Abstract
Early in its life cycle, the marine mollusc Elysia chlorotica Gould forms an intracellular endosymbiotic association with chloroplasts of the chromophytic alga Vaucheria litorea C. Agardh. As a result, the dark green sea slug can be sustained in culture solely by photoautotrophic CO2 fixation for at least 9 months if provided with only light and a source of CO2. Here we demonstrate that the sea slug symbiont chloroplasts maintain photosynthetic oxygen evolution and electron transport activity through photosystems I and II for several months in the absence of any external algal food supply. This activity is correlated to the maintenance of functional levels of chloroplast-encoded photosystem proteins, due in part at least to de novo protein synthesis of chloroplast proteins in the sea slug. Levels of at least one putative algal nuclear encoded protein, a light-harvesting complex protein homolog, were also maintained throughout the 9-month culture period. The chloroplast genome of V. litorea was found to be 119.1 kb, similar to that of other chromophytic algae. Southern analysis and polymerase chain reaction did not detect an algal nuclear genome in the slug, in agreement with earlier microscopic observations. Therefore, the maintenance of photosynthetic activity in the captured chloroplasts is regulated solely by the algal chloroplast and animal nuclear genomes.
The majority of animal-algal symbioses are cellular associations with a unicellular alga either residing between animal cells or within a vacuole produced by the animal (Douglas, 1994). In contrast, the ascoglossan sea slug Elysia chlorotica Gould establishes an intracellular symbiotic association with chloroplasts from the siphonaceous, chromophytic alga Vaucheria litorea C. Agardh (West, 1979; West et al., 1984). Juvenile sea slugs feed on V. litorea filaments and phagocytotically incorporate the chloroplasts into the cytoplasm of one of two morphologically distinct epithelial cells that line the tubules of the digestive system (Graves et al., 1979; West, 1979). During this process the chloroplast endoplasmic reticulum, a structural characteristic of chromophytic plastids (Lee, 1989), is lost resulting in symbiotic plastids with their outer envelope in direct contact with the animal cytoplasm (Graves et al., 1979; Mujer et al., 1996; Rumpho et al., 2000). Heterokont algae (chromophytes or autotrophic stramenopiles) such as V. litorea do not typically contain nucleomorphs, and electron microscopy studies have not revealed any unusual nucleomorph-type structures or algal nuclei in the sea slugs (Graves et al., 1979; Mujer et al., 1996; Rumpho et al., 2000). It is important to note that the captured chloroplasts are functional, i.e. they are capable of light dependent oxygen evolution (Graves et al., 1979; West, 1979).
When maintained in the laboratory in artificial seawater (ASW), E. chlorotica sustains itself apart from any algal food source for at least 9 months when provided with only light and a source of CO2 (Mujer et al., 1996; Pierce et al., 1996). Whether in their native salt marsh or in culture, the life cycle of the sea slugs lasts 8 to 10 months. There is no evidence for plastid division in the animals and the plastids are not transmitted in the eggs; thus, the endosymbiosis must be re-established with each generation (West, 1979; West et al., 1984). Symbiotic associations of this type occur in other ascoglossan species, but they are far more transient (Greene, 1970; Trench, 1975; Clark and Busacca, 1978; Rumpho et al., 2000). The E. chlorotica/V. litorea symbiosis represents the longest known functional association of its kind (West, 1979; Pierce et al., 1996).
The longevity and functional capacity of E. chlorotica is surprising considering the complexity of chloroplast function and regulation evidenced, in part, by the unsuccessful attempts to culture isolated chloroplasts on a long-term basis in an artificial system (Nass, 1969; Ridley and Leech, 1970; Giles and Sarafis, 1971). Seventy percent to 90% of all polypeptides needed for plastid function have a nuclear origin in plants (Reith, 1995; Palmer and Delwiche, 1996; Martin and Herrmann, 1998). Even in chromophytic algae whose chloroplast genomes tend to have a greater coding capacity than chlorophytes (due in part to low intron no. and relatively small inverted repeats), only 120 to 130 gene products are plastid encoded, accounting for only about 13% of all gene products required for plastid function (Reith, 1995; Martin and Herrmann, 1998). Furthermore, although the gene products D1, D2, PsaA/B, and several other polypeptides that assemble to form the photosynthetic complexes are plastid encoded, they depend on nuclear regulation at either or both the transcriptional and translational levels (Stern et al., 1997; Merchant and Dreyfuss, 1998). In turn this nuclear regulation can be influenced by additional environmental and physiological factors (Aro et al., 1993; Christopher and Mullet, 1994; Russell et al., 1995).
The complex nucleocytosolic/chloroplast interactions required for plastid function in plant and algal species presents E. chlorotica with what would seem to be insurmountable obstacles for maintaining plastid function in a foreign environment for such an extended period of time. Despite this, previous results indicated that several chloroplast encoded polypeptides (specifically, D1, D2, CP43, the large subunit of Rubisco [Rubisco LS]), and one probably nuclear encoded protein (related to a fucoxanthin chlorophyll [chl] a/c-binding protein [FCP]) are present in the molluscan chloroplasts (Mujer et al., 1996; Pierce et al., 1996). At least two of these proteins, D1 and Rubisco LS, are synthesized de novo in the animal (Mujer et al., 1996; Pierce et al., 1996). In addition, levels of D1 transcripts were detected in the endosymbiotic chloroplasts for as long as 7 months (Mujer et al., 1996). Taken together, these data suggest that in addition to photosynthesis, the captured plastids are functionally capable of transcription and translation.
To further understand how chloroplast activity can be maintained in an animal cell, we have extended these earlier studies and measured oxygen evolution and photosynthetic electron transport (PET) capacity of E. chlorotica chloroplasts through 9 months after removal of the sea slugs from an algal food source. We have found a preliminary correlation between the presence of photosystem (PS) proteins and activity. We also provide the first molecular evidence supporting earlier structural analysis that the photosynthetically active animals do not contain an intact algal nuclear genome.
RESULTS
Rates of Photosynthesis and Respiration in Intact Animals
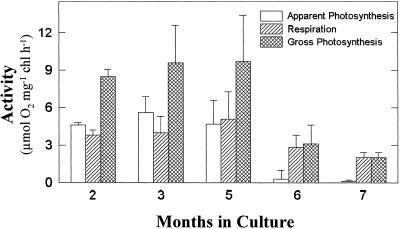
E. chlorotica's symbiont plastids remained photosynthetically competent and capable of splitting water through 7 months in culture in the absence of an algal food source (Fig. 1). Oxygen evolution rates were fairly low relative to typical values obtained for plant and algal specimens including V. litorea filaments, which exhibited rates between 98 and 120 μmol O2 evolved mg−1 chl h−1. The lower sea slug photosynthetic rates probably reflect an underestimate of the true rates due to gaseous diffusion limitations to O2 and CO2 through the mucus-covered sea slug body, the rapid respiration and O2 utilization of the animals even in the light, and the variability in exposing the animals to the actinic light source (the animals tend to fold up). Decreasing illumination below the maximal output of the light source (1,500 μmol photons m−2 s−1) resulted in a decrease in apparent photosynthetic rates (data not shown), suggesting maximal rates may not have been obtained.
