ABSTRACT
Icariin (ICA) is a flavonoid glucoside derived from the Epimedium plant genus, which has potent regenerative properties and is used in western medicine to treat impotence. Recently, ICA has generated great interest in improving hepatic stellate cell function and cardiac rejuvenation. However, how this natural component functions in hematopoiesis remains unexplored. Here we have examined the role of ICA on hematopoietic stem cells (HSCs) using the cancer-prone disease model of Fanconi anemia (FA), an inherited bone marrow failure syndrome with extremely high risk of leukemic predisposition. We show that ICA reverses the less quiescent status of HSCs deficient for the Fanca or Fancd2 gene, and improves the ability of these mutant stem cells to form colony formation units (CFU) in vitro and reconstitutes hematopoiesis in transplanted recipients. Further analysis reveals that ICA upregulates enzyme activity of the chromatin binding protein SIRT6 in Fanca−/− and Fancd2−/− HSCs, both of which have an intrinsic low SIRT6 activity. Furthermore, forced expression of SIRT6 blocks the natural decline of quiescent HSCs in Fanca−/− or Fancd2−/− mice and improves the repopulating capacity of these mutant HSCs in irradiated recipients. Mechanistically, ICA enhances SIRT6-mediated H3K9 deacetylation on the promoter of NF-κB and represses the expression of NF-κB target genes. Together, our findings indicate that ICA improves the function of HSCs by stimulating SIRT6 activity and contributes to the regenerative effect of ICA.
KEYWORDS: Fanconi anemia, hematopoietic stem cell, SIRT6, NF-κB
Introduction
Hematopoietic stem cells (HSCs) are a rare population of pluripotent cells that can self-renew and differentiate into various types of cells of the blood lineages [1]. HSC homeostasis is essential for the continuous replenishment of the hematopoietic system throughout the entire lifespan of an organism [1–3]. Under steady physiologic conditions, the most primitive HSCs are in a quiescent state and reside in the bone marrow (BM) niche where they preserve the capacity to self-renew and continue to produce all types of blood cells throughout a prolonged life span without depleting the regenerative cell pool [2,4]. Disruption of HSC quiescence prematurely exhausts the stem cell pool and causes hematologic failure in human diseases like Fanconi anemia (FA) [5,6].
FA is a genetic disorder caused by the defects in at least 21 genes (FANCA-V) [7–16]. One of the common clinical features of FA is hematologic manifestations. In fact, a majority of FA patients invariably experience progressive BM failure, and oftentimes progress to myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) [7,12,16]. Marrow dysfunction occurs at early stage, is associated with HSC loss and accounts for the majority of FA childhood mortality [7,11,16]. On the other hand, rapid hematopoietic cell loss then forces compensatory chronic proliferation of the mutant hematopoietic stem and progenitor cells (HSPCs) leading to malignant transformation [11]. Significant evidence supports that FA proteins play specific roles in hematopoiesis by governing the responses of hematopoietic cells to both genotoxic and cytotoxic stresses [11,17,18]. Therefore, research on improving the FA stem cell function is an impactful area of research due to their therapeutic potential.
Icariin (ICA) is a flavonoid isolated from the traditional Chinese herbal medicine Epimedium brevicornum maxim, which has potent antioxidant, anti-inflammatory and regenerative properties [19]. It has been used in traditional Chinese medicine to enhance erectile function, and treat variety of cardiovascular diseases. Recent rodent studies suggest a protective effect of ICA on diverse cellular stresses in various cell types [19–26]. For example, ICA suppresses hepatic stellate cell activation by regulating Nuclear factor-kappa B (NF-κB) activity [19,20]. Another study reveals that ICA intervenes in cardiac inflammation-associated aging through upregulation of enzyme activity of sirtuin-6 (SIRT6), a chromatin binding protein [22,23] and inhibition of NF-κB activation [23]. ICA can also attenuate oxidative stress-induced cardiac apoptosis through protection of mitochondria and stimulation of extracellular signal-regulated kinases (ERK) [24]. Furthermore, ICA was found to influence adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture [25]. Although a few studies have shown a potential anti-proliferative activity on leukemia/lymphoma cell lines [26,27], how ICA impacts hematopoiesis remains unknown. In the present study, we demonstrate that ICA improves the function of Fanca−/− and Fancd2−/− HSCs through SIRT6-mediated repression of NF-κB signaling pathway.
Results
ICA restores quiescence of FA HSCs
In attempt to search for new chemopreventive and regenerative agents that are effective and less toxic in hematopoietic improvement for patients with BM failure syndromes, such as FA, in which HSC defect is considered as a major cellular hallmark [28], we investigated the regenerative role of Icariin (ICA) in FA HSCs. ICA is a flavonoid isolated from the traditional Chinese herbal medicine Epimedium brevicornum maxim, and has been reported to be effective for the treatment of a variety of cardiovascular diseases [22,23]. To test the effect of Icariin on HSCs in vivo, we daily intraperitoneally (i.p.) injected Fanca−/− or Fancd2−/− mice and their wild-type (WT) littermates with ICA (100 mg/kg/d) for consecutive 7 days. Analysis of peripheral blood (PB) showed that all the hematological parameters, including platelet and erythrocyte count, did not appear to be affected by ICA treatment (Table S1). In addition, we did not observe any changes in the numbers of total nucleated cells in the bone marrow (BM) after ICA administration (Fig. 1A).
Figure 1.
