.1111/ddg.12290. [DOI] [PubMed] [Google Scholar]
- Woodley DT. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatologic clinics. 2017;35:95–100. doi: 10.1016/j.det.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Developmental cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Advances in wound care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Owens DM. The skin: a home to multiple classes of epithelial progenitor cells. Stem cell reviews. 2008;4:113–118. doi: 10.1007/s12015-008-9022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EV, Bryant SV. Developmental regulation of a matrix metalloproteinase during regeneration of axolotl appendages. Developmental biology. 1994;166:696–703. doi: 10.1006/dbio.1994.1348. [DOI] [PubMed] [Google Scholar]
- Yang EV, Gardiner DM, Carlson MR, Nugas CA, Bryant SV. Expression of Mmp-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Developmental dynamics : an official publication of the American Association of Anatomists. 1999;216:2–9. doi: 10.1002/(SICI)1097-0177(199909)216:1<2::AID-DVDY2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Yang J, Plikus MV, Komarova NL. The Role of Symmetric Stem Cell Divisions in Tissue Homeostasis. PLoS computational biology. 2015;11:e1004629. doi: 10.1371/journal.pcbi.1004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth defects research Part C, Embryo today : reviews. 2012;96:325–333. doi: 10.1002/bdrc.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii Y, Matsuzaki T, Ishida H, Ihara S. Wound healing ability of Xenopus laevis embryos. II. Morphological analysis of wound marginal epidermis. Development, growth & differentiation. 2005;47:563–572. doi: 10.1111/j.1440-169X.2005.00831.x. [DOI] [PubMed] [Google Scholar]
- Zgheib C, Xu J, Liechty KW. Targeting Inflammatory Cytokines and Extracellular Matrix Composition to Promote Wound Regeneration. Advances in wound care. 2014;3:344–355. doi: 10.1089/wound.2013.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]