ABSTRACT
Most keloids are clinically observed as solid nodules or claw-like extensions. However, they appear hypoechoic on ultrasound images and are therefore easily confused with liquid features such as blood or vessels. The pathological manifestations of typical keloids also include prominent, thick blood vessels. The existing classification of scars fails to reflect the natural history of keloids. The outer characteristics of a typical keloid include bright red hyperplasia with abundant vessels, suggesting the importance of vascular components in the process of scar formation and prompting consideration of the role of inflammation in the development of granular hyperplasia. Additionally, we further considered the potential effectiveness of oral isotretinoin for severe keloids secondary to severe acne. We also explored different principles and applications related to 5-fluorouracil (5-FU), pulsed dye laser (PDL), and CO2 laser treatments for scars.
KEYWORDS: keloid, scar, vessel, granulation, inflammation, acne, isotretinoin, 5-FU, PDL
1. Vascular components and granulation of scars/keloids
Keloids are mostly considered solid, hard plaques and nodules that are unrelated to blood and blood vessels. However, clinical B-ultrasound, which is used to measure the depth of a keloid, shows a low echo, leading to the assumption of liquid components, which is not consistent with keloid features.1 The textbook histopathological features of keloids include several uniform, red-stained, randomly arranged collagen fiber bundles and cracks that are visible in the mature keloid dermis. In fissures caused by shrinkage, blood vessels and red blood cells are clearly visible, suggesting that keloids contain granulation and vascular components.2,3 Additionally, interwoven vascular components are visible in the pathologic structure of hypertrophic