Figure 1.
Changes in photosynthetic and respiratory activity in E. chlorotica symbiotically associated with V. litorea chloroplasts and cultured over a 7-month period in the absence of algae. Apparent photosynthesis was calculated by measuring the rate of CO2- and light-dependent O2 evolution. Respiration rates were based on the uptake of O2 in the dark. Gross photosynthesis was estimated by summing the apparent photosynthesis and respiration rates. Data represent means ± se of at least three sea slugs for each time point.
After 5 months of endosymbiotic association, photosynthetic rates dropped significantly in the sea slugs and dark respiration rates exceeded the apparent photosynthetic rates. However, respiration rates also decreased over time in culture, indicating an overall decline in metabolic activity as the animals aged. The decline in activity after 5 months preceded, by 1 to 2 months, a measurable decrease in chl concentration in the sea slugs (Table I). Eighty-five percent of the original chl concentration was maintained through 6 months in culture, ultimately decreasing to 50% by month 9. An increase in the ratio of chl a to chl c was observed at month 7 as a result of a larger decrease in the accessory pigment chl c (80% decline), compared with the reaction center pigment chl a (45% decline). Both pigments remained at a steady, but low, level through the final 3 months. As expected, the concentration of chl in the paler chromophytic alga (0.27 μg chl mg−1 fresh weight) was significantly less than in the densely green sea slugs (1.28 μg chl mg−1 fresh weight at month 1). However, the algal chl a to chl c ratio (17.1) was approximately the same as the sea slug's through the first 6 months in culture, ranging from a low of 14.4 to a high of 17.1 (Table I).
Table I.
Concentration of chl a and chl c in E. chlorotica cultured in the absence of algae for 9 months
| Symbiotic Association | Chl a | Chl c | Chl a/c |
|---|---|---|---|
| months | μg chl mg−1 fresh wt | ||
| 1 | 1.20 | 0.083 | 14.4 |
| 2 | 1.08 | 0.063 | 17.1 |
| 3 | 1.21 | 0.081 | 14.8 |
| 4 | 1.09 | 0.065 | 16.8 |
| 5 | 1.09 | 0.070 | 15.6 |
| 6 | 1.02 | 0.069 | 14.9 |
| 7 | 0.50 | 0.012 | 41.3 |
| 8 | 0.49 | 0.011 | 45.9 |
| 9 | 0.66 | 0.017 | 39.5 |
Intact animals were extracted with 90% acetone and chl a and c quantified by A664 and A630 measurements according to Sterman (1988). Values represent the average of at least three sea slugs for each time point. Corresponding data for V. litorea filaments are chl a = 0.27 μg chl mg−1 fresh wt, chl c = 0.016 μg chl mg−1 fresh wt, and chl a/c = 17.1.
PET Activity
Individual PS activities and whole chain PET were measured in isolated thylakoids to eliminate any permeability problems with the intact animals and to determine the competency of each PS after several months of symbiotic association and separation from the algal nucleus. The ability to isolate chloroplasts from E. chlorotica is limited by the copious amount of mucus produced by the animals (Rumpho et al., 1994); consequently, thylakoid yields were low per extraction despite pooling several sea slugs. These low yields limited the extent of analysis per extraction and collection, thereby necessitating analysis from multiple collections. In any case reproducible results were obtained with subsequent extractions or collections and a consistent pattern for PSI and PSII activity was observed.
At 6 months, the symbiont plastid thylakoids still exhibited rates of PET (whole chain and PSI) comparable with that of the algal thylakoids (Table II), even though CO2 fixation rates had begun to decline (Fig. 1). Whole chain PET rates declined after 6 months, but this decline did not appear to be a specific oxygen evolving complex (OEC) limitation since rates did not increase if the complex was bypassed by using 1,5-diphenylcarbazide (DPC) as the electron donor. In addition, OEC activity was detected through 8 months by measuring PET only through PSII from water to FeCN, although rates were consistently lower in the sea slugs compared with the algal thylakoids. In contrast to whole chain and PSII activity, PSI activity remained very high through 7 months symbiotic association, even exceeding (at months 6 and 7) the average rates measured for algal thylakoids.
Table II.
Photosynthetic electron transport activity of thylakoids isolated from symbiont plastids in E. chlorotica cultured up to 8 months in the absence of algae and from V. litorea chloroplasts
| Partial Reaction | Electron Donor | Electron Acceptor | Months Symbiotic Association
|
Algal Thylakoids | ||
|---|---|---|---|---|---|---|
| 6 | 7 | 8a | ||||
| μmol O2 mg−1 chl h−1 | ||||||
| PSII + PSI (including OEC) | Water | Methylviologen (MV) | 26.4 | 6.3 | 3.7 | 16.0 |
| PSII + PSI (after OEC) | DPC | MV | 7.0 | 2.3 | 3.2 | 11.5a |
| PSII (including OEC) | Water | FeCN | 8.8 | nmb | 4.7 | 47.9a |
| PSI | Dichlorophenolindophenol | MV | 236.0 | 266.5 | 22.4 | 100.5 |
Thylakoids were isolated by differential centrifugation from Percoll-purified chloroplasts. Oxygen exchange values for the sea slug chloroplasts are representative of several pooled animals from each month's collection. Values represent the average of two independent experiments, unless indicated otherwise.
Values represent a single collection of sea slugs or algal extraction.
nm, Not measured.
Total Protein Analysis
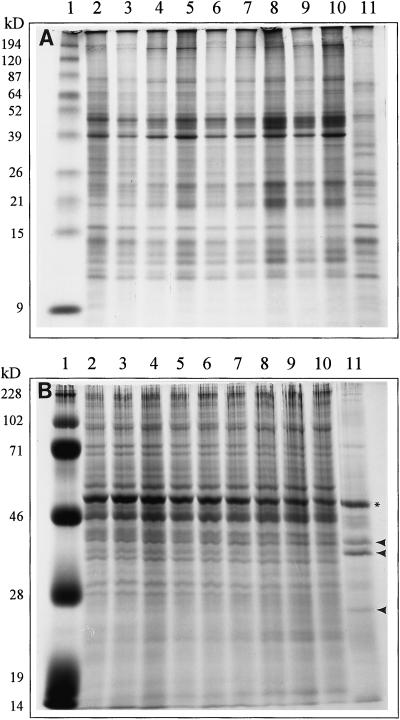
The polypeptide profile of sea slug-enriched thylakoid membrane preparations did not vary significantly over a 9-month time period (Fig. 2A). When compared with thylakoid proteins from V. litorea, there were numerous comigrating bands, especially in the mid-to-low Mr range. There were also significant differences between the slug and algal thylakoid fractions. The most obvious differences centered on a cluster of peptides ranging from about 39 to 50 kD, which increased in relative concentration over time in the sea slug and always exceeded algal levels. These are likely due to contamination of this membrane fraction, a result of the difficulty of extracting chloroplast membranes from the animal.
Figure 2.
