Abstract
Oxidative stress is implicated in the pathogenesis of various neurodegenerative diseases, including Alzheimer’s disease. Oxidative stress is a result of a disruption of the equilibrium between antioxidants and oxidants, in favor of oxidants. Since mitochondria are major sites of production and reduction of reactive oxygen species (ROS), measurement of ROS levels can help us determine if mitochondrial functional integrity has been compromised. In this protocol, we describe a method to measure the level of ROS in the nematode Caenorhabditis elegans, using chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA).
Keywords: C. elegans, ROS, Mitochondria, DCF, Oxidative stress, CM-H2DCFDA
Background
The life cycle of ROS is closely associated with the mitochondria. Superoxides are produced as a result of the inevitable electron leak at complex I and complex III from the electron transport chain in the mitochondria. This superoxide is then dismutated to hydrogen peroxide in the mitochondrial matrix and mitochondrial intermembrane space. The superoxide and hydrogen peroxide generated via these processes are considered to be ROS, with the mitochondria being responsible for 90% of endogenous ROS ( Balaban et al., 2005 ). Therefore, measuring ROS levels will provide insight into the status of mitochondrial health. Here, we provide a protocol to determine the levels of ROS in the nematode C. elegans using CM-H2DCFDA, a cell-permeant chloromethyl derivative of a reduced form of fluorescein. Once inside the cell, the CM-H2DCFDA is cleaved by intracellular esterases and the resulting nonfluorescent H2DCF, upon oxidation by ROS, gets converted into the highly fluorescent 2’,7’-dichlorofluorescein (DCF). Imaging and measurement of fluorescence intensity of H2DCFDA in live C. elegans can be confounded by the presence of autofluorescence from the animal’s intestine. Our protocol utilizes worm lysates to measure ROS levels, thereby bypassing this issue (Sarasija and Norman, 2015).
Materials and Reagents
100 mm, 60 mm Petri dishes (Kord-Valmark Labware Products, catalog numbers: 2900, 2901)
15-ml centrifuge tubes (Globe Scientific, catalog number: 6285)
22 x 22 mm coverslip (Globe Scientific, catalog number: 1404-10)
Glass Pasteur pipettes (Krackeler Scientific, catalog number: 6-72050-900)
Vacuum filtration (GE Healthcare, Whatman, catalog number: 6722-5001)
1.5 ml Micro Centrifuge tube (CELLTREAT Scientific, catalog number: 229443)
50 ml conical tubes (Corning, catalog number: 430829)
15 ml conical tubes (Corning, catalog number: 430791)
C. elegans strains and OP50 (Caenorhabditis Genetics Center (CGC), University of Minnesota)
Deionized water (dH2O)
BCA protein assay (Pierce, Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 23227)
Chloro methyl-2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) (Thermo Fisher Scientific, InvitrogenTM, catalog number: C6827)
Polybead Polystyrene 0.10 μm microspheres (Polysciences, catalog number: 00876-15)
Agarose (RPI, catalog number: A20090-500.0)
Clear nail polish (generic)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: BP358-10)
Agar (Fisher Scientific, catalog number: BP1423-2)
Bacto peptone (BD, BactoTM, catalog number: 211677)
Calcium chloride dihydrate (CaCl2·2H2O) (Fisher Scientific, catalog number: C79-500)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Fisher Scientific, catalog number: BP213-1)
Potassium phosphate dibasic (K2HPO4) (Fisher Scientific, catalog number: BP363-1)
Cholesterol (Fisher Scientific, catalog number: C314-500)
Potassium phosphate monobasic (KH2PO4) (Fisher Scientific, catalog number: P285-500)
Potassium chloride (KCl) (Fisher Scientific, catalog number: BP366-1)
5-Fluoro-2’-deoxyuridine (FUDR) (bioWORLD, catalog number: 40690016-1)
Bacto tryptone (BD, BactoTM, catalog number: 211705)
Bacto yeast extract (BD, BactoTM, catalog number: 212750)
Sodium hydroxide (NaOH) (Fisher Scientific, catalog number: BP359-500)
Sodium phosphate dibasic anhydrous (Na2HPO4) (Fisher Scientific, catalog number: BP332-1)
Bleach (generic, plain)
Hydrogen chloride (HCl) (Fisher Scientific, catalog number: A144-500)
Standard Nematode Growth Media (NGM) plates (see Recipes)
Sterile solutions (see Recipes)
5-Fluoro-2’-deoxyuridine containing NGM plates (see Recipes)
-
Sterile stocks for NGM (see Recipes)
1 M CaCl2
1 M MgSO4
1 M K2HPO4
1 M KH2PO4
1 M KPO4