scars. As described in textbooks, vertically oriented blood vessels, collagen nodules and fibroblasts are features of keloids.3 We have found in clinical practice that applying a high-pressure local injection of hormones at the edge of a keloid causes the injection to spread laterally along the skin inside the scar, suggesting the presence of channels. Therefore, scars/keloids contain extensive granulation and several vascular components that should not be overlooked.
2. The natural history of scars/keloids
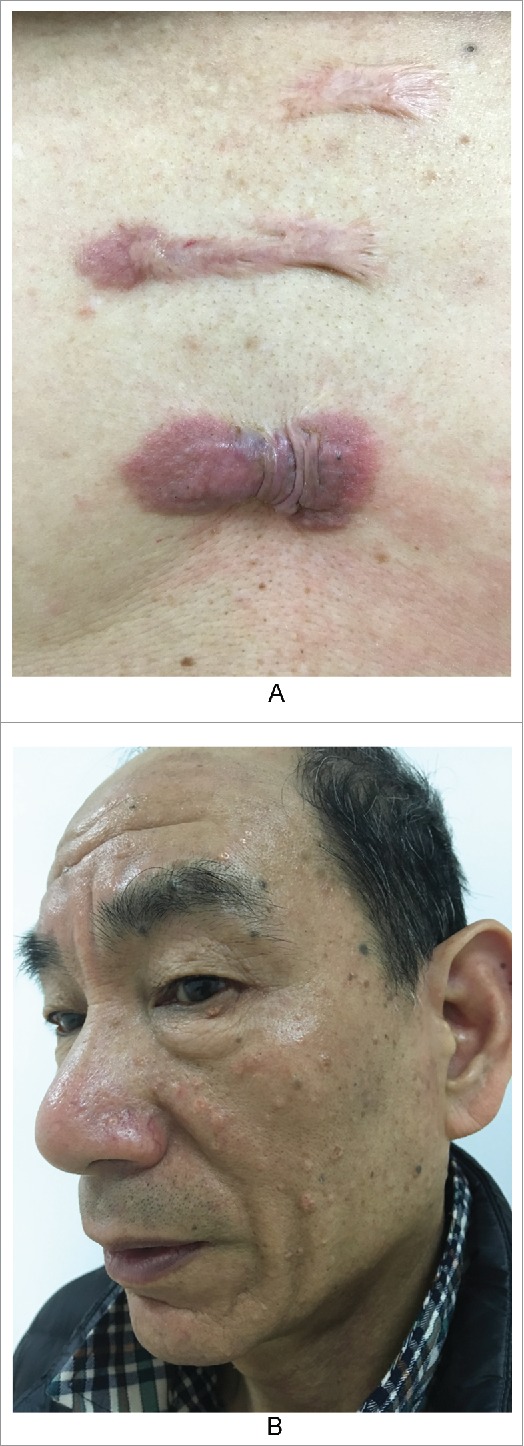
The outer features of typical keloid lesions are bright red raised patches or nodules that grow rapidly. Their inner rings are relatively static and show slow proliferation and dark color, and the middle portions are gray, similar to atrophic scars (Fig. 1A). These features suggest that keloids develop in stages based on their natural history and that in hypertrophic scars, which we usually focus on, proliferation or atrophy is only one part of this natural history.3-5 In clinical practice, we observed a patient with a keloid on the left breast due to suppurative mastitis that had been in the stationary phase for approximately thirty years. However, when she was sixty years old, the scar tissue appeared to reactivate, showing re-proliferation at the edge of the keloid with itching symptoms (Fig. 1B). It is necessary to determine the cause of such phenomena.
Figure 1.
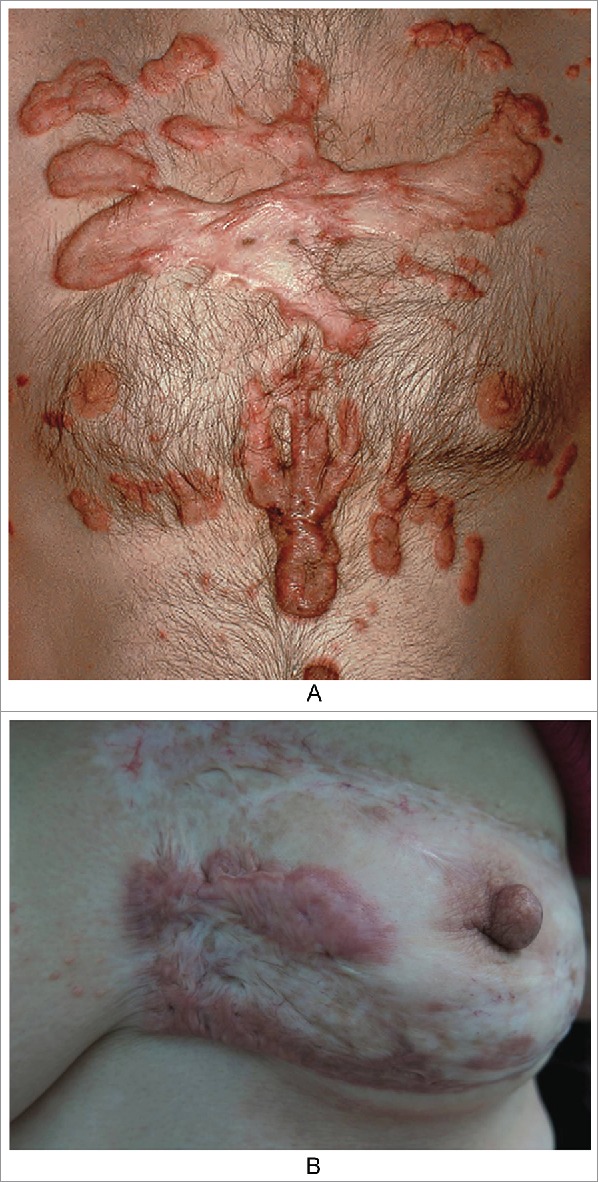
the nature history of keloid. A. From outside to inside: the bright red uplift patches/nodules, growing rapidly; inner ring static relatively,with dark color; then the middle shows atrophy.(from Bolognia JL,Jorizzo JL,Schaffer JV .Dermatology (3rd Edition). Amsterdam(NL):Elsevier;2012.p1622.Fig.98.2B) B. The atrophic scar in the breast with the re-proliferation at the edge.