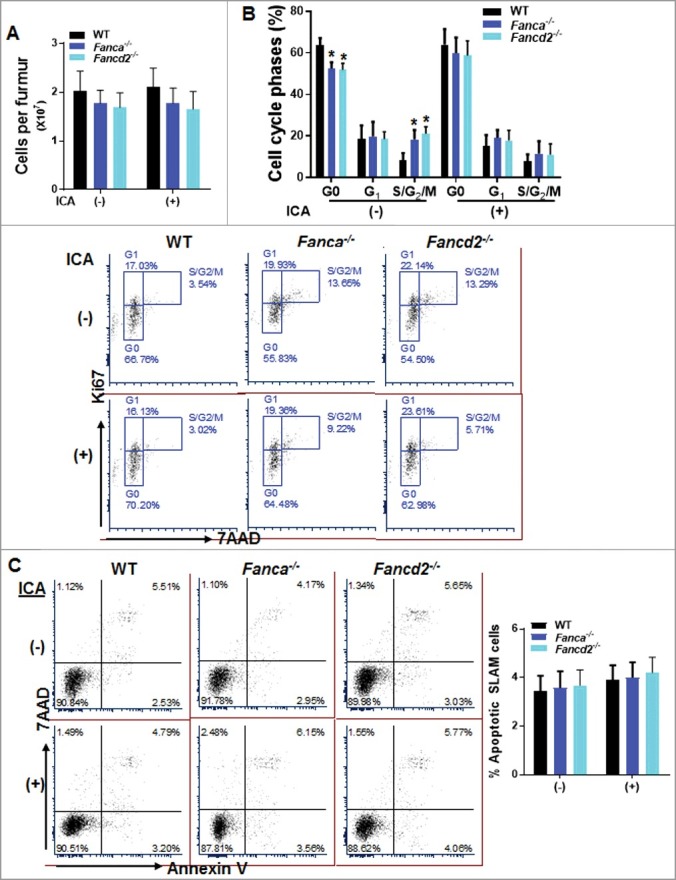
ICA restores quiescence of FA HSPCs. (A) ICA treatment does not change absolute bone marrow cell numbers in mice. Whole bone marrow cells (WBMCs) isolated from ICA treated or untreated 8-week-old Fanca−/− or Fancd2−/− mice and their wild-type (WT) littermates were enumerated. Results are means ±SD of 3 independent experiments (n = 6). (B) ICA treatment reverses less quiescent status of FA HSPCs. Low density bone marrow cells (LDBMCs) were harvested from mice described in (A) followed by cell cycle analysis using Ki67 and 7AAD staining. BM SLAM (Lin−Sca1+c-kit+CD150+CD48−) cells were gated for cell cycle analysis. Representative flow plots (Lower) and quantification (Upper) are shown. (C) ICA treatment is not toxic to FA HSPCs. Cells described in (B) were subjected to flow cytometry analysis for Annexin V/7AAD. BM SLAM cells were gated for apoptosis analysis. Representative flow plots (Left) and quantification (Right) are shown. Results are means ±SD of 3 independent experiments (n = 6).
Since quiescence is an important feature of HSC homeostasis [29], and since FA HSCs are known to be less quiescent than their WT counterparts [30], we next performed cell cycle analysis to determine whether ICA has impact on the quiescence status of HSCs. By using Annexin V and 7AAD staining, we found a reduction of HSCs in S and G2/M phase in FA, and WT mice although to a less extent, treated with ICA, which was accompanied with an increase in the proportion of quiescent HSCs (G0 phase) in these ICA-treated mice (Fig. 1B). Importantly, we noticed that the effect of ICA on HSC quiescence was more profound in Fanca−/− and Fancd2−/- mice compared to that in WT mice (Fig. 1B). In addition, we did not observe obvious ICA-induced toxicity in WT or Fanca−/− and Fancd2−/− mice, as ICA treatment did not lead to increased apoptosis in the phenotypic (Lin−Sca1+c-kit+CD150+CD48−, SLAM) [31] HSC compartment (Fig. 1C). Therefore, these data suggest that ICA has a positive effect on HSC quiescence.
ICA improves FA HSC function
Since increased HSC cycling leading to premature HSC exhaustion is considered as an important pathological cause of BM failure in FA, and since we observed improved quiescence in the phenotypic HSC compartment in ICA-treated mice, we asked whether ICA could improve FA HSC function. By utilizing the well-established colony forming unit (CFU) assay, we found that the number of colonies formed by LSK (Lin−Sca1+c-kit+) cells isolated from ICA-treated Fanca−/− or Fancd2−/− mice was significantly higher than those formed by the cells from the untreated mice (Fig. 2A). More importantly, the LSK cells from the ICA-treated Fanca−/− or Fancd2−/− mice showed a marked increase in serial replating activity compared to the untreated control LSK cells (Fig. 2A), indicative of a rescued replicative exhaustion.
Figure 2.
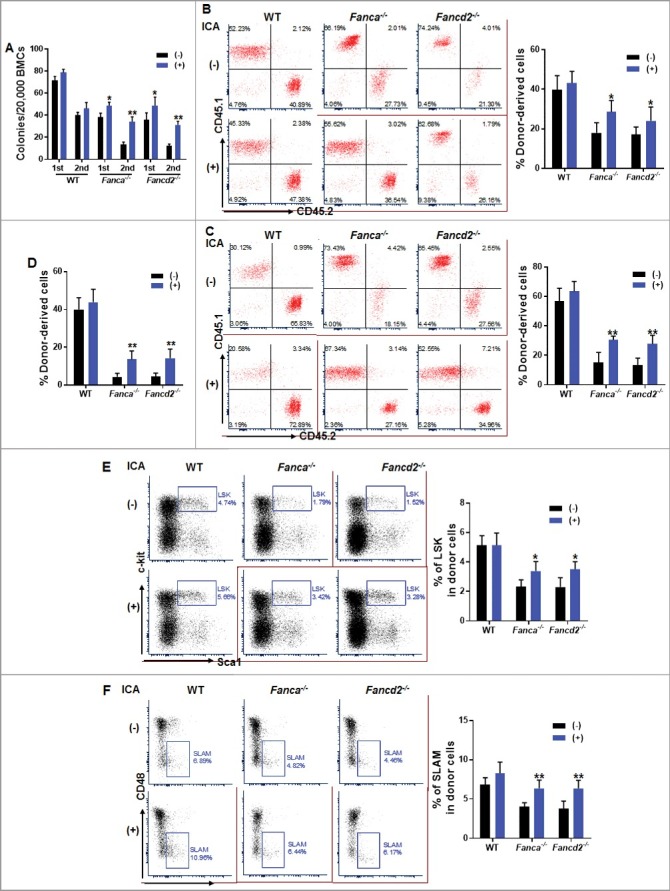
ICA improves FA stem cell function. (A) ICA treatment improves FA progenitor activity in vitro. LSK (Lin−Sca1+c-kit+) cells isolated from ICA treated or untreated 8-week-old Fanca−/− or Fancd2−/− mice as well as their WT littermates were plated in cytokine-supplemented methycellulose medium. Colonies from the 1st plating were pooled for 2nd plating. Total colony numbers were enumerated on day 7 after plating. Results are means ± SD of three independent experiments (n = 6 per group). (B, C) ICA improves FA HSCs in vivo repopulating capacity. 1,000 LSK cells isolated from ICA treated or untreated 8-week-old WT, Fanca−/− or Fancd2−/− mice along with 3 × 105 congenic bone marrow cells from BoyJ mice were transplanted into lethally irradiated BoyJ recipients. Donor-derived chimera 4 weeks (B) and 16 weeks (C) post BMT in the recipient bone marrow were analyzed by flow cytometry. Quantification (Left) and representative flow plots (Right) are shown. Results are means ±SD of 3 independent experiments (n = 6). (D-F) ICA improves FA HSC long-term repopulating capacity. 3 million WBMCs from the recipients described in (B) were transplanted into sublethally irradiated (7.0 Gy) BoyJ recipients. Donor-derived chimera (D) and percentage of LSK (E) and SLAM cells (F) in CD45.2+ compartment were analyzed by flow cytometry 16 weeks post BMT. Representative flow plots (Left) and quantifications (Right) are shown. Results are means ±SD of 3 independent experiments.