LB medium (see Recipes)
M9 buffer (see Recipes)
Bleach solution (see Recipes)
10 N NaOH (see Recipes)
10x PBS (see Recipes)
Equipment
Single channel pipettes (Rainin, models: PR-10, PR-20, PR-200, PR-1000)
Finnpipette II Multichannel pipettes (Fisher Scientific, model: FisherbrandTM FinnpipetteTM II, catalog number: 21377830)
Fisher Scientific Sonic Dismembrator with microtip probe (Fisher Scientific, model: Sonic Dismembrator Model 100)
Thermo Electron Corporation IEC Centra CL2 Centrifuge (Thermo Fischer Scientific, Thermo ScientificTM, model: IEC Centra CL2)
Eppendorf 5415D Centrifuge (Eppendorf, model: 5415 D)
Castle Steam Sterilizer Autoclave (Getinge, model: 433/533HC-E)
Zeiss SteREO Discovery. V8 microscope with SCHOTT Ace® I light source (ZEISS, model: SteREO Discovery.V8)
Molecular Devices FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, model: FlexStation® 3)
PYREX® Griffin beakers (Corning, catalog number: 1000-PACK)
PYREX® Reusable Media Storage Bottles (Fisher Scientific)
Software
SoftMax® Pro 6 Software (Molecular Devices)
Microsoft Office 2011 Excel (Microsoft Corporation, Redmond, USA)
GraphPad Prism 5 software package (GraphPad Software Inc., San Diego, USA)
Procedure
-
Growth and synchronization of nematode population
Pick L4 larvae of animals of desired genetic backgrounds onto 100 mm or 60 mm NGM plates freshly seeded with E. coli (OP50). Use at least two 100 mm or four 60 mm NGM plates for each nematode strain with 5-10 and 20-30 animals each, respectively.
Incubate the animals at 20 °C for 3-4 days till plates contain a large number of eggs.
Wash the eggs and nematodes off the plates into individual 15-ml centrifuge tubes for each genotype using about 4 ml of M9 per plate of nematodes. Spin these down for 3 min at 6,180 × g in a clinical centrifuge equipped with a swing basket rotor and aspirate out the M9. Add 3-4 ml of the bleach solution to each tube containing the worm pellet and vortex at full speed for 15 sec every 2 min for 6 min. Add M9 to fill each tube and spin at 6,180 × g for 1 min. Repeat the wash with M9 three times and move the worm eggs/carcass pellet to a fresh half-filled 15-ml tube of M9. To synchronize, allow the animals to hatch in the absence of food by nutating at 20 °C for anywhere between 16-48 h (make sure that within an experiment all strains are synchronized for similar amounts of time).
-
Due to lack of food, all the animals will arrest at L1 stage of larval growth. Spin these tubes down at 6,180 × g for 1.5 min and put the worms down on individual OP50 seeded NGM plates and keep at 20 °C (approximately 6,000 to 10,000 worms per 100 mm plate). These animals will reach the L4 larval stage at ~42 h, at which point they should be moved to FUDR plates to prevent them from producing progeny (and to avoid complication of ROS production of progeny). These sterilized animals will be analyzed the next day (they will be day 1 adult animals at ~66 h) for the assay.
Note: We have observed no difference in phenotypes between animals age synchronized by starving for 24 h vs. 48 h.
-
Worm lysate preparation
Wash the day 1 adult animals off of the plates with 10 ml of M9 into individual 15-ml centrifuge tubes using glass Pasteur pipettes. Add another 5 ml of M9 to each tube and allow worms to sediment gravitationally on the bench for ~10 min (to reduce the sedimentation of OP50). Aspirate out as much of the M9 with OP50 without compromising the worm ‘pellet’ as possible, retaining approximately 0.5-1 ml of worm slurry. Add more M9 to the tube to bring up the volume back to 15 ml and repeat three more times to remove as much of the OP50 containing M9 as possible.
Spin the animals down for a minute at 6,180 × g and aspirate to retain just the worm pellet. Add 15 ml of 1x PBS to each tube, mix to disperse the worm pellet and then spin down for 3 min at 6,180 × g. Aspirate out this PBS and resuspend the worm pellet in a fresh 100 μl of PBS.
Move the nematodes in PBS to 1.5-ml centrifuge tubes and place them at -80 °C for 15 min to freeze crack the animals. Place the tubes in a bucket of ice and sonicate each tube for 10 sec on ice at continuous operation with the power set to 3 on the dismembrator. Spin the sonicated mixture down at 20,000 × g for 10 min at 4 °C to separate the worm lysate from the worm carcass pellet. Save as much of the worm lysate (~100 μl) as possible from each tube.