3. Pivotal role of inflammation in scars/keloids
A scar forms due to inflammation and trauma. After trauma, many inflammatory factors are released, and the process of granulation and tissue repair is also activated. In this article, we focus on the role of post-traumatic inflammation. The presence of persistent inflammation leads to granulation and tissue repair, angiogenesis, and then scar formation; if this process is relatively slow, then continuous hyperplasia will result in a hypertrophic scar.6
Greaves NS et al. 2013.7 reported that after trauma, coagulation and inflammatory reactions are initiated first, and various inflammatory factors released by the tissue promote the aggregation of neutrophils, which are later replaced by macrophages. Through the effects of various chemotactic factors and cytokines, fibroblasts and endothelial cells gradually accumulate at the wound and continue to proliferate. Mediated by inflammatory factors (including NO, histamine, Ang-1, prostacyclin and vascular endothelial growth factor), the capillaries at the edge of the wound expand, and their permeability increases, which is beneficial to the extravasation of endothelial cells and their migration to peripheral vessels. The process is further mediated by platelet-derived factors, extracellular matrix components (heparin and fibronectin), platelet-derived growth factor (PDGF), interleukin (IL)-8, and fibroblast growth factor 2 (FGF-2). FGF-2 induces integrin expression to promote collagen degradation, which induces matrix metalloproteinase 2 (MMP-2) localization and promotes endothelial cell migration. Once the endothelial cells are implanted in the wound, they proliferate and form new capillary lumens, which are involved in the formation of granulation tissue and in the construction of a complete circulatory system. Migrating proliferated fibroblasts and macrophages, new blood vessels, and embedded collagen matrix and hyaluronic acid constitute the granulation tissue. Through the remodeling process, the cells gradually diminish, and a scar eventually manifests.