Coomassie Brilliant Blue-stained polypeptide patterns of enriched-thylakoid (A) and soluble (B) extracts from E. chlorotica over a 9-month period cultured in the absence of algae and from V. litorea. Lane 1, Molecular mass markers in kD; lanes 2 through 10, E. chlorotica months 1 through 9 in culture; lane 11, V. litorea. Sample size, 50 μg protein per lane. B, Star, 52-kD Rubisco LS protein; arrowheads, 38-, 36-, and 26-kD polypeptides, which predominate in the alga, but are absent or greatly reduced in the sea slugs.
As expected, the algal soluble protein patterns differed vastly from those of E. chlorotica since the soluble fraction contained cytosolic proteins as well as chloroplast stromal proteins (Fig. 2B). The main exception was a prominent 52-kD protein in both organisms that hybridized with antibody to Rubisco LS (data not shown). Three polypeptides with molecular masses of 38, 36, and 26 kD were detected as major polypeptides in the algal extract and absent or very reduced in the sea slug extracts.
Immunoblot Analysis of Chloroplast Proteins
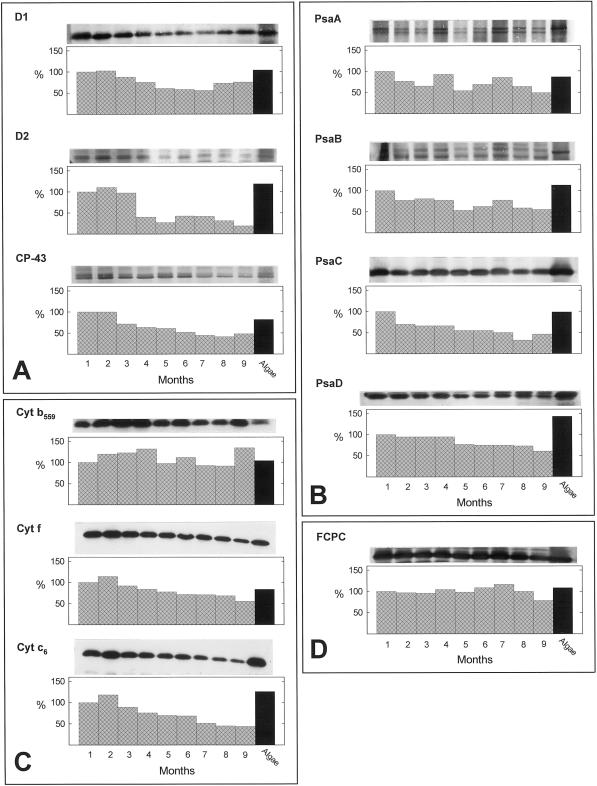
Several PET proteins were identified and quantified by immunoblotting of algal and sea slug solubilized thylakoid extracts. Three major PSII polypeptides D1, D2, and CP-43 were detected in the sea slug over the entire 9 months (Fig. 3A). Of the two core proteins, D2 exhibited the greatest relative decrease, 81% by month 9. D1 never decreased below 50% of the level at month 1. Similarly, the chl a-binding protein, CP-43, showed a steady decline in relative concentration over the 9 months, but remained close to 50% of the level at month 1. Since the algal levels of each of the three PSII proteins approximated those at month 1 in the sea slug, similar trends were seen when comparing changes in the sea slug over time or relative to the alga.
Figure 3.
Immunoblot analysis of chloroplast polypeptides from E. chlorotica over a 9-month culture period in the absence of algae, and V. litorea. The western blot is shown above the corresponding graph of densitometric quantification of each protein. Antibodies used are listed to the left of each panel. For PsaA and PsaB, the larger molecular mass band of the two shown was quantified. A, PSII proteins; B, PSI proteins; C, mobile electron carriers, and D, LHCP. Lanes 1 through 9, E. chlorotica months 1 through 9 of culture; algae lane, V. litorea. The value obtained for month 1 in E. chlorotica (lane 1) was set at 100% and all other values calculated relative to this control.
Both PSI core complex polypeptides (PsaA and PsaB) exhibited doublets in the sea slug and algal extracts although the level of the lower molecular mass band was greatly reduced in the alga (Fig. 3B). This may be due to differential proteolysis in the two organisms and possibly influenced by the levels of the other smaller PSI polypeptides around the core complex as demonstrated in other organisms (Sun et al., 1997). PsaA/B in E. chlorotica never declined below 45% to 50% of month 1 levels or algal levels.
The PSI FA-FB-binding protein, PsaC, exhibited an initial decline of 30% by month 2, but thereafter levels declined slower and stepwise an additional 23% over the remaining months (Fig. 3B). Algal PsaC levels approximated sea slug month 1 levels. The PSI ferrodoxin (Fd)-binding protein, PsaD, remained at a steady level until month 5 in the sea slugs. A total decline of only 39% was detected by month 9 (Fig. 3B). However, algal PsaD was about 50% greater than month 1 levels in the sea slug. Thus, by month 9, PsaD had decreased in the sea slug about 80% compared with the alga.
Three chloroplast cytochromes (cyts) were also detected in E. chlorotica extracts over the entire 9-month period using a heme stain and identified as cyt b559, cyt f, and cyt c6 based on immunostaining (cyt f) and/or published Mr values (cyt b559 and cyt c6). Of the three proteins, the PSII associated mobile electron carrier, cyt b559, was the most stable and remained about equal to or greater than levels measured at month 1 or in the alga (Fig. 3C). Cyt f exhibited a small, steady decline over time with levels in E. chlorotica remaining greater than or equal to the alga for 4 of the 9 months. Cyt c6 exhibited the greatest decline over time relative to month 1 (66%) or the algal sample (85%). Sea slug cyt c6 levels never equaled that observed for the alga (Fig. 3C).
Levels of a protein immunoreactive to an antibody generated against diatom FCP remained relatively unchanged in E. chlorotica for 9 months and comparable with that detected in the algal extract (Fig. 3D). V. litorea does not contain fucoxanthin, but instead uses vaucheriaxanthin as its major xanthophyll. Regardless of accessory pigment, the chl a/c-binding proteins are highly conserved (Green and Durnford, 1996; Durnford et al., 1999). The molecular mass of the immunoreactive band in the sea slugs and algae (19 kD) was similar to that reported for the diatom FCP (20 kD; Green and Durnford, 1996).
De Novo Polypeptide Patterns
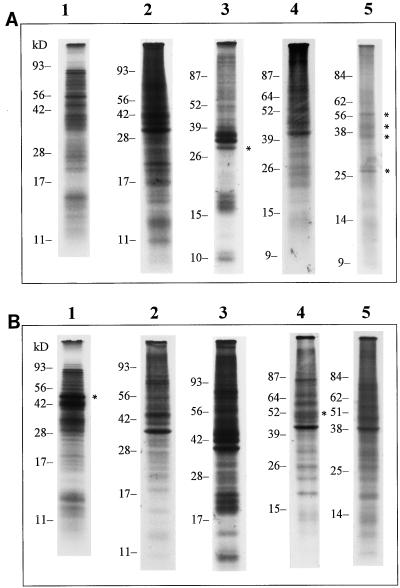
E. chlorotica (at 3, 5, 7, and 9 months of culture) and V. litorea filaments were labeled with [35S]Met to compare the translational ability of the symbiont plastids with that of chloroplasts maintained in their native cellular environment and under the control of the algal nucleus. Although the number of thylakoid polypeptides actively translated in E. chlorotica decreased with time of association, at least four major peptides detected in the algal extract were also detected through 9 months in the sea slugs (Fig. 4A). Several other minor peptides were labeled at months 3, 5, and 7. The greatest decline in de novo synthesis was observed for polypeptides less than about 25 kD beginning with month 7. Between about 25 and 65 kD, several bands were detected even at 9 months. The 32-kD band immunoprecipitated with antibody against the D1 protein (Pierce et al., 1996 and data not shown), but the other polypeptides remain unidentified.