Use 10 μl of each worm lysate sample to determine the protein concentration using the BCA Protein Assay Kit (Pierce) following manufacturer’s instructions. (On average, you should have about 100 μg of protein per 100 μl of lysate.)
Determine the volume of lysate needed for 25 μg of protein for each sample. Add this volume to a new tube and bring up all the samples to 50 µl by adding an appropriate volume of PBS.
-
Chloromethyl-H2DCFDA dye preparation and Fluorescence measurement
Dissolve each tube of 50 μg of Chloromethyl-H2DCFDA in 45 µl of DMSO.
Dilute this further with 300 µl of PBS to obtain 250 μM of Chloromethyl-H2DCFDA.
Incubate the 50 µl lysates containing 25 μg of protein or no worm control (PBS) with 100 μl of 250 μM of Chloromethyl-H2DCFDA in PBS in triplicate at 37 °C for 4 h. The total volume of the reaction mixture with lysate and dye will be 150 μl. Discard any dye solution not used to avoid freeze-thaw cycles.
Measure fluorescence intensity using a Flex Station 3 Reader (Molecular Devices) at an excitation of 485 nm and emission of 535 nm.
-
Perform 3 separate biological replicates.
Note: Worm lysates for ROS measurement cannot be frozen for future use.
Data analysis
Using Microsoft Excel, normalize all fluorescence intensity values for samples by subtracting the average no-worm control fluorescence intensity from them.
These values can then be further normalized to the fluorescence intensity of wild type.
The fluorescence intensity of each strain normalized to wild type is then entered into GraphPad Prism and analyzed using one-way ANOVA with Bonferroni post-test.
Notes
Check on nematodes every day to make sure that they are not at the risk of starving. If the bacterial lawn on the plates looks thin, nematodes need to be promptly moved to new OP50 seeded NGM plates by picking. Starved animals should never be used for assaying.
While sedimenting the nematodes by gravity separation, hold the tubes up against the light to make sure nematodes have settled down before aspirating.
Make sure the sonicator tip is submerged in the PBS-worm mixture during sonication.
BCA protein kit suggests a 1:10 dilution of the worm lysate, do not do this; use lysate undiluted. We discovered that the protein concentration of worm lysates tend to be low and therefore a 10 fold dilution resulted in protein concentrations that were too low to be measured via a BCA assay.
We use a concentration of FuDR that has been previously used (Park, 2009). Although we have not noted mitochondrial function defects in our analyses, it should be noted that FuDR might affect the mitochondria. For example, it has been reported that FuDR treatment can extend the lifespan of complex I gas-1 mutants, but does not show a robust effect on wild type animals (van Raamsdonk and Hekimi, 2011).
For further information on C. elegans growth and maintenance, please refer to the chapter on ‘Maintenance of C. elegans’ on WormBook available at http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.pdf.
Please refer to Sarasija and Norman (2015) for representative data.
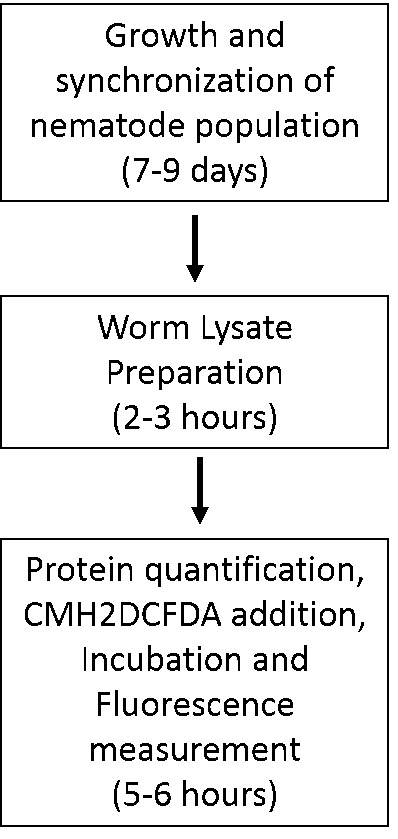
The schematic below can aid the reader in planning for these experiments (Figure 1).
Figure 1. Flowchart depicting stages and timelines of assay execution.
Recipes
Note: For additional information, please refer to He, 2011.