Case reports
Example 1. Trauma occurred on the left ear due to a low suture, but no scar formed after healing (Fig. 2). Why was scar formation not observed in the region with high tension and a low suture but was observed in the region of low tension? Because the area without the suture healed late, and inflammation persisted, thus stimulating hyperplasia of the granulation tissue. Therefore, a triangular gap without the suture was observed in the top left area, resulting in a scar after healing.
Figure 2.
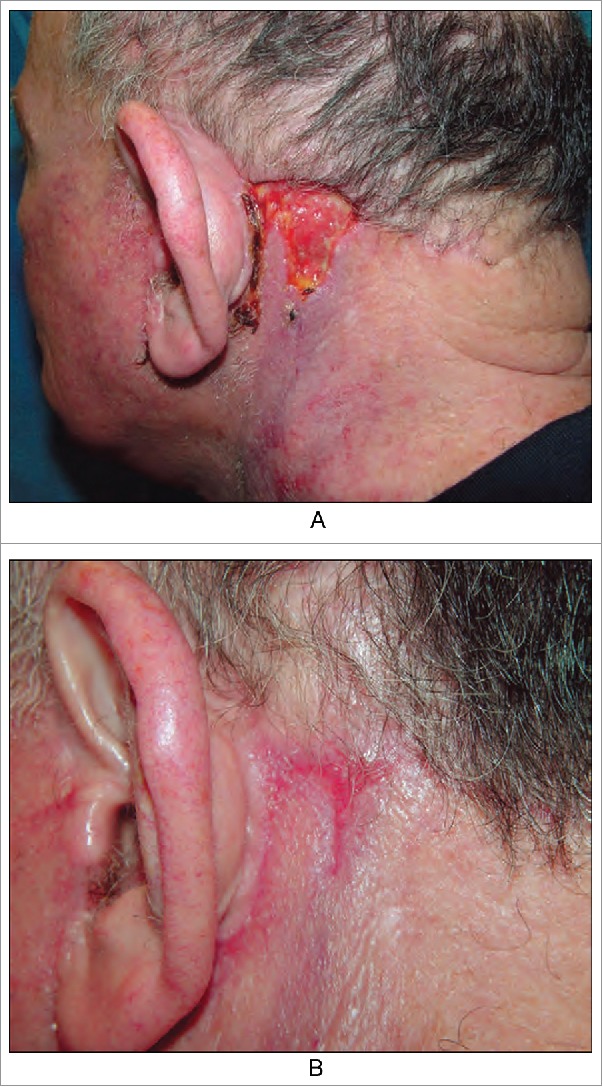
We emphasize repeated inflammation stimulates to the granulation tissue hyperplasia in the area without suture, so leaving scars after healing. (from Bolognia JL,Jorizzo JL,Schaffer JV .Dermatology (3rd Edition). Amsterdam(NL):Elsevier;2012.p2315.Fig.141.4)
Example 2. Redness completely dissipated after compression of inflammatory papules, suggesting that many blood vessels were present within these lesions (Video), which is similar to observations of suppurative granulomas with a large number of vascular components but no pustules. This reflects the link between inflammation and vascular proliferation.8
4. Sebaceous secretions promote inflammation
We observed that sebaceous secretions were the main cause of inflammation in acne in many clinical cases.
Example 3. Several nodules and cysts on the submandibular neck and old scars and keloids on the neck were observed in a patient with inflammatory acne. Obvious cord-like hyperplasia on the back was also evident (Fig. 3AB). This is a continuous and gradual process. Determining how to block this process is challenging and will be the focus of this article.
Figure 3.
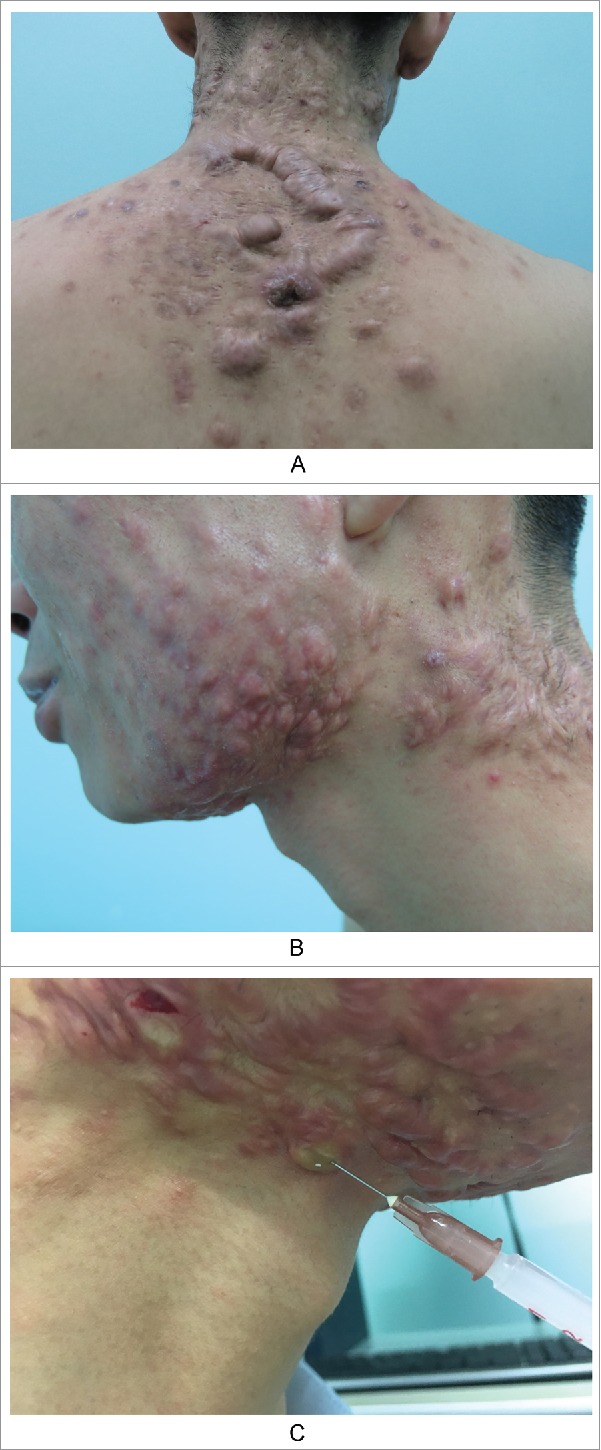
Keloids secondary to severe acne. A. Obvious cord-like hyperplasia on the back; B. Nodules and cysts on the submandibular neck, old scars/keloids on the neck and neck side; C. The contents of sebaceous glands were squeezed out due to the high injection pressure, when we injected the hormone into scar nodules.