Figure 4.
De novo synthesis of enriched-thylakoid (A) and soluble (B) polypeptides by E. chlorotica over a 9-month culture period in the absence of algae, and by V. litorea filaments. Animals and algae were labeled with [35S]Met and fractionated as described in “Materials and Methods.” Lane 1, V. litorea; lanes 2 through 5, E. chlorotica labeled at 3, 5, 7, and 9 months of culture. A, lane 3, asterisk, 32-kD D1 protein; lane 5, asterisk, four major membrane polypeptides synthesized by the sea slugs after 9 months symbiotic association; B, lanes 1 and 4, asterisk, 52-kD Rubisco LS protein in algal and sea slug extracts, respectfully. Samples were loaded on an equal radioactivity basis (100,000 dpm/lane).
Soluble translation products in E. chlorotica varied less over time of culture than the thylakoid products, but considerable variation between E. chlorotica and V. litorea was evident (Fig. 4B). One common soluble fraction peptide noted in E. chlorotica and V. litorea was a predominant 52-kD protein. This peptide has been identified as Rubisco LS by immunoblotting (Pierce et al., 1996 and data not shown). Rubisco LS is synthesized at high rates over the entire 9 months in E. chlorotica and appears to be translated at levels comparable with that seen in V. litorea.
Absence of Algal nDNA in E. chlorotica
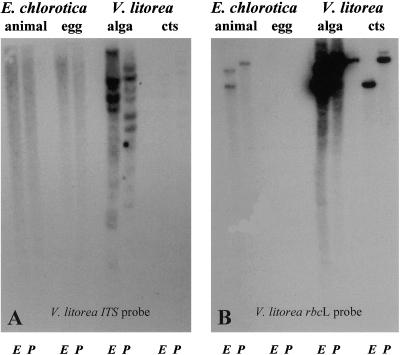
DNA from E. chlorotica adult animals and eggs and V. litorea filaments and chloroplasts was subjected to restriction digest and Southern-blot analysis using probes to V. litorea nuclear internal transcribed spacer (ITS) and chloroplast Rubisco LS (rbcL). A positive hybridization signal was detected with the ITS fragment only with V. litorea total DNA when the blot was exposed to film for 64 h (Fig. 5A). When the blot was intentionally overexposed, the same pattern of ITS hybridization signal was also detected in the V. litorea chloroplast DNA (ctDNA) sample, but no hybridization signals were seen in either of the E. chlorotica samples (data not shown). The presence of an ITS signal in the V. litorea ctDNA is a reflection of nDNA contamination, which is common in our hands and for V. litorea in general (Kowallik, 1989). To verify that the sea slugs examined did contain endosymbionts, rbcL was used as a control and gave a positive signal for sea slug DNA as well as total algal and algal ctDNA, as expected (Fig. 5B). The rbcL signals for total algal DNA were stronger after an 8-h exposure relative to algal ctDNA because less algal ctDNA was loaded and the majority of the total algal DNA fraction is actually ctDNA. V. litorea filaments are coenocytic with numerous ellipsoid chloroplasts found peripheral to a large central vacuole (Lee, 1989). No chloroplasts are found in sea slug eggs and no rbcL signal was detected even when the blots were overexposed (data not shown). The larger rbcL signal in the E. chlorotica “animal” and V. litorea “alga” EcoRI digested samples represents incomplete digestion due to the large number of EcoRI sites in the nDNA relative to the ctDNA. The stray rbcL hybridization signal to the right of the V. litorea “alga” lane resulted from spillover of the sample. This spillover was also evident in the ITS Southern blot when it was overexposed (data not shown).
Figure 5.
Southern blots of E. chlorotica and V. litorea. DNA (2 g/sample except V. litorea ctDNA, which was 400 ng/sample) was isolated from photosynthetically active adult sea slugs (animal), sea slug eggs (egg), algal filaments (algae), and algal chloroplasts (cts), digested with EcoRI (E) or PstI (P), transferred to membranes, and probed with V. litorea ITS (A) or V. litorea rbcL (B) coding region. The ITS and rbcL autoradiograms shown are 64 and 8 h exposures, respectively.
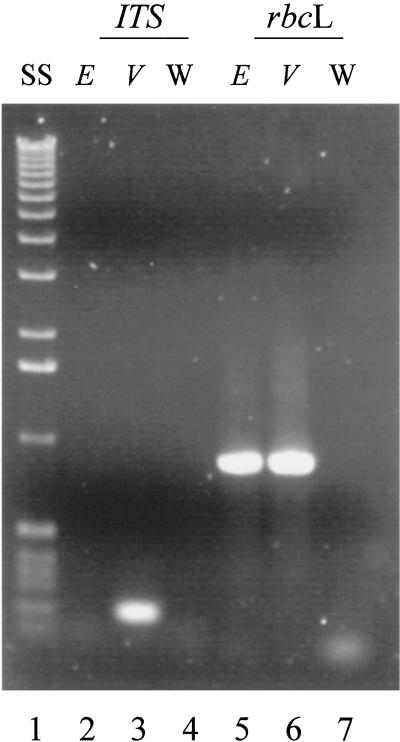
PCR reactions were also utilized as an independent and more sensitive means for determining if the sea slugs contain even small amounts of an intact algal nuclear genome. When V. litorea DNA was utilized as a template, primer sets for rbcL and ITS both gave strong positive signals (approximately 900 and 200 bp, respectively; Fig. 6). In contrast, when E. chlorotica DNA was utilized, a positive signal was observed only for rbcL. Negative controls did not yield positive signals for either primer set.
Figure 6.
Ethidium bromide stained 1% (w/v) agarose gel showing PCR reactions of photosynthetically active E. chlorotica (E) and V. litorea (V) DNA and negative water control (W) using primers specific for the V. litorea ITS region (lanes 2–4) or rbcL (lanes 5–7). Also shown is 0.5 g of size standards (1-kb ladder, Gibco-BRL, no. 15615-016); SS, lane 1.
Chloroplast Genome Size
In the absence of an algal nuclear genome in the sea slug, it is possible that V. litorea chloroplasts have an unusual chloroplast genome, possibly with a significantly increased coding capacity compared with that of other algae and this is the major contributing factor to the long-term functional activity of the symbionts. V. litorea ctDNA was digested to completion with the restriction endonuclease PstI and the fragment sizes summed to reveal a genome size of 119.1 kb (Fig. 7). This value is typical for chromophytes and suggests that there is nothing remarkable about the size of the V. litorea chloroplast genome.