To substantiate these in vitro observations, we determined the in vivo hematopoietic repopulating capacity of ICA-treated HSCs by transplanting 1,000 LSK cells from ICA-treated or untreated Fanca−/− and Fancd2−/− mice as well as their WT littermates, along with 3 × 105 bone marrow cells from congenic BoyJ mice, into lethally irradiated BoyJ recipients. Consistent with previous report [30,32,33], Fanca−/− and Fancd2−/− LSK cells exhibited defect in reconstituting hematopoiesis of the irradiated recipient mice (Fig. 2B, 2C). Significantly, donor-derived chimera in recipients transplanted with ICA-treated Fanca−/− or Fancd2−/− LSK cells was markedly increased compared to those transplanted with untreated control cells both 4 weeks and 16 weeks post BMT (Fig. 2B, 2C). ICA treatment did not alter lineage differentiation, as the percentage of donor-derived cells stained positive for CD3e, B220 and Gr1/Mac1 was found approximately equal in the recipients transplanted with ICA treated or untreated LSK cells (Fig. S1).
To determine whether ICA can improve long-term repopulating capacity of FA HSCs, we performed secondary bone marrow transplantation (BMT) by injecting whole bone marrow cells (WBMCs) from primary recipients into sublethally irradiated BoyJ recipients. Donor-derived chimera analysis at 16 weeks post BMT indicated a significantly improved long-term hematopoietic reconstitution of ICA-treated Fanca−/− or Fancd2−/− HSCs (Fig. 2D). Consistently, the percentages of donor-derived LSK and SLAM cells in the secondary recipients were also significantly increased in the recipients transplanted with ICA-treated Fanca−/− or Fancd2−/− cells (Fig. 2E, 2F). Taken together, these results indicate that ICA improves the function of FA HSCs.
ICA upregulates SIRT6 enzyme activity
SIRT6 (sirtuin 6) is a chromatin binding protein known as a key regulator of many important biological processes, including DNA repair, telomere maintenance, and cellular metabolic processes, depending on its enzymatic activity, subcellular localization and target specificity [21,22]. It has been shown that loss of SIRT6 results in premature aging in mice, and SIRT6 overexpression prolongs the mouse lifespan [21]. Recent studies reveal that ICA upregulates the enzyme activity of SIRT6 and prevents inflammation-associated aging [22,23]. We therefore attempted to determine SIRT6 levels in FA HSPCs. By employing a well-established Sandwich ELISA assay, we found that the levels of SIRT6 in LSK cells isolated from Fanca−/− or Fancd2−/− mice was significantly lower compared to that in WT LSK cells (Fig. 3A). Interestingly, ICA treatment markedly increased SIRT6 level in Fanca−/− and Fancd2−/− LSK cells (Fig. 3B). The deacetylase activity of SIRT6 is highly specific for H3K9 acetylation, which is considered the readout for activity of SIRT6 [34]. We thus analyzed H3K9 acetylation by immunoblotting. The results showed that the levels of acetylated H3K9 in Fanca−/− and Fancd2−/− BM Lin− cells (enriched for HSPCs) were much higher than that in WT cells. ICA treatment significantly reduced H3K9 acetylation in FA cells (Fig. 3C). These findings indicate that FA HSPCs have an intrinsic low SIRT6 activity, and ICA can stimulate SIRT6 activity in FA HSPCs.
Figure 3.