Example 4. A patient presented with keloids due to previous acne characterized by irregular nodules and plaques with hard, bright red lesions on both sides of the face (Fig. 4A). After six months of treatment, the scars flattened. Epidermoid cysts were present around the scars (Fig. 4B). In addition to the classical manifestations of epidermoid cysts, peripheral inflammatory responses and inflammatory activity were evident, leading to granulation and tissue repair and, ultimately, scarring.
Figure 4.
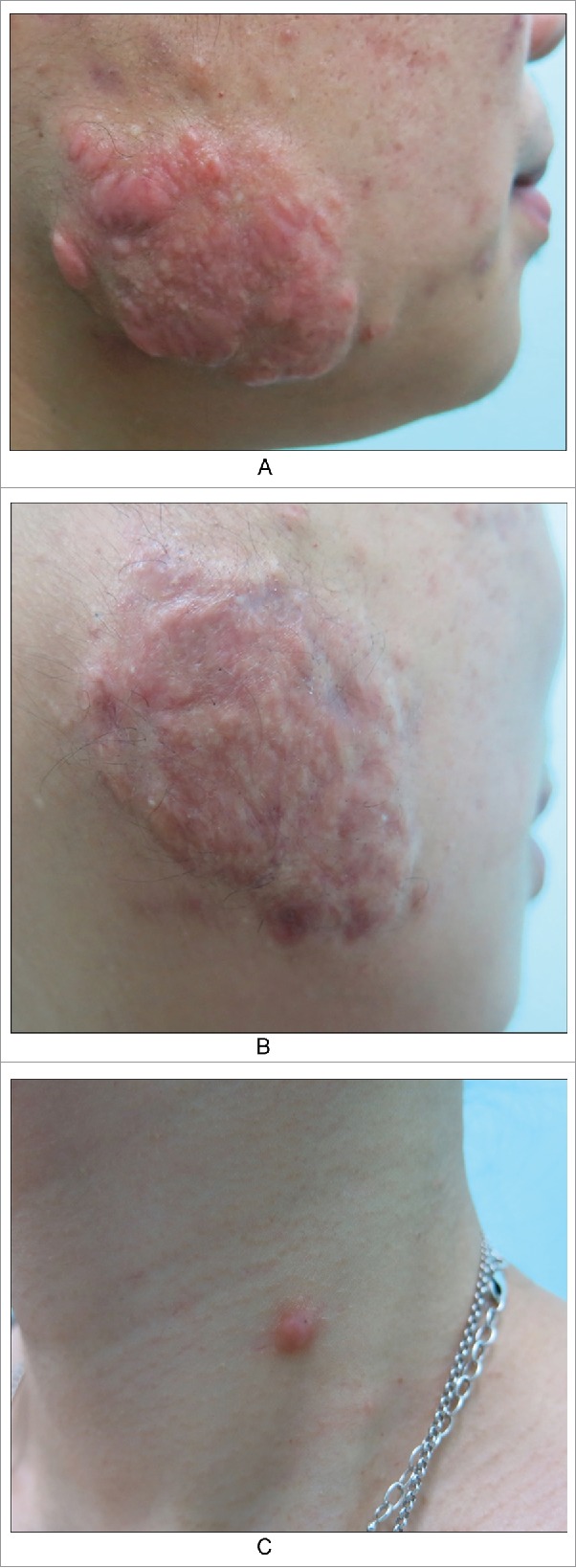
A keloid after acne in cheek. A. Before; B. After six months of treatment, scars flattened. But epidermoid cysts were found around the scar. C. A epidermoid cyst which is already scarring.