Figure 7.
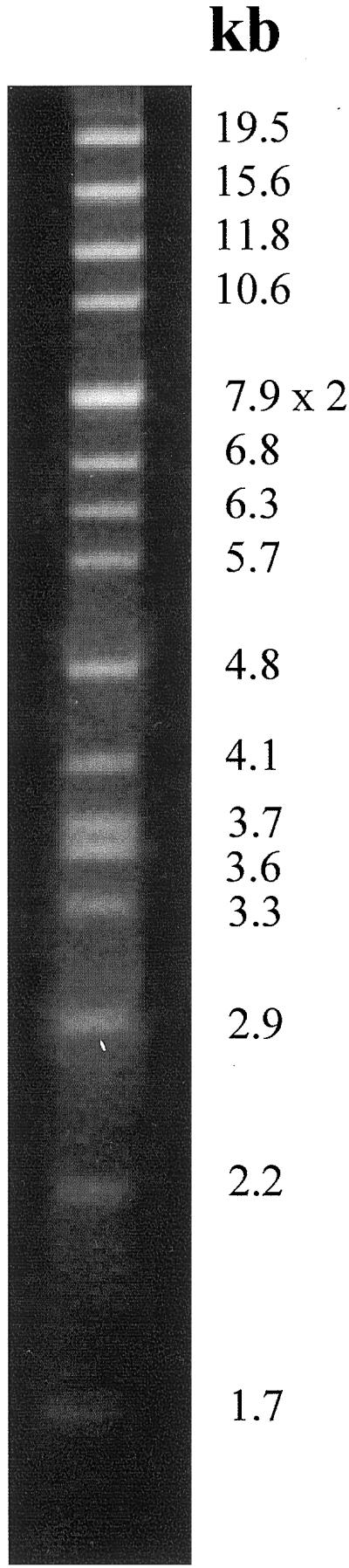
V. litorea ctDNA digested with PstI and electrophoresed through 1% (w/v) agarose. Fragment sizes (in kb) in descending order are 19.5, 15.6, 11.8, 10.6, 7.9 × 2, 6.8, 6.3, 5.7, 4.8, 4.1, 3.7, 3.6, 3.3, 2.9, 2.2, 1.7, and 0.7 (not visible on picture) for a total chloroplast genome size of 119.1 kb.
DISCUSSION
The E. chlorotica/V. litorea symbiosis is the longest-lived functional mollusc/plastid association known (West, 1979; Pierce et al., 1996). Although it is possible that the chloroplasts provide some other benefit to these shell-less molluscs (such as camouflage), this and previous studies (Mujer et al., 1996; Pierce et al., 1996) indicate that the plastids are photosynthetically active for at least 5 months and minimally functional for at least 9 months, following incorporation by the sea slug. Here we have more closely dissected the nature of this functional interaction and provide a correlation between the presence of PS proteins and photosynthetic activity.
The symbiotic chloroplasts contained levels of plastid encoded PS proteins similar to that in the alga for at least 5 months following incorporation. These included the core proteins, D1 and D2 for PSII and PsaA and PsaB for PSI, which are generally accepted as being essential (Nechushtai et al., 1996; Satoh, 1996). Chl-binding proteins (CP-43 and CP-47) are also typically found associated with the PSII complex (Bricker and Ghanotakis, 1996), as well as cyt b559 (Whitmarsh and Pakrasi, 1996). Antibodies to CP-43 and heme staining for cyt b559 revealed that both were present at relatively high levels throughout the entire symbiotic association. In addition to PsaA/B in PSI, PsaC, and PsaD, the terminal electron acceptor and Fd-docking site of PSI, respectively (for review, see Nechushtai et al., 1996), were also present in the symbionts.
The decrease in oxygen evolution rates and PET activity after about 5 months of incorporation paralleled reductions in PS core polypeptides, especially D2, which exhibited the greatest decline over time of any PS polypeptide examined. The lability of PSII-D2 and the stability of PSI-PsaA/B were also documented previously when a shorter time period was examined (Mujer et al., 1996). The maintenance of high levels of PsaA through D by protein stability or de novo synthesis, supports the high PSI PET rates measured through 7 months, possibly fueling cyclic photophosphorylation.
One protein predicted to be nuclear-encoded in the algae, a light harvesting complex protein (LHCP) immunoreactive to diatom FCP, was found at greater than or equal abundance in the endosymbionts compared with algal plastids for at least 7 out of 9 months (Fig. 3D). The turnover rate of the LHCPs in V. litorea or chromophytes in general has not been studied in detail (Green and Durnford, 1996; Pichersky and Jansson, 1996). The persistence of FCP in the symbionts is either due to extreme pigment-protein complex stability or fcp genes have been transferred to the sea slug nucleus where they are expressed along with the appropriate targeting signals to route them to the chloroplasts.
A link between sea slug longevity and plastid degradation is not evident, but it should be noted that a loss of pigmentation was observed in the sea slugs similar to senescence of a leaf (Table I). Pigmentation loss occurred at about the same time symbiont plastid protein turnover increased (7 months) and shortly after a decline in photosynthetic activity (5–6 months). This suggests that as the natural life cycle of the sea slug proceeds, the host may no longer be able to provide the necessary cellular environment/components for chloroplast protein production to maintain the symbiont plastids' photosynthetic machinery. Pierce et al. (1999) have attributed the synchronized mass death of the sea slugs at about 9 to 10 months in culture or in the sea to increased activity of a virus endemic in the E. chlorotica population.
In view of the complex nuclear/plastid interactions documented for numerous plant and algal species, a functionally autonomous plastid seems unlikely. The simplest explanation for functional plastids would be the presence of an algal nuclear genome in the animal. No algal nuclei or nucleomorphs have been observed by electron microscopy in this or other ascoglossan/plastid symbiosis (Kawaguti and Yamasu, 1965; Graves et al., 1979; Rumpho et al., 2000). However, this conclusion was still based on visual observations and a final definitive answer required biochemical and/or molecular evidence. Here we have presented the first molecular evidence employing a gene probe and primers to the unique ribosomal ITS region of V. litorea nDNA. The absence of positive ITS1 signals in sea slug tissue at the same time positive plastid rbcL signals were detected demonstrated that symbionts were present in the tissue, but no algal nDNA was present. In contrast, positive signals were obtained for both probes using the control algal total DNA. We chose the nuclear ribosomal DNA ITS region because of its rapid evolutionary change, which would prevent the V. litorea ITS probe from hybridizing to slug ITS and because of its presence in multiple copies (White et al., 1990; Coleman et al., 1994; Zechman et al., 1994; Manhart et al., 1995).
