Abstract
Background
Sentinel node biopsy using radioisotope and blue dye remains a gold standard for axillary staging in breast cancer patients with low axillary burden. However, limitations in the use of radioisotopes have resulted in emergence of novel techniques. This is the first in vivo study to assess the feasibility of combining the two most common novel techniques of using a magnetic tracer and indocyanine green (ICG) fluorescence.
Materials and methods
A total of 48 mice were divided into eight groups. Groups 1 and 2, the co-localization groups, received an injection of magnetic tracers (Resovist® and Sienna+®, respectively) and ICG fluorescence; distilled water was used as the solvent of ICG. Groups 3 and 4, the diluted injection groups, received an injection of magnetic tracers (Resovist and Sienna+, respectively) and saline for dilution. Groups 5, 6, and 7, the control groups, received magnetic tracer (Resovist, Sienna+) and ICG alone, respectively. Fluorescent intensity assessment and iron quantification of excised popliteal lymph nodes were performed. Group 1′, a co-localization group, received an injection of magnetic tracers (Resovist) and ICG′ fluorescence: saline was used as the solvent for ICG.
Results
Lymphatic uptake of all tracers was confined to the popliteal nodes only, with co-localization confirmed in all cases and no significant difference in fluorescent intensity or iron content of ex vivo nodes between the groups (except for Group 1′). There was no impact of dilution on the iron content in the diluted Sienna+ group, but it significantly enhanced Resovist uptake (P=0.005). In addition, there was a significant difference in iron content (P=0.003) in Group 1′.
Conclusion
The combination of a magnetic tracer (Resovist or Sienna+) and ICG fluorescence is feasible for sentinel node biopsy and will potentially allow for precise transcutaneous node identification, in addition to accurate intraoperative assessment. This radioisotope-free “combined technique” warrants further assessment within a clinical trial.
Keywords: sentinel node biopsy, combined technique, fluorescence dye, magnetic nanoparticles, dilutional effect
Introduction
Sentinel node biopsy (SNB) emerged in the early 1990s as a targeted alternative to axillary node clearance for staging the axilla, whereby subcutaneous injection of blue dye and subsequently radioisotopes was used to identify the first draining lymph nodes from the primary malignancy. The absence of metastatic spread to the lymph nodes on SNB allowed for the avoidance of axillary node clearance. The reduced morbidity of the procedure resulted in SNB using a radioactive tracer and blue dye (dual technique) becoming the standard of care for axillary staging in clinically node-negative breast cancer patients. The use of the “dual technique” provides a high sentinel node identification rate and low false-negative rate.1 However, it is accepted that access to radioisotopes is limited and their associated legislation for handling and disposal restricts use of the “dual technique”.2 The use of blue dye is not without risk when considering the possibility of anaphylaxis. As a consequence of the drawbacks of the “dual technique”, other radioisotope-independent techniques have emerged.3,4 The most clinically evaluated within the literature are the magnetic technique – using a magnetic tracer and handheld magnetometer – and indocyanine green immunofluorescence using a photodynamic eye (PDE) camera.4 These techniques have been evaluated within meta-analyses and demonstrated to show non-inferiority to the “dual technique”. The optimization of magnetic tracers within preclinical settings has also been evaluated in terms of volumes and particle sizes.5–7 However, all these analyses have possessed methodological limitations, most strikingly that none has consisted of a robust randomized controlled trial against the gold standard. These novel alternative techniques possess both pros and cons compared to the standard “dual technique” and over each other. It is, therefore, conceivable that as with the combined use of radioisotope and blue dye, multimodal, radioisotope-free techniques could be used to overcome the limitations of any single technique. In order for this to be possible, it is essential that techniques consistently allow for the co-localization of sentinel nodes. Within the protocols of conducted clinical trials,8–10 the magnetic tracer (Sienna+®; Endomagnetics, Cambridge, UK) has been diluted in normal saline for injection. This has increased the volume administered and evidence suggests it may impact adversely upon residual iron remnants on magnetic resonance imaging.11 The ability of indocyanine green (ICG) fluorescence and magnetic tracers to co-localize sentinel nodes and the impact of dilution of magnetic tracers were evaluated within a murine model in this study.