Example 5. A patient presented with an epidermoid cyst with a black opening (Fig. 4C). We could not extract the contents through a needle, which appeared to be new, raw granulation tissue, reflecting the scarring process.
Example 6. A patient with keloids on the chest showed improvement after treatment with glucocorticoid and 5-fluorouracil (5-FU), but many blackheads and epidermoid cysts remained (Fig. 5AB). The patient stated that his keloids were caused by folliculitis.
Figure 5.
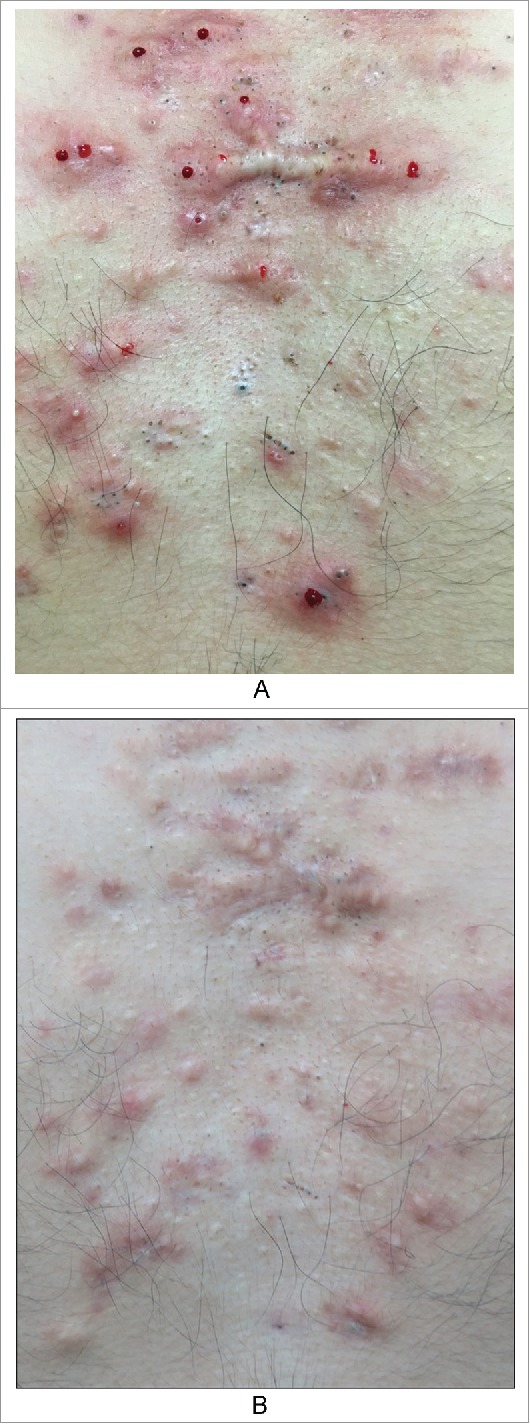
The patient with keloids in the chest. A. Before B. After four months treament of glucocorticoid and 5-fluorouracil, leaving a lot of blackheads and epidermoid cysts.
5. Keloid/hypertrophic scar treatment
Exploring the natural history of scar hyperplasia can guide treatment. First, early trauma healing should be identified, and infection should be prevented. Infection can initiate inflammation and therefore lead to scar formation. Second, vessel occlusion should be performed early. Pulsed dye laser (PDL) or intense pulse light (IPL) with the corresponding wavelengths can be used to treat vascular components. For generalized acne, oral isotretinoin can be used to inhibit inflammation.