Having ruled out any algal nucleocytosolic influence in the sea slugs, the question of what sustains the E. chlorotica/V. litorea system for such extended periods of time, even relative to other mollusc/plastid symbioses, shifts to the autonomy of the chromophytic plastids. A completely autonomous plastid is one possibility. It may be more than a coincidence that Codium fragile, one of the shorter-lived plastid endosymbionts (Greene, 1970), also possesses the smallest chloroplast genome reported (89 kb) (Manhart et al., 1989). V. litorea's plastid genome (119.1 kb) is significantly larger than C. fragile's, but much smaller than the red alga, Porphyra purpurea (191 kb), which represents the largest chloroplast genome studied (Reith and Munholland, 1995). V. litorea's ctDNA is even smaller than the 124.6-kb ctDNA of Vaucheria bursata, a freshwater species (Linne von Berg and Kowallik, 1992), but it is very close in size to that of the ctDNA of the diatom, Odontella sinensis, which has been completely sequenced and found to be 119,704 bp in length with 174 genes and open reading frames (Kowallik et al., 1995). Although it is doubtful that the ctDNA of V. litorea will contain enough genes to make the plastids completely autonomous, complete sequencing will allow a detailed comparison with other chloroplast genomes.
In the absence of any algal nuclear influence and assuming a typical, less-than-completely autonomous plastid, possible mechanisms being explored that might contribute to the functional symbiosis include unusual stability of nuclear encoded plastid proteins in the animal, unanticipated activity of transcription/translation/PS complexes in the absence of normally critical nuclear encoded subunits, targeting of animal cytosolic or mitochondrial proteins with related functions to the chloroplast, and lateral transfer of algal genes to the animal nucleus (for review, see Rumpho et al., 2000).
By combining information from oxygen exchange and PET measurements, immunostaining, the highly similar trends in peptide banding patterns between E. chlorotica and algal de novo and constitutive thylakoid preparations, and the previous work demonstrating accumulation of chloroplast transcripts and de novo protein biogenesis (Mujer et al., 1996; Pierce et al., 1996), it appears that a significant number of processes related to gene expression and protein biosynthesis are all occurring in the symbiotic plastids for several months, enabling long-term photosynthetic activity and survival. For a plastid within its usual cellular environment to produce and process transcripts and synthesize, fold, and process polypeptides into their active forms, nuclear assistance is required in most higher plant systems (Barkan et al., 1994; Hayes et al., 1996). Understanding how this all occurs in a marine mollusc harboring symbiont algal plastids with a relatively small chloroplast genome and no detectable algal nucleocytosolic influence continues to be investigated.
MATERIALS AND METHODS
Sea Slug and Algal Cultures
Elysia chlorotica Gould specimens originated from collections made in November 1997 and October 1998 from an intertidal marsh on Martha's Vineyard Island, MA. The animals were maintained photoautotrophically in aerated aquaria containing ASW (925 mosmol kg-1, Instant Ocean, Aquarium Systems, Mentor, OH) at 10°C on a 14-h photoperiod. Sea slugs were either sampled live for photosynthetic measurements and chloroplast isolation or sacrificed monthly for 9 months and analyzed later. For the monthly harvests, 20 to 25 individual animals were blotted dry, pooled, frozen, and powdered immediately in liquid nitrogen, and stored at −80°C until analyzed. “Months Symbiotic Association” or “Months in Culture” in the text and figures refers to the number of months from the time of collection from the intertidal marsh in which the sea slugs are no longer associated with any algae. Vaucheria litorea C. Agardh is maintained in culture in enriched one-quarter-strength ASW (250 mosmol kg-1) and a modified f/2 medium as described previously (Pierce et al., 1996), except that the cultures are illuminated by natural lighting and aeration is limited to daily manual swirling.
Oxygen Evolution Measurements
Rates of O2 exchange were measured at 15°C with an oxygen electrode (2.5 mL vol, DW1, Hansatech, King's Lynn, UK) illuminated with a high-intensity tungsten-halogen light source (1,500 μmol photons m−2 s−1; LS2, Hansatech). Individual animals were separated from the stirring apparatus by a screen and suspended in 1 mL of ASW supplemented with 10 mm NaHCO3. Samples were incubated in the dark for 1 min prior to illumination. Rates were recorded for 5 to 10 min, after which illumination ceased and dark respiration rates (O2 uptake) were recorded for at least 5 min. Apparent photosynthesis was calculated by measuring the rate of CO2- and light-dependent O2 evolution, whereas respiration rates were based on the uptake of O2 in the dark. Gross photosynthesis was calculated by summing the apparent photosynthesis and dark respiration rates. At least three sea slugs were analyzed for each time point. Rates were calculated on a total chl basis with chl a and c quantified by A664 and A630 measurements following homogenization in 90% (v/v) acetone (Sterman, 1988).
Electron Transport Measurements
Chloroplasts were isolated from live sea slugs and algal filaments and purified on Percoll gradients (Pierce et al., 1996). Intact chloroplasts were lysed by hypertonic freeze-thawing and thylakoid membranes were collected by centrifugation at 35,000g for 5 min. The washed thylakoids were resuspended in reaction buffer (50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.6, 100 mm sorbitol, 5 mm NaCl, and 5 mm MgCl2) and used immediately at a concentration of about 50 μg chl mL−1. All PET measurements were made with an oxygen electrode at 15°C and 1,500 μmol photons m−2 s−1 (Hind, 1993).
Whole chain PET including the OEC, but excluding Fd and Fd-NADP reductase, was measured with water as the electron donor and MV as the electron acceptor. Endogenous catalase activity was inhibited by adding 5 mm NaN3. For whole chain PET excluding the OEC, electron flow from water was inhibited by incubating the thylakoids at 50°C for 2 min. The reaction medium was then supplemented with 5 mm NH4Cl (uncoupler), 0.5 mm DPC (electron donor), 2 mm NaN3, and 50 μm MV. Electron transport through PSI (Mehler reaction) was measured as described for whole chain PET except, after 5 min illumination, 10 μm dichloromethylurea was added to inhibit PSII and 2 mm ascorbate and 50 μm dichlorophenolindophenol were added to provide electrons for PSI. Electron flow from water through PSII including the dichloromethylurea-sensitive site was measured by additions to the reaction media of 5 mm NH4Cl, 1 mm p-phenylenediamine and excess FeCN (4 mm).
Soluble and Thylakoid Membrane-Enriched Proteins
Powdered samples from the monthly collections were aliquoted (200 mg fresh weight) and proteins isolated as described by Russell et al. (1995). The powdered tissue was homogenized in a microcentrifuge tube containing 400 μL (1:2, w/v) of isolation buffer (330 mm sorbitol, 25 mm HEPES-KOH, pH 7.0, 5 mm MgCl2, 10 mm NaCl, 100 mm N-acetyl-l-Cys, and 1 mm phenylmethylsulfonyl fluoride). Enriched-thylakoid membranes (hereafter, thylakoid proteins) were collected by centrifugation at 10,000g for 5 min at 4°C. The resultant supernatant (containing soluble cytosolic proteins and the chloroplast stroma) was collected and used as the soluble fraction. Thylakoid pellets were washed once in isolation buffer lacking sorbitol and N-acetyl-l-Cys, and resuspended in isolation buffer containing 1% (v/v) Triton X-100 followed by continuous shaking for at least 2 h at 4°C. Total protein was determined using the Bio-Rad (Hercules, CA) protein stain with bovine serum albumin as the standard and corrected for Triton X-100, when necessary.