-
Standard Nematode Growth Media (NGM) plates
1 L 0.5 L 0.25 L NaCl 3 g 1.5 g 0.75 g Bacto-agar 17 g 8.5 g 4.25 g Bacto-peptone 2.5 g 1.25 g 0.625 g Store at 4 °C -
Sterile solutions
-
Autoclave on liquid cycle (45 min exposure), allow to cool to ~60 °C and then use sterile technique and add the following:
1 L 0.5 L 0.25 L 1 M CaCl2 1 ml 0.5 ml 0.25 ml 1 M MgSO4 1 ml 0.5 ml 0.25 ml 1 M KPO4 25 ml 12.5 ml 6.25 ml 5 mg/ml Cholesterol 1 ml 0.5 ml 0.25 ml Swirl to mix thoroughly after each addition. After all additions are made, pour plates
To make liquid NGM, follow the same protocol but omit the addition of Bacto-agar (and obviously no need to pour into plates). Grow up OP50 in LB media overnight at room temperature and seed plates
-
-
5-Fluoro-2’-deoxyuridine containing NGM plates
-
Follow the protocol for making standard NGM plates. Add the appropriate FUDR amount (from the table below) to 1 ml of M9 and dissolve with vortexing
1 L 0.5 L 0.25 L FUDR 500 mg 250 mg 125 mg Add this FUDR solution to the final liquid NGM and mix well by swirling gently and pour to make 2 mM FUDR plates. FUDR is a pyrimidine analog that inhibits DNA synthesis and thereby prevents gamete formation in the animals, effectively sterilizing them
Therefore, OP50 used to seed plates containing FUDR will have to be grown overnight with shaking at 37 °C and then spun down to concentrate ten fold and 100 µl of this is used to seed each FUDR plates
-
-
Sterile stocks for NGM
-
1 M CaCl2
In a beaker (sterile if possible), add:
110.9 g CaCl2
dH2O to 250 ml
Dissolve thoroughly and use vacuum filtration to sterilize, label, and store at room temperature
-
1 M MgSO4
In a beaker (sterile if possible), add:
120.3 g MgSO4
dH2O to 250 ml
Dissolve thoroughly and use vacuum filtration to sterilize, label, and store at room temperature
-
1 M K2HPO4
174.18 g bring up to 1 L with dH2O
-
1 M KH2PO4
136.01 g bring up to 1 L with dH2O and heat
1 M KPO4 pH 6.0
Measure out 132 ml of 1 M K2HPO4 and 868 ml of 1 M KH2PO4
Combine, and autoclave on liquid cycle (45 min exposure)
Store at room temperature
-
-
LB medium
1 L dH2O 1,000 ml NaCl 10 g Bacto tryptone 10 g Bacto yeast extract 5 g Adjust pH from ~6.9 to 7.5 with NaOH
Aliquot 250 ml per bottle and autoclave on liquid cycle (15 min exposure)
-
M9 buffer (1 L) (store at room temperature)
dH2O 1,000 ml NaCl 5 g KH2PO4 3 g Na2HPO4 6 g 1 M MgSO4 1 ml *add after autoclaving Split between 2 bottles: 500 ml each. Autoclave on liquid cycle (15 min exposure)
-
Bleach solution (store at room temperature)
25 ml final volume 50 ml final volume dH2O 18 ml 36 ml Bleach 7 ml 14 ml 10 N NaOH 400 μl 800 μl -
10 N NaOH
20 g NaOH
dH2O up to 50 ml
Store at room temperature
Notes:
Exercise CAUTION: This reaction is EXOTHERMIC!
50 ml conical tubes should be fine, but make sure lid is tight, keep ice around to cool tube down to prevent conical breakage (expansion by heat).
-
10x PBS
Phosphate buffered saline, recipe from Harlow and Lane:
To make 1 L:
NaCl 80 g KCl 2 g Na2HPO4 14.4 g KH2PO4 2.4 g Dissolve in 800 ml dH2O
Adjust pH to 7.4 with HCl
Bring volume to 1 L and autoclave on liquid cycle (45 min exposure)
Acknowledgments
The authors would like to acknowledge J. T. Laboy for her help in ordering and preparing reagents, and P. McKeown-Longo, Y. Tang, M. Barroso and their lab members for support. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). The Alzheimer’s Association (NIRG-09-132122) and NIH (GM088213) supported this work. The authors declare that there are no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Balaban R. S., Nemoto S. and Finkel T.(2005). Cell 120(4): 483-95. [DOI] [PubMed]
- 2. He F.(2011). Common worm media and buffers. Bio-protocol Bio101: e55. [Google Scholar]
- 3. Park S. K., Tedesco P. M. and Johnson T. E.(2009). Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1 . Aging Cell 8(3): 258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarasija S. and Norman K. R.(2015). A γ-secretase independent role for presenilin in calcium homeostasis impacts mitochondrial function and morphology in Caenorhabditis elegans . Genetics 201(4): 1453-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stiernagle T.(2006). Maintenance of C. elegans. WormBook(Ed.). The C. elegans Research Community: doi/ 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Raamsdonk J. M. and Hekimi S.(2011). FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech Ageing Dev 132(10): 519-521. [DOI] [PMC free article] [PubMed] [Google Scholar]