6. Therapies targeting blood vessels
The wavelength of the PDL is 585–600 nm. This wavelength can penetrate 2 mm subcutaneously to reach blood vessels. However, it is not necessarily better to use longer wavelengths to reach deeper layers of the skin. In addition to the depth, the maximum absorption effect should occur at the hemoglobin target chromophore. The absorption of hemoglobin at the wavelength of 585–600 nm is optimal. These two factors determine the optimal treatment of blood vessels by PDLs.
International guidelines on hypertrophic scars from 2014 state that the effects of PDLs, CO2 fractional lasers (clinically, fractional lasers are less effective for inhibiting scarring, and the effects of PDLs are clearer), 5-FU and hormones are all precise. However, 5-FU has a greater effect on blood vessels.9 Low-dose 5-FU (less than 1 mg/mL) can prevent fibroblast proliferation, cell cycle arrest and apoptosis at the G2/M phase, but it cannot immediately lead to fibroblast death.10 Injection of 5-FU and corticosteroids to inhibit scar vascular hyperplasia can achieve satisfactory results.11
7. Reducing sebaceous gland secretions to treat keloids after severe acne
Dermatologists are concerned about scarring after acne heals. The guidelines for acne (2016)12 define acne lesions based on comedones, inflammatory papules, pustules and nodules (cysts). Previously, nodules and cysts were defined as two distinct lesions because the nodules or cysts associated with acne cannot be strictly differentiated. Factors that may be associated with clinical extrusion and damage transform nodular lesions into cysts.
For clinical treatment, hormones are injected into scar nodules, and the contents of the sebaceous glands are expelled through high-pressure injections (Fig. 3C). An inflammatory response, as a foreign body reaction, occurs around the injection site. Ma Ying et al13 suggested that inflammation is inherent during the entire course of acne. They also emphasized anti-inflammatory therapy as a treatment. Their article noted that studies by Norris JF14 and others confirmed that the inflammatory response is involved in subclinical skin lesions and that the early inflammatory response eventually leads to the emergence of clinical inflammatory lesions. Some scholars15 have detected IL-1α in isolated sebaceous glands and in the body, which resulted in excessive proliferation and abnormal differentiation of keratinocytes, thereby promoting the growth of clinical microbes. Jeremy16 and others found that the normal pilosebaceous unit of acne patients was extensively involved in inflammation, and the expression levels of CD4+ T-lymphocytes, macrophages, IL-1 and epidermal α-integrin were significantly increased, thus confirming the role of inflammation in micro acne. This prolonged inflammatory response leads to granulation hyperplasia and tissue repair, thus causing scarring and fibrosis. From this point of view, considering the persistence of inflammatory factors in keloids, we can alleviate inflammation by reducing sebaceous gland secretion. Therefore, oral isotretinoin is effective for the healing of generalized persistent acne scars. As another example, a patient with keloids on his chest for 20 years that had become painful during the previous 6 months (Fig. 6A) mentioned that he could eject sebum-like material from the reactive keloid. Extensive sebaceous gland hyperplasia was evident on his face (Fig. 6B). This disease was previously considered to be related to a tumor. However, scholars now believe that this condition only reflects hyperplasia of sebaceous glands. Therefore, the use of oral isotretinoin to inhibit sebaceous gland secretions is a reasonable therapeutic strategy.
Figure 6.
Patient showing keloid and sebaceous gland hyperlplasia. A. The keloids in his chest for 20 years. B. Multiple sebaceous gland hyperplasia in his face.
No clear standard is available for the prevention and treatment of acne scars and keloids in the guidelines for acne (2016).12 In the 2014 edition of the International Scar Guide,17 no description is available regarding the treatment of acne scars. Only the Chinese Guideline of Acne (2014)18 mentions that “oral isotretinoin treatment is required for acne that tends to scar”, but it does not clarify the basis of this recommendation, and no exact assessment was used to determine clinical efficacy. We hope that through this paper, practitioners can clearly understand the key role of inflammatory factors in the process of hypertrophic scar/keloid formation after acne, the key role of comedo/sebaceous gland secretions in inducing inflammation, and the subsequent role of isotretinoin in the treatment and prevention of keloids/hypertrophic scars after acne heals.