Immunoblot Analysis and Quantification
Thylakoid proteins (50 μg protein lane−1) were separated by SDS-PAGE employing a 9% to 18% (w/v) linear polyacrylamide gradient (Laemmli, 1970). Gels were either stained with Coomassie Brilliant Blue G-250 or the proteins were transferred to polyvinylidene fluoride membrane (Immobilon-P, Millipore, Bedford, MA) by electroblotting at 15°C and 75 V for 3 h (Towbin et al., 1979). Proteins were visualized using the alkaline phosphatase system as recommended by the manufacturer (Promega, Madison, WI). Polyclonal, heterologous antibodies directed against D1, D2, and CP43 (all from barley), FCP (diatom), PsaA and PsaB (domain specific Synechocystis sp. PCC 6803 synthetic peptides [Sun et al., 1997]), PsaC and PsaD (Synechocystis sp. PCC 6803), and cyt f (Chlamydomonas reinhardtii), were raised in rabbits and generously provided to us by a number of sources (please see “Acknowledgments”). Immunoblots were quantified densitometrically (AlphaImager 2000, Alpha Innotech, San Leandro, CA). The quantity of each polypeptide at month 1 was set at 100% (control) and all other samples were calculated relative to their respective control.
Super Signal Chemiluminescent Detection System
Cyts were detected using the chemiluminescent substrate Luminol (Pierce Chemical, Rockford, IL), which reacts with proteins containing heme-functional groups. Due to the hydrophobic nature of these proteins they were transferred as described above, but in the presence of 0.5% to 1% (w/v) SDS and onto nitrocellulose membrane (Schleicher and Schuell). Once transferred, the blots were incubated in Luminol for 5 min, exposed to x-ray film, and the cyts identified by comparisons with known molecular weights, protein fraction from which the samples originated, and immunostaining in the case of cyt f.
De Novo Protein Synthesis
For each time point, four to six sea slugs were placed in glass scintillation vials containing 3 mL of ASW and 50 μCi mL−1 [35S]Met, and illuminated for 7 h at 18°C. V. litorea (450 mg fresh weight) was treated as above, but labeled for 4 h. Following labeling, thylakoid and soluble proteins were isolated and separated by SDS-PAGE as described above. Equal radioactivity (100,000 dpm) was loaded for each sample. Gels were fixed and processed for fluorography according to Mujer et al. (1996).
DNA Isolation, Sizing, and Southern Blotting
DNA was isolated from E. chlorotica adult animals, E. chlorotica eggs, V. litorea filaments, and V. litorea chloroplasts (Palmer, 1986; Mujer et al., 1996). Aliquots of DNA (2 μg sample−1 except V. litorea ctDNA, which was 400 ng sample−1) were digested with EcoRI or PstI and processed for Southern analysis as described (Mujer et al., 1996). For probes, the entire ITS from V. litorea genomic DNA was amplified using ITS4 and ITS5 primers of White et al. (1990). Primers to ITS1 only (5′-CCAACATATTCATCCTC and 5′- ATTGCACCATTGCTGGC) were constructed from this sequence and used to produce a 203-bp ITS1 probe by amplification of V. litorea genomic DNA. Primers to rbcL (5′-CCTTAATACAACTGCAG and 5′-CCTTTATTTACAGCATAC) were designed using sequence information obtained from V. litorea ctDNA clones (accession no. AF207527) and used to produce an 892-bp rbcL probe. PCR reactions for ITS and rbcL were performed using the primers listed above with 2 ng of genomic DNA from photosynthetically active E. chlorotica (3 months in culture), V. litorea, or water control as templates following standard protocols (35 cycles, 1′-1′-2′ extension times at 94°, 50°, and 72°C, respectively) using Taq polymerase (Gibco-BRL, Gaithersburg, MD). For chloroplast genome sizing, V. litorea ctDNA was digested with PstI and analyzed as described in Palmer (1986).
ACKNOWLEDGMENTS
We thank the following for generously providing antibodies for these studies: John Mullet (Texas A&M University), Steven Theg (University of California, Davis), Paul Falkowski (Brookhaven National Laboratory), John Golbeck (Penn State University), Beverley Green (University of British Columbia), Parag Chitnis (Iowa State University) and Sabeeha Merchant (University of California, Los Angeles [UCLA]). We would also like to thank Beth Dreyfuss (UCLA) for communicating the cyt staining protocol.
Footnotes
This work was supported in part by the American Chemical Society Herman Frasch Foundation (grant no. 343–HF92 in Agricultural Chemistry to M.E.R.), by the National Science Foundation (grant nos. IBN–9505416 to S.K.P. and M.E.R. and IBN–9808904 to M.E.R. and J.R.M.), by the Texas A&M University Interdisciplinary Research Initiative (grants to M.E.R. and J.R.M.), and by the Texas Agricultural Experiment Station.
LITERATURE CITED
- Aro E, Virgin I, Andersson B. Photoinhibition of Photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Barkan A, Walker M, Nolasco M, Johnson D. A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 1994;13:3170–3181. doi: 10.1002/j.1460-2075.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker TM, Ghanotakis DF. Introduction to oxygen evolution and the oxygen-evolving complex. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Vol. 4. Boston: Kluwer Academic Publishers; 1996. pp. 113–136. [Google Scholar]
- Christopher DA, Mullet JE. Separate photosensory pathways co-regulate blue light/ultraviolet-A-activated psbD-psbC transcription and light-induced D2 and CP43 degradation in barley (Hordeum vulgare) chloroplasts. Plant Physiol. 1994;104:1119–1129. doi: 10.1104/pp.104.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KB, Busacca M. Feeding specificity and chloroplast retention in four tropical Ascoglossa, with a discussion of the extent of chloroplast symbiosis and the evolution of the order. J Mollusc Stud. 1978;44:272–282. [Google Scholar]
- Coleman AW, Suarez A, Goff LJ. Molecular delineation of species and syngens in volvocacean green algae (chlorophyta) J Phycol. 1994;30:80–90. [Google Scholar]
- Douglas AE. Symbiotic Interactions. New York: Oxford University Press; 1994. [Google Scholar]
- Durnford DG, Deane JA, Tan S, McFadden GI, Gantt E, Green BR. A phylogenetic assessment of the eukaryotic light-harvesting antenna proteins, with implications for plastid evolution. J Mol Evol. 1999;48:59–68. doi: 10.1007/pl00006445. [DOI] [PubMed] [Google Scholar]
- Giles KL, Sarafis V. On the survival and reproduction of chloroplasts outside the cell. Cytobios. 1971;4:61–74. [Google Scholar]
- Graves DA, Gibson MA, Bleakney JS. The digestive diverticula of Alderia modesta and Elysia chlorotica. Veliger. 1979;21:415–422. [Google Scholar]
- Green BR, Durnford DG. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:685–714. doi: 10.1146/annurev.arplant.47.1.685. [DOI] [PubMed] [Google Scholar]
- Greene RW. Symbiosis in sacoglossan opisthobranchs: functional capacity of symbiotic chloroplasts. Marine Biol. 1970;7:138–142. [Google Scholar]