Materials and methods
Tracers
Three tracers were used in this study: 1) the superparamagnetic iron oxide tracer, Resovist® (Bayer Pharma AG, Tokyo, Japan), containing a magnetic core of maghemite (γ-Fe2O3) covered with a carboxydextran coating and an iron concentration of 28 mg/mL; 2) the superparamagnetic iron oxide tracer, Sienna+ (Endomagnetics); and 3) the fluorescent dye ICG, containing serum protein capable of absorbing near-infrared light at the wavelength of 805 nm and emitting it at ~820 nm. The difference between Sienna+ and Resovist is that the latter contains mannitol, resulting in a difference in osmolality.
Animals and surgery
Ethical permission for animal experimentation was granted by the University of Tokyo Ethical Review Board (reference number KA16-4), and all procedures followed were in accordance with the institutional guidelines and ensured humane care of animals. The animals used were 48 female SLC/ICR mice (Japan SLC, Inc., Tokyo, Japan) of age 8–9 weeks, which were sacrificed under deep anesthesia and the popliteal and inguinal nodes were excised.
Imaging systems
A PDE camera (UI-3240CP-NIR-DL; iDS, Obersulm, Germany) equipped with focus lens (LM12HC-SW; Kowa Optical Products Co., Ltd., Nagoya, Japan) and longpass filter (LIX810; ASAHI SPECTRA Co., Ltd., Tokyo, Japan), which cuts off the near-infrared light with a wavelength shorter than 810 nm, was used to observe the fluorescence emitted by ICG. A red light-emitting diode with a low-pass filter – passing only near-infrared light below 780 nm – was used for the excitation of ICG, ensuring the PDE camera obtained fluorescence intensity without the influence of the excitation light coming from the light-emitting diode.
Experimental protocol
To assess the co-localization of magnetic tracers and ICG, the animals were divided into eight groups, each containing six mice. Mice were anesthetized using a mixture of 2%–3% isoflurane and 300 mL/min air via an automatic delivery system (Isoflurane Vaporizer; SN-487; Shinano Manufacturing, Tokyo, Japan) during massages and observations using the PDE camera. The mice were repeatedly monitored during the procedure to maintain adequate depth of anesthesia.
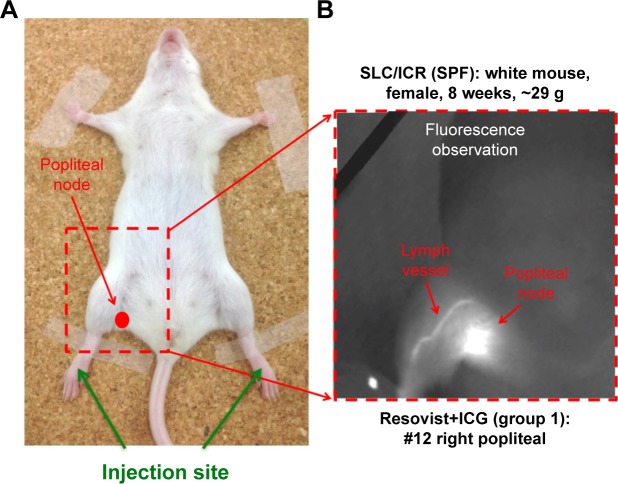
Groups 1 and 2 constituted the magnetic tracers and ICG co-localization sentinel node groups. An injection of 5 μL magnetic tracer and 5 μL diluted ICG (5 mg/mL) was given bilaterally into the rear footpad (Figure 1A), followed by 5 min of massage to promote tracer uptake. Fluorescence was immediately observed using the PDE camera to assess lymphatic uptake of the fluorescent tracer (Figure 1B). All mice were subsequently sacrificed 15 min after the injection, and inguinal and popliteal lymph nodes were excised for iron quantification using a superconducting quantum interference device (SQUID, MPMS-5S; Quantum Design, Inc., San Diego, CA, USA) and the fluorescence intensity was assessed using a PDE camera (Figure 2). The magnetic moments of the excised lymph nodes measured by SQUID magnetometry included not only superparamagnetic components of the nanoparticles but also diamagnetic components of the biomedical tissues. To quantify the iron content in the nodes, the magnetic moment of a phantom containing 139 μg of iron (5 μL of Resovist/Sienna+) was used as a reference. Taking into account the subtraction of the diamagnetic components and comparing the measured moments and reference data by use of the least squares method, we obtained the iron content in the nodes. Group 1′, in which ICG was diluted in saline instead of distilled water, followed the standard protocol used for groups 1 and 2.
Figure 1.
(A) Location of the injection site and right popliteal node. (B) Transcutaneous fluorescence visible using a near-infrared camera after ICG administration.
Abbreviation: ICG, indocyanine green.