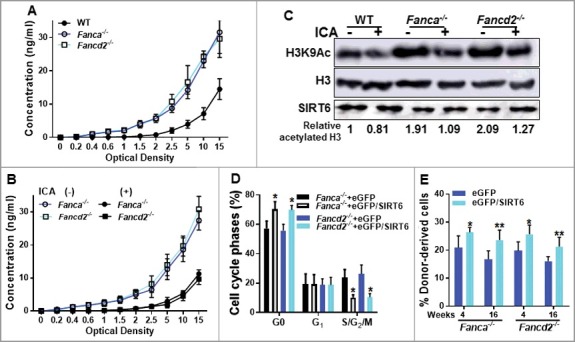
ICA upregulates SIRT6 enzyme activity. (A) Decreased SIRT6 levels in Fanca−/− and Fancd2−/− HSPCs cells. LSK cells from Fanca−/− or Fancd2−/− mice and their WT littermates were subjected to SIRT6 detection using Sandwich ELISA kit. Results are means ±SD of 3 independent experiments (n = 4). (B) ICA treatment upregulates SIRT6 enzyme activity in Fanca−/− and Fancd2−/− LSK cells. LSK cells from ICA-treated or untreated Fanca−/−, Fancd2−/− mice and their WT littermates were subjected to SIRT6 enzyme activity detection using Sandwich ELISA kit. Results are means ±SD of 3 independent experiments (n = 4). (C) Increased level of acetylated H3K9 in Fanca−/− and Fancd2−/− HSPCs cells. Whole cell lysates (WCL) were extracted from Lin− (Lineage depleted) cells isolated from ICA-treated or untreated WT, Fanca−/−, Fancd2−/− mice followed by immunoblotting using antibodies against H3K9Ac, H3 and SIRT6. Acetylated H3K9 was normalized to total H3 protein. The relative levels of H3K9Ac are indicated below the blot. (D) Re-expression of SIRT6 increases quiescence of Fanca−/− and Fancd2−/− HSCs. LSK cells isolated from Fanca−/− and Fancd2−/− mice were transduced with lentiviral vector expressing eGFP or eGFP/SIRT6. Sorted eGFP+ cells were subjected to cell cycle analysis by Ki67 and 7AAD staining. Quantifications are shown. (E) Re-expression of SIRT6 enhances repopulating capacity of Fanca−/− and Fancd2−/− HSCs. 2,000 eGFP+ cells described in (D) along with 3 × 105 congenic bone marrow cells from BoyJ mice were transplanted into lethally irradiated (7.0 Gy) BoyJ recipients. Donor-derived chimera (GFP+) were determined by flow cytometry 16 weeks post BMT. Results are means ±SD of 3 independent experiments (n = 6).
We next asked whether increased expression of SIRT6 could improve the function Fanca−/− and Fancd2−/− HSCs. Indeed, we found that ectopic expression of SIRT6 in Fanca−/− or Fancd2−/− HSCs by lentiviral transduction (Fig. S2) significantly increased quiescent Fanca−/− and Fancd2−/− HSCs compared to those transduced with the eGFP control vector (Fig. 3D). Functionally, overexpression of SIRT6 enhanced repopulating capacity of Fanca−/− and Fancd2−/− HSCs both short (4 weeks) and long (16 weeks) terms (Fig. 3E). Interestingly, ectopic expression of SIRT6 did not further improve the repopulating ability of WT HSCs (Fig. S3). These results suggest that ICA improves the function of FA HSCs probably through a mechanism involving stimulation of SIRT6 activity.
Upregulated SIRT6 by ICA inhibits NF-κB in FA HSPCs
SIRT6 is a specific histone H3 lysine 9 (H3K9) deacetylase that modulates chromatin structure at telomeres [34]. H3K9 deacetylation has been shown to play a critical role in gene repression [35]. Recent studies have indicated that SIRT6 deacetylates H3K9 on promoters of NF-κB target genes to destabilize NF-κB [36]. We first detected the expression of a NF-κB subunit, RelA. Quantitative PCR analysis indicated a higher level of RelA expression in Fanca−/- and Fancd2−/− HSPCs compared to their WT counterpart. ICA treatment reduced RelA expression to almost the WT cell level (Fig. 4A). Since NF-κB is known to be hyperactive in FA cells [37], we then asked whether SIRT6 might be required for deacetylation of H3K9 at the promoter of NF-κB and subsequent repression of NF-κB target genes in HSCs. Indeed, we found that ICA enhanced the deacetylation of H3K9 on the promoter of NF-κB in Fanca−/−, Fancd2−/− Lin− cells (enriched for HSPCs), as detected by chromatin immunoprecipitation (ChIP) assay (Fig. 4B). ICA also enriched H3K9 deacetylation on the NF-κB promoter in WT cells, albeit not statistically significant. Concomitantly, the expression of NF-κB target genes, including Gadd45β, Sod2, Stat1, and Tnfrsf1β [38], was repressed in ICA-treated LSK cells (Fig. 4C). Furthermore, it appeared that inhibition of NF-κB by ICA was mediated by upregulation of SIRT6, as knockdown of SIRT6 (Fig. S4) almost completely abolished the effect of ICA on H3K9 deacetylation of the NF-κB promoter (Fig. 4D) and subsequent repression of the NF-κB target genes (Fig. 4E). Together, these results indicate that upregulated SIRT6 by ICA inhibits NF-κB in FA HSPCs.
Figure 4.
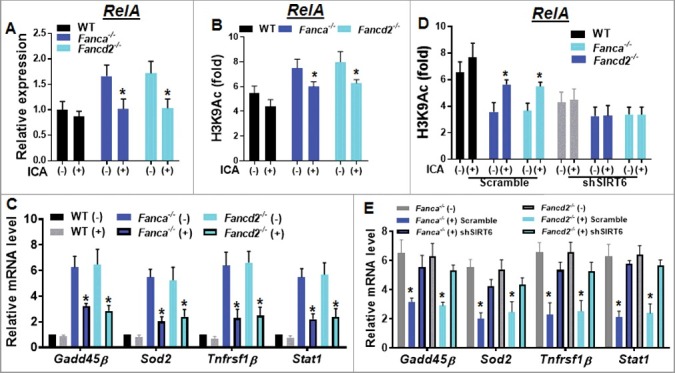
Upregulated SIRT6 by ICA inhibits NF-κB in FA HSPCs. (A) Higher RelA expression in Fanca−/− and Fancd2−/− HSPCs. RNA were extracted from Lin− cells isolated from ICA treated or untreated WT, Fanca−/− or Fancd2−/− mice followed by quantitative PCR analysis using primers for RelA (Table S2). Samples were normalized to the level of GAPDH mRNA. (B) ICA enhances the deacetylation of histone H3 lysine 9 (H3K9) on the promoter of NF-κB (RelA). Cells described in (A) were subjected to chromatin immunoprecipitation using α-H3K9Ac and α-H3 antibodies. H3K9 acetylation at RelA gene promoter is shown relative to untreated control samples and normalized to total H3 levels. (C) ICA represses NF-κB target gene expression. RNA was extracted from LSK cells described in (A) and subjected to qPCR analysis using primers listed in Table S2. Samples were normalized to the level of GAPDH mRNA. (D) The enhanced deacetylation of H3K9 on the promoter of NF-κB by ICA is mediated by upregulation of SIRT6. Lin− cells isolated from Fanca−/−, Fancd2−/− and their WT littermates were transduced with lentiviral vector expressing shRNA targeting SIRT6 or control sequence. Sorted GFP+ cells were chromatin immunoprecipitated with α-H3K9Ac and α-H3 antibodies. H3K9 acetylation at RelA gene promoter is shown relative to untreated control samples and normalized to total H3 levels. (E) Knockdown SIRT6 abolishes ICA-induced repression of NF-κB target genes. RNA was extracted from GFP+ cells described in (D) followed by qPCR using primers listed in Table S2. Samples were normalized to the level of GAPDH mRNA. Results are means ±SD of 3 independent experiments (n = 3).