- Hayes R, Kudla J, Schuster G, Gabay L, Maliga P, Gruissem W. Chloroplast mRNA 3′-end processing by a high molecular weight protein complex is regulated by nuclear encoded RNA binding proteins. EMBO J. 1996;15:1132–1141. [PMC free article] [PubMed] [Google Scholar]
- Hind G. Thylakoid components and processes. In: Hall DO, Scurlock JMO, Bolhar-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual. New York: Chapman & Hall; 1993. pp. 283–298. [Google Scholar]
- Kawaguti S, Yamasu T. Electron microscopy on the symbiosis between an elysioid gastropod and chloroplasts of a green alga. Biol J Okayama Univ. 1965;11:57–65. [Google Scholar]
- Kowallik K. Molecular aspects and phylogenetic implications of plastid genomes of certain chromophytes. In: Green JC, Leadbeater BSC, Diver WL, editors. The Chromophyte Algae: Problems and Perspectives. Vol. 38. Oxford: Clarendon Press; 1989. pp. 101–124. [Google Scholar]
- Kowallik K, Stroebe B, Schaffran I, Freier U. The chloroplast genome of a chlorophyll a + c containing alga, Odontella sinensis. Plant Mol Biol Rep. 1995;13:336–342. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;22:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee RE. Phycology. New York: Cambridge University Press; 1989. [Google Scholar]
- Linne von Berg K-H, Kowallik KV. Structural organization of the chloroplast genome of the chromophytic alga Vaucheria bursata. Plant Mol Biol. 1992;18:83–95. doi: 10.1007/BF00018459. [DOI] [PubMed] [Google Scholar]
- Manhart JR, Fryxell GA, Celia Villac M, Segura LY. Psuedo-nitzschia pungens and P. multiseries (bacillariophyceae): nuclear ribosomal DNAs and species differences. J Phycol. 1995;31:421–427. [Google Scholar]
- Manhart JR, Kelly K, Dudock BS, Palmer JD. Unusual characteristics of Codium fragile chloroplast DNA revealed by physical and gene mapping. Mol Gen Genet. 1989;216:417–421. doi: 10.1007/BF00334385. [DOI] [PubMed] [Google Scholar]
- Martin W, Herrmann RG. Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol. 1998;118:9–17. doi: 10.1104/pp.118.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Dreyfuss BW. Posttranslational assembly of photosynthetic metalloproteins. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:25–51. doi: 10.1146/annurev.arplant.49.1.25. [DOI] [PubMed] [Google Scholar]
- Mujer CV, Andrews DL, Manhart JR, Pierce SK, Rumpho ME. Chloroplast genes are expressed during intracellular symbiotic association of Vaucheria litorea plastids with the sea slug Elysia chlorotica. Proc Natl Acad Sci USA. 1996;93:12333–12338. doi: 10.1073/pnas.93.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass MM. Uptake of isolated chloroplasts by mammalian cells. Science. 1969;165:1128–1131. doi: 10.1126/science.165.3898.1128. [DOI] [PubMed] [Google Scholar]
- Nechushtai R, Eden A, Cohen Y, Klein J. Introduction to photosystem I: reaction center function, composition and structure. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Vol. 4. Boston: Kluwer Academic Publishers; 1996. pp. 289–311. [Google Scholar]
- Palmer JD. Isolation and structural analysis of chloroplast DNA. Methods Enzymol. 1986;118:167–186. [Google Scholar]
- Palmer JD, Delwiche CF. Second-hand chloroplasts and the case of the disappearing nucleus. Proc Natl Acad Sci USA. 1996;93:7432–7435. doi: 10.1073/pnas.93.15.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Jansson S. The light-harvesting chlorophyll a/b-binding polypeptides and their genes in angiosperm and gymnosperm species. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Vol. 4. Boston: Kluwer Academic Publishers; 1996. pp. 507–521. [Google Scholar]
- Pierce SK, Biron RW, Rumpho ME. Endosymbiotic chloroplasts in molluscan cells contain proteins synthesized after plastid capture. J Exp Biol. 1996;199:2323–2330. doi: 10.1242/jeb.199.10.2323. [DOI] [PubMed] [Google Scholar]
- Pierce SK, Maugel TK, Rumpho ME, Hanten JJ, Mondy WL. Annual viral expression in a sea slug population: life cycle control and symbiotic chloroplast maintenance. Biol Bull. 1999;197:1–6. doi: 10.2307/1542990. [DOI] [PubMed] [Google Scholar]
- Reith M. Molecular biology of rhodophyte and chromophyte plastids. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:549–575. [Google Scholar]
- Reith M, Munholland J. Complete nucleotide sequence of the Porphyra purpurea chloroplast genome. Plant Mol Biol Rep. 1995;13:333–335. [Google Scholar]
- Ridley SM, Leech RM. Division of chloroplasts in an artificial environment. Nature. 1970;227:463–465. doi: 10.1038/227463a0. [DOI] [PubMed] [Google Scholar]
- Rumpho ME, Mujer CV, Andrews DL, Manhart JR, Pierce SK. Extraction of DNA from mucilaginous tissues of a sea slug (Elysia chlorotica) BioTech. 1994;17:1097–1101. [PubMed] [Google Scholar]
- Rumpho ME, Summer EJ, Manhart JR. Solarpowered sea slugs: mollusc/algal chloroplast symbiosis. Plant Physiol. 2000;123:1–10. doi: 10.1104/pp.123.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton GGR, Chow WS, Anderson JM, Osmond CB. Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol. 1995;107:943–952. doi: 10.1104/pp.107.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K. Introduction to the photosystem II reaction center: isolation and biochemical and biophysical characterization. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Vol. 4. Boston: Kluwer Academic Publishers; 1996. pp. 193–211. [Google Scholar]
- Sterman NT. Spectrophotometric and fluorometric chlorophyll analysis. In: Lobban CS, Chapman DJ, Kramer BP, editors. Experimental Phycology. Cambridge: Cambridge University Press; 1988. pp. 35–46. [Google Scholar]
- Stern DB, Higgs DC, Yang J. Transcription and translation in chloroplasts. Trends Plant Sci. 1997;2:308–315. [Google Scholar]
- Sun J, Xu Q, Chitnis VP, Jin P, Chitnis PR. Topography of the photosystem I core proteins of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1997;272:21793–21802. doi: 10.1074/jbc.272.35.21793. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trench RK. Of “leaves that crawl”: functional chloroplasts in animal cells. Soc Exp Biol Cambridge Symp. 1975;29:229–266. [PubMed] [Google Scholar]
- West HH. Chloroplast symbiosis and development of the ascoglossan opisthobranch Elysia chlorotica. PhD thesis. Boston: Northeastern University; 1979. [Google Scholar]
- West HH, Harrigan J, Pierce SK. Hybridization of two populations of a marine opisthobranch with different developmental patterns. Veliger. 1984;26:199–206. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Whitmarsh J, Pakrasi HB. Form and function of cytochrome b-559. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Vol. 4. Boston: Kluwer Academic Publishers; 1996. pp. 249–264. [Google Scholar]
- Zechman FW, Zimmer EA, Theriot EC (1994) Use of ribosomal DNA internal transcribed spacers for phylogenetic studies in diatoms. 30: 507–512