Supplementary Material
References
- 1.Lobos N, Wortsman X, Valenzuela F, Alonso F. Color doppler ultrasound assessment of activity in keloids. Dermatol Surg. 2017;0:1–9. doi: 10.1097/DSS.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi: 10.3390/ijms18030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Amsterdam: (NL: ): Elsevier; 2012. p. 1621–5. [Google Scholar]
- 4.CaiJinglong Modern Scar Therapeutics. Beijing: (CHN: ): People's Medical Publishing House; 1998. [Google Scholar]
- 5.CaiJinglong Modern Science of Keloids. 2nd ed. Beijing: (CHN: ): People's Medical Publishing House; 2008. [Google Scholar]
- 6.Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review). Int J Mol Med. 2009;24(3):283–93. doi: 10.3892/ijmm_00000231. PMID:19639219. [DOI] [PubMed] [Google Scholar]
- 7.Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206–17. doi: 10.1016/j.jdermsci.2013.07.008. PMID:23958517. [DOI] [PubMed] [Google Scholar]
- 8.Katta R, Bickle K, Hwang L. Pyogenic granuloma arising in port-wine stain during pregnancy. Br J Dermatol. 2001;144(3):644–5. doi: 10.1046/j.1365-2133.2001.04114.x. PMID:11260044. [DOI] [PubMed] [Google Scholar]
- 9.Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment: a systematic review. Acta Derm Venereol. 2015;95(7):778–82. doi: 10.2340/00015555-2106. PMID:25805099. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Wong YP, Cai YJ, Lung I, Leung CS, Burd A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br J Dermatol. 2010;163(6):1181–5. doi: 10.1111/j.1365-2133.2010.09939.x. PMID:20633010. [DOI] [PubMed] [Google Scholar]
- 11.Wu XL, Liu W, Cao YL. Clinical study on keloid treatment with intralesional injection of low concentration 5-fluorouracil. Chinese J Plast Surg. 2006;22(1):44–46. (in Chinese) [PubMed] [Google Scholar]
- 12.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, Bowe WP, Graber EM, Harper JC, Kang S, et al.. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–73. doi: 10.1016/j.jaad.2015.12.037. PMID:26897386. [DOI] [PubMed] [Google Scholar]
- 13.Ying MA, Leihong X. The new understanding of the pathogenesis of acne and treatment goals. J Clin Dermatol. 2015;44(1):66–69. (in Chinese) [Google Scholar]
- 14.Norris JF, Cunliffe WJ. A histological and immunocytochemical study of early acne lesions. Br J Dermatol. 1988;118(5):651–9. doi: 10.1111/j.1365-2133.1988.tb02566.x. PMID:2969256. [DOI] [PubMed] [Google Scholar]
- 15.Brănişteanu DE, Cotrutz CE, Luca MC, Molodoi DA, Stoica LE, Ianoşi SL, Cianga CM, Brănişteanu DC. Morphopathological stigmata in acne fulminans. Rom J Morphol Embryol. 2015;56(3):1185–90. PMID:26662157. [PubMed] [Google Scholar]
- 16.Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27. doi: 10.1046/j.1523-1747.2003.12321.x. PMID:12839559. [DOI] [PubMed] [Google Scholar]
- 17.Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, Murcia C. International advisory panel on scar management. updated international clinical recommendations on scar management: part 2-algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825–31. doi: 10.1111/dsu.0000000000000050. PMID:25068544. [DOI] [PubMed] [Google Scholar]
- 18.Leihong X, et al.. Acne treatment guidelines expert group in China. guideline for diagnosis and treatment of acne in China(the 2014 revised edition). J Chin Dermatol. 2015;44(1):52–57. (in Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