Discussion
Adult HSCs reside in specialized microenvironments, where they are maintained in a dormant state, a stem cell-specific function known as quiescence [3,39]. Quiescence has been postulated to prevent HSC exhaustion [2], and contribute to HSC longevity and function [40]. Increased cycling of HSCs may result in premature exhaustion of the HSC pool leading to BM failure, such as the devastating genetic disorder FA [30]. The current study identifies a novel function of ICA, a Flavonol glycoside, which improves FA HSC function through enhancing SIRT6-mediated H3K9 deacetylation and NF-κB inhibition. There are several findings that highlight the significance of this study: 1) ICA treatment reverses the less quiescent status of FA HSCs; 2) ICA improves in vitro progenitor capacity and in vivo repopulating ability of FA HSCs; 3) ICA stimulates the enzyme activity of SIRT6 in FA HSPCs; 4) Forced expression of SIRT6 enhances FA HSC function; 5) Upregulated SIRT6 mediated by ICA inhibits NF-κB in FA HSPCs.
Patients with FA often develop BM failure during the first few years of life, likely caused by HSC depletion. One intriguing finding of this study is that ICA can reverses the less quiescent status of FA HSCs. Since premature exhaustion of the HSC pool leads to BM failure [17], preventing FA HSCs from excessive cycling by ICA may serve as a potential therapeutic approach to improve FA HSC function. It is in this context that we identify ICA as a potent small molecule that prevents FA HSC depletion, and thus constitutes a promising candidate for treatment of FA BMF.
SIRT6 is a member of the highly conserved sirtuin family of NAD+-dependent enzymes, which plays a key role in DNA repair, telomere maintenance, and cellular metabolic processes [21,22,41]. It has been shown that SIRT6 deficiency increases genomic instability, premature ageing and cancer development [42–44]. SIRT6 is also known to be critical in HSC homeostasis. Recent study demonstrates that deletion of Sirt6 leads to impaired HSC quiescence [45]. Our present study reveals an intrinsic reduction of SIRT6 activity in Fanca−/− and Fancd2−/− HSCs, which may, at least in part, account for HSC defects observed in FA. Indeed, we demonstrate that this SIRT6 defect is correlated with excessive HSC cycling and compromised HSC function. Further, ectopic expression of SIRT6 increases quiescence of the Fanca−/− and Fancd2−/− HSCs and enhances the ability of these FA mutant HSCs to reconstitute hematopoiesis in the irradiated recipient mice. Interestingly, while ICA also increases SIRT6 activity in WT HSCs, ICA treatment does not further enhance the repopulating capacity of WT HSCs. This suggests that the threshold level of SIRT6 enzyme activity required for maintaining HSC function may be already achieved in WT HSCs. Although further studies are required to define the underlying mechanism that links FA deficiency and reduced SIRT6 enzyme activity, our study, along with previous reports from others [42–44], suggests that the FA pathway collaborates with other DNA damage response (DDR) factors in HSC maintenance, and that disruption of the FA pathway account for the hematologic defects found in both human and mouse [11].
NF-κB is a master transcription factor that controls cell fate, survival, and differentiation [46,47], and has been indicated in various physiologic processes, including immunity, inflammation, and development [46–48]. Persistent NF-κB activation is commonly observed in inflammatory diseases and malignancies [49], including FA. Indeed, a substantial increase in the expression of genes involved in the NF-κB signaling pathway was observed in Fanca−/− and Fancc−/− multipotential progenitor cells (MPPs) [38]. Another study shows that TNF-α-induced Fancc−/− pre-leukemic stem cells requires NF-κB signaling for survival [37]. SIRT6 decetylates H3K9ac or H3K56ac, and acts as a transcriptional co-repressor of NF-κB, c-JUN, MYC and hypoxia-inducible factor-1α (HIF-1α) pathways [36,42,50–52], all of which are implicated in the regulation of adult stem cell function and maintenance [50–52]. The observation that ICA inhibits NF-κB signaling pathway through SIRT6-mediated deacetylation of H3K9 at the promoter of NF-κB leading to the repression of NF-κB target gene expression may shed light on understanding leukemia evolution in FA. In this context, our finding raises an important question as to whether activation of SIRT6 by ICA has preventive potential in FA disease progression.
Materials and methods
Mice and treatments
Fanca+/+, Fanca−/− and Fancd2+/+, and Fancd2−/− mice were generated by interbreeding the heterozygous Fanca+/− or Fancd2+/− mice [53,54], respectively. 6–8 week-old BoyJ mice were used as bone marrow transplant recipients. All the animals including BoyJ mice were maintained in the animal facility at West Virginia University (WVU).
ICA (Sigma-Aldrich, St Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) to 10−3 M and stored at −20˚C. For in vitro studies, 10−3 M ICA was diluted with Earle's balanced salt solution (116 mM NaCl, 5.4 mM KCl, 1 mM NaH2PO4, 0.9 mM CaCl2, 0.8 mM MgSO4, and 10 mg/L phenol red) or full culture medium [4.5 g/mL glucose, 5% foetal bovine serum (Gibco, USA), and 5% horse serum (Gibco, USA) in Dulbecco's modified Eagle's medium (DMEM)] to the final concentration of 10−5 M. For in vivo studies, ICA dosage was adapted and modified based on previous studies [55–57]. Specifically, 6-8-week-old Fanca−/− or Fancd2−/− mice or their age-matched male wild-type littermates were daily intraperitoneal (i.p.) injected with 100 mg/kg of ICA for consecutive 7 days.
Flow cytometry analysis
The lineage marker (Lin) mixture (BD Biosciences, San Jose, CA, USA) for BM cells from treated or untreated mice included the following biotinylated antibodies: CD3ε (145-2C11), CD11b (M1/70), CD45R/B220 (RA3-6B2), mouse erythroid cells Ly-76 (Ter119), Ly6G and Ly-6C (RB6-8C5). Other conjugated antibodies (BD Biosciences) used for surface staining included: CD45.1 (A20), CD45.2 (A104), Sca1 (D7), c-kit (2B8), CD48 (HM48-1) and CD150 (9D1). Biotinylated primary antibodies were detected by incubation of antibody coated cells with streptavidin-PerCP or FITC (BD Biosciences) in a two-step staining procedure. For some of the experiments, pacific blue conjugated CD45.2 (A104, BioLegend, San Diego, CA, USA) was used to determine donor-derived cells.
For apoptosis staining, surface markers stained SLAM cells from ICA treated or untreated mice were subjected to Annexin V and 7AAD staining using BD ApoAlert Annexin V Kit (BD Pharmingen, San Jose, CA) in accordance with the manufacturer's instruction. Apoptosis was analyzed by quantification of Annexin V-positive cell population by flow cytometry.
For cell cycle analysis, surface markers stained SLAM cells from ICA treated or untreated mice were incubated with anti-mouse Ki67 antibody and 7AAD (BD Pharmingen, San Jose, CA) followed by flow cytometry analysis.
Isolation of bone marrow cells and BM transplantation
The femora and tibiae were harvested from the mice immediately after their sacrifice with CO2. Bone marrow (BM) cells were flushed from bones into Iscove's modified Dulbecco's medium (IMDM; Invitrogen) containing 10% FCS, using a 21-guage needle and syringe. Low-density BM cells (LDBMCs) were separated by Ficoll Hypaque density gradient (Sigma-Aldrich, St. Louis, MO) and washed with IMDM medium. Low density bone marrow cells (LDBMCs) were depleted of lineage-committed cells using a lineage cell depletion kit (Miltenyi Biotec Inc, San Diego, CA) in accordance with the manufacturer's instruction.
For bone marrow transplant (BMT), 1,000 ICA-treated LSK cells from Fanca−/−, and Fancd2−/− mice as well as their WT littermates was transplanted along with 3 × 105 bone marrow cells from congenic BoyJ mice, into lethally irradiated (2 split doses of 7.0 Gy and 4.75 Gy, 3 hours apart) BoyJ recipients. For secondary BMT, whole bone marrow cells (WBMCs) from primary recipients were pooled and injected into sublethally irradiated (7.0 Gy) BoyJ recipients.
Colony-forming unit assay
1,000 LSK (Lin−Sca1+c-kit+) cells isolated from the experimental mice were plated in a 35-mm tissue culture dish in 4 mL of semisolid medium containing 3 mL of MethoCult M3134 (Stem Cell Technologies, Vancouver, BC, Canada) and the following growth factors: 100 ng/mL SCF, 10 ng/mL IL-3, 100 ng/mL GM-CSF, and 4 units/mL erythropoietin (Peprotech, Burlington, NC). On day 7 after plating, erythroid and myeloid colonies were enumerated. For serial plating, cells from primary CFU assays were pooled and re-plated to evaluate secondary CFUs. Hematopoietic clonal growth results were expressed as means (of triplicate plates) ± SD of three experiments.
SIRT6 level detection
The levels of SIRT 6 in FA HSPCs were measured using Mouse SIRT6/Sirtuin 6 ELISA Kit (Sandwich ELISA, LSBio, Seattle, WA) following manufacturer's instruction. Briefly, whole cell lysates were collected from LSK cells isolated from experimental mice. 100 μl of sample, standard or blank were added to each well of 96-well plate followed by incubation at 37 degree for 1 hour. The liquid was removed by aspiration and 100 μl of detection reagent A was added to the well followed by incubation at 37 degree for 1 hour. Each well was then washed by wash buffer for 3 times. 100 μl of detection reagent B will be then added to each well and incubated at 37 degree for 30 minutes followed by washing for 5 times. 90 μl of TMB substrate was added to each well followed by incubation at 37 degree for 1–20 minutes. The reaction was stopped by adding 50 μl of stop solution and read immediately at 450 nm.
Acetylation of H3K9
To determine SIRT6 enzyme activity, we perfomed immunoblotting for the level of acetylated H3K9 as previously described [34]. Briefly, sorted LSK cells isolated from ICA treated or untreated Fanca−/− or Fancd2−/− as well as their WT littermates were washed with ice-cold PBS, and resuspended in ice-cold lysis buffer containing 50 mmol/ L Tris-HCL (Ph 7.4), 0.1% NP40, and 1 mol/L NaCl supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Cell debris was removed from the lysates by centrifuging them at 14,000 rpm for 30 min. Protein concentration was quantified by using Bio-Rad reagent and resolved on SDS-PAGE and transferred onto nitrocellulose membrances. Immunoblots were then incubated with primary antibodies specific for acetylated H3K9, histone H3, SIRT6 or GAPDH (Abcam, Cambridge, MA). Acetylated H3K9 was normalized to total H3 protein and the relative amount was quantified by densitometry using ImageJ software (NIH).
Deacetylation of Histone H3 Lysine 9 on the promoter of NF-κB (RelA)
Lin− cells from ICA-treated or untreated Fanca−/− or Fancd2−/− mice and their WT littermates were treated with TNF-α (20 ng/mL) in vitro for 1 hour. DNA was then crosslinked for 10 min with 1% formaldehyde and stopped in 0.125 M glycine. Purified chromatin was sonicated to 300 ∼bp using the Bioruptor (Diagenode, Denville, NJ) and incubated with α-H3K9Ac and α-H3 antibodies. Following reverse crosslinking, ChIP-associated sequences were detected by quantitative real-time PCR as described above. PCR primers sequences for promoter region of RelA are provided in Table S2.
RNA Isolation, Reverse Transcriptase (RT)-PCR
Total RNA from indicated cell compartment isolated from experimental mice was prepared with RNeasy kit (Qiagen, Valencia, CA) following the manufacturer's procedure. Reverse transcription was performed with random hexamers and Superscript II RT (Invitrogen, Grand Island, NY) and was carried out at 42 °C for 60 min and stopped at 95 °C for 5 min. First-strand cDNA was used for real-time PCR using primers listed in Table S2. Samples were normalized to the level of GAPDH mRNA.
Plasmids and antibodies
SIRT6 short hairpin RNA (shSIRT6-1 and shSIRT6-2) or non-targeting shRNA (shCont) were purchased from Sigma (Sigma-Aldrich, St Louis, MO, USA). Sequences of shSIRT6-1 and shSIRT6-2 are 5′-AGAGGAATGTCCCAAGTGT-3′ and 5′-GTTTGACACCACCTTCGAG-3′. Sequence of shCont is 5′-GCAACAAGATGAAGAGCACCAA-3′.
For lentiviral vector construction, FLAG-tagged full-length SIRT6 cDNA were cloned into the pLVX-eGFP vector (Gateway) [28]. The plasmids (10 μg each) were used to produce lentiviral supernatant.
Statistical analysis
Paired or unpaired student's t-test was used for two-group comparisons. Survival data were plotted by the Kaplan-Meier curve method and analyzed by the log-rank test. Values of p<0.05 were considered statistically significant. Results are presented as mean ± SD. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001.
Supplementary Material
Funding Statement
This work was supported by the NIH [grant number U54 GM104942]
Acknowledgments
We thank Dr. Madeleine Carreau (Laval University) for Fanca+/− mice, Dr. Markus Grompe (Oregon Health & Sciences University) for Fancd2+/− mice. This work was supported by the National Natural Science Foundation of China (NNSFC# 81370608; 81470288 and U1401221). W.D. is supported by West Virginia University (WVU) Health Science Center (HSC) and School of Pharmacy (SOP) Startup Funds, NIH/NIGMS WV CTSI Award (U54 GM104942) and a West Virginia University General International Grant, and a Leukemia Research Foundation (LRF) Award.
Disclosure of Potential Conflicts of Interest
There is no conflict of interest to disclose on the part of any authors.
References
- [1].Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4): 631–644. doi: 10.1016/j.cell.2008.01.025. PMID:18295580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. doi: 10.1038/nrg2269. PMID:18202695 [DOI] [PubMed] [Google Scholar]
- [3].Wilson A, Trumpp A. Bone-marrow hematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. PMID:16491134 [DOI] [PubMed] [Google Scholar]
- [4].Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. PMID:8689561 [DOI] [PubMed] [Google Scholar]
- [5].Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/ waf1. Science. 2000;287(5459):1804–1808. doi: 10.1126/science.287.5459.1804. PMID:10710306 [DOI] [PubMed] [Google Scholar]
- [6].Hock H, Hamblen MJ, Rooke HM, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431(7011):1002–1007. doi: 10.1038/nature02994. PMID:15457180 [DOI] [PubMed] [Google Scholar]
- [7].Bagby GC. Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10(1):68–76. doi: 10.1097/00062752-200301000-00011. PMID:12483114 [DOI] [PubMed] [Google Scholar]
- [8].Bluteau D, Masliah-Planchon J, Clairmont C, et al. Biallelic inactivation of REV7 is associated with Fanconi anemia. J Clin Invest. 2017;127(3):1117. doi: 10.1172/JCI92946. PMID:28248207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11(7):467–480. doi: 10.1038/nrc3088. PMID:21701511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dong H, Nebert DW, Bruford EA, et al. Update of the human and mouse Fanconi anemia genes. Hum Genomics. 2015;9:32. doi: 10.1186/s40246-015-0054-y. PMID:26596371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Du W, Erden O, Pang Q. TNF-α signaling in Fanconi anemia. Blood Cells Mol Dis. 2013;52(1):2–11. doi: 10.1016/j.bcmd.2013.06.005. PMID:23890415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19(24):2925–2940. doi: 10.1101/gad.1370505. PMID:16357213 [DOI] [PubMed] [Google Scholar]
- [13].Meyer S, Neitzel H, T¨onnies H. Chromosomal aberrations associated with clonal evolution and leukemic transformation in Fanconi anemia: clinical and biological implications. Anemia. 2012;2012:349837. doi: 10.1155/2012/349837. PMID:22675616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park JY, Virts EL, Jankowska A, et al. Complementation of hypersensitivity to DNA interstrand crosslinking agents demonstrates that XRCC2 is a Fanconi anaemia gene. J Med Genet. 2016;53:672–680. doi: 10.1136/jmedgenet-2016-103847. PMID:27208205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sawyer SL, Tian L, Kähkönen M, et al. Biallelic Mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov. 2015;5(2):135–142. doi: 10.1158/2159-8290.CD-14-1156. PMID:25472942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tischkowitz MD, Hodgson SV. Fanconi anaemia. J Med Genet. 2003;40(1): 1–10. doi: 10.1136/jmg.40.1.1. PMID:12525534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du W, Adam Z, Rani R, et al. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10(11):1909–1921. doi: 10.1089/ars.2008.2129. PMID:18627348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walter D, Lier A, Geiselhart A, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–552. doi: 10.1038/nature14131. PMID:25707806 [DOI] [PubMed] [Google Scholar]
- [19].Ma P, Zhang S, Su X, et al. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am J Transl Res. 2015;7(10):2105–2014. PMID:26692955 [PMC free article] [PubMed] [Google Scholar]
- [20].Hu Y, Sun B, Liu K, et al. Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway. Inflammation. 2016;39(1):228–236. doi: 10.1007/s10753-015-0242-x. PMID:26307750 [DOI] [PubMed] [Google Scholar]
- [21].Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. PMID:22395773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci. 2014;39:72–81. doi: 10.1016/j.tibs.2013.12.002. PMID:24438746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen Y, Sun T, Wu J, et al. Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. Biomed Res Int. 2015;2015:895976. PMID:25688369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song YH, Cai H, Zhao ZM, et al. Icariin attenuated oxidative stress induced-cardiac apoptosis by mitochondria protection and ERK activation. Biomed Pharmacother. 2016;83:1089–1094. doi: 10.1016/j.biopha.2016.08.016. PMID:27551754 [DOI] [PubMed] [Google Scholar]
- [25].Zhang S, Feng P, Mo G, et al. Icariin influences adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture and clinical research adipogenic. Biomed Pharmacother. 2017;88:436–442. doi: 10.1016/j.biopha.2017.01.050. PMID:28122309 [DOI] [PubMed] [Google Scholar]
- [26].Lin CC, Ng LT, Hsu FF, et al. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol Physiol. 2004;31(1-2):65–69. doi: 10.1111/j.1440-1681.2004.03951.x. PMID:14756686 [DOI] [PubMed] [Google Scholar]
- [27].Wang Z, Zhang H, Dai L, et al. Arsenic Trioxide and Icariin show synergistic anti-leukemic activity. Cell Biochem Biophys. 2015;73(1):213–9. doi: 10.1007/s12013-015-0660-2. PMID:25721870 [DOI] [PubMed] [Google Scholar]
- [28].Du W, Amarachintha S, Wilson A, et al. The immune receptor Trem1 cooperates with diminished DNA damage response to induce preleukemic stem cell expansion. Leukemia. 2017;31(2):423–433. doi: 10.1038/leu.2016.242. PMID:27568523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kobayashi M, Srour EF. Regulation of murine hematopoietic stem cell quiescence by Dmtf1. Blood. 2011;118(25):6562–6571. doi: 10.1182/blood-2011-05-349084. PMID:22039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Du W, Amarachintha S, Erden O, et al. Fancb deficiency impairs hematopoietic stem cell function. Sci Rep. 2015;5:18127. doi: 10.1038/srep18127. PMID:26658157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. PMID:15989959 [DOI] [PubMed] [Google Scholar]
- [32].Parmar K, Kim J, Sykes SM, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28(7):1186–1195. doi: 10.1002/stem.437. PMID:20506303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cheng NC, van de Vrugt HJ, van der Valk MA, et al. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum Mol Genet. 2000;9(12):1805–1811. doi: 10.1093/hmg/9.12.1805. PMID:10915769 [DOI] [PubMed] [Google Scholar]
- [34].Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylases that modulates temomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. PMID:18337721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. PMID:17320507 [DOI] [PubMed] [Google Scholar]
- [36].Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. PMID:19135889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhou Y, Du W, Koretsky T, et al. TAT-mediated intracellular delivery of NPM-derived peptide induces apoptosis in leukemic cells and suppresses leukemogenesis in mice. Blood. 2008;112(6):2474–83. doi: 10.1182/blood-2007-12-130211. PMID:18574026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Du W, Amarachintha S, Sipple J, et al. Inflammation-mediated notch signaling skews fanconi anemia hematopoietic stem cell differentiation. J Immunol. 2013;191(5):2806–2817. doi: 10.4049/jimmunol.1203474. PMID:23926327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. PMID:747780 [PubMed] [Google Scholar]
- [40].Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. PMID:19725055 [DOI] [PubMed] [Google Scholar]
- [41].Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. PMID:22367546 [DOI] [PubMed] [Google Scholar]
- [42].Sebastian C, Zwaans BM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. PMID:23217706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. PMID:16439206 [DOI] [PubMed] [Google Scholar]
- [44].Toiber D, Erdel F, Bouazoune K, et al. SIRT6 recruits SNF2H to DNA break sites,preventing genomic instability through chromatin remodeling. Mol Cell. 2013;51:454–468. doi: 10.1016/j.molcel.2013.06.018. PMID:23911928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang H, Diao D, Shi Z, et al. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell. 2016 Apr 7;18(4):495–507. doi: 10.1016/j.stem.2016.03.005. PMID:27058938 [DOI] [PubMed] [Google Scholar]
- [46].Stanic AK, Bezbradica JS, Park JJ, et al. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004;172:2265–2273. doi: 10.4049/jimmunol.172.4.2265. PMID:14764695 [DOI] [PubMed] [Google Scholar]
- [47].Rizo A, Vellenga E, de Haan G, et al. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum Mol Genet. 206;15:210–219. doi: 10.1093/hmg/ddl175. PMID:16987886 [DOI] [PubMed] [Google Scholar]
- [48].Gerondakis S, Banerjee A, Grigoriadis G, et al. NF-κB subunit specificity in hemopoiesis. Immunol Rev. 2012;246:272–285. doi: 10.1111/j.1600-065X.2011.01090.x. PMID:22435561 [DOI] [PubMed] [Google Scholar]
- [49].Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. PMID:15385993 [DOI] [PubMed] [Google Scholar]
- [50].Laurenti E, Varnum-Finney B, Wilson A, et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. PMID:19041778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stein SJ, Baldwin AS. Deletion of the NF-kB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood. 2013;121:5015–5024. doi: 10.1182/blood-2013-02-486142. PMID:23670180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. PMID:20804974 [DOI] [PubMed] [Google Scholar]
- [53].Wong JC, Alon N, Mckerlie C, et al. Targeted disruption of exons 1 to 6 of the Fanconi Anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet. 2003;12:2063–2076. doi: 10.1093/hmg/ddg219. PMID:12913077 [DOI] [PubMed] [Google Scholar]
- [54].Houghtaling S, Timmers C, Noll M, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17(16):2021–2035. doi: 10.1101/gad.1103403. PMID:12893777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pan Y, Zhang WY, Xia X, et al. Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley rats. Biol Pharm Bull. 2006;29(12):2399–2403. doi: 10.1248/bpb.29.2399. PMID:17142971 [DOI] [PubMed] [Google Scholar]
- [56].Zhang ZB, Yang QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8(5):601–605. doi: 10.1111/j.1745-7262.2006.00197.x. PMID:16751992 [DOI] [PubMed] [Google Scholar]
- [57].Mok SK, Chen WF, Lai WP, et al. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br J Pharmacol. 2010;159(4):939–949. doi: 10.1111/j.1476-5381.2009.00593.x. PMID:20128811 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