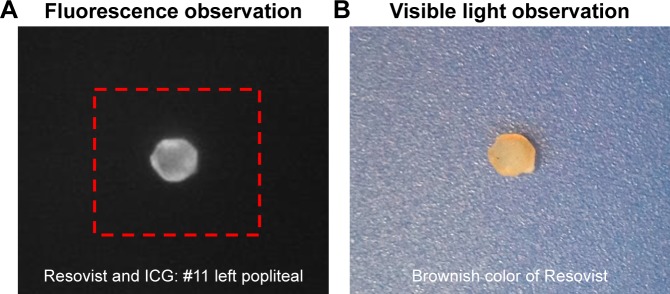
Figure 2.
(A) Fluorescence and (B) visible observation of the excised popliteal node in the experiment with Resovist+ICG.
Abbreviation: ICG, indocyanine green.
Groups 3 and 4 constituted the diluted magnetic tracer groups. An injection of 5 μL of magnetic tracer and 5 μL saline (100% dilution, 14 mg/mL) was given bilaterally into the rear footpad, followed by 5 min of massage to promote tracer uptake. The standard protocol applied to groups 1 and 2 was followed, without the assessment of fluorescence.
The other three groups acted as controls. Groups 5 and 6 consisted of mice receiving injection of magnetic tracer (5 μL Resovist and Sienna+, respectively) alone and followed the standard protocol applied to groups 1 and 2, without the assessment of fluorescence. Group 7 consisted of mice injected with 5 μL of ICG (5 mg/mL) alone, and observations using the PDE camera started immediately after the injection and continued for 15 min to observe the emission intensity of ICG. Inguinal and popliteal lymph nodes were excised and ex vivo quantification analyses of the fluorescence using PDE camera were conducted. Particle size and zeta potential of each injected tracer were measured by the ELSZ-2 zeta potential and particle size analyzer (Otsuka Electronics Co., Ltd., Osaka, Japan).
Statistical analysis
Unpaired t-tests (SPSS, Chicago, IL, USA) were performed to compare the fluorescent intensity and iron content of ex vivo nodes (n=12 for each group). The relationship between fluorescent intensity and iron content was evaluated using Spearman’s correlation.
Results
Fluorescence intensity
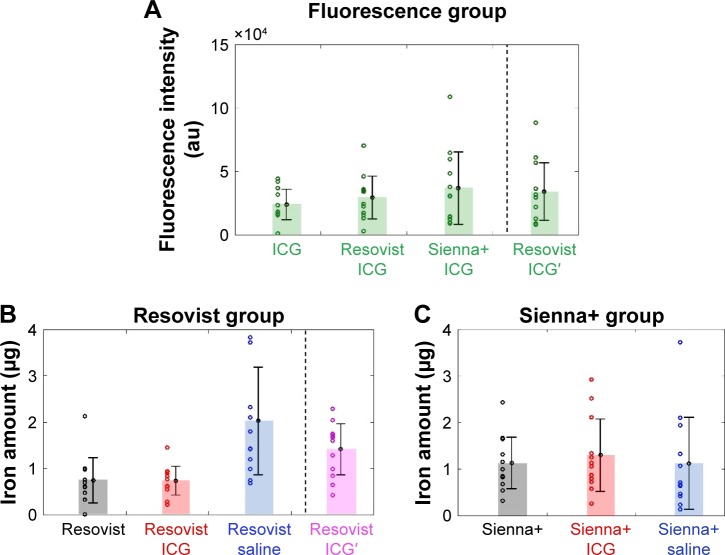
The magnetic tracer plus ICG (groups 1, 1′, and 2) and ICG only (Group 7) groups demonstrated visible fluorescence in bilateral popliteal nodes in all 24 mice. Figure 1A and B demonstrates ex vivo node fluorescence and a visible popliteal node – plus lymphatic vessel – transcutaneously on the PDE camera in the combined use of Resovist and ICG, respectively. The mean fluorescence intensities recorded were 29,430 (SD 17,683), 33,354 (SD 21,123), 36,869 (SD 29,843), and 23,987 (SD 12,583) for groups 1, 1′, 2, and 7, respectively (P=0.25 in groups 1 and 7, P=0.11 in groups 1′ and 7, and P=0.13 in groups 2 and 7), as shown in Figure 3A. No uptake was visible in the inguinal lymph nodes, except for one case (unilateral node) using Sienna+ and ICG (Group 2).
Figure 3.
Accumulation of the tracers in the popliteal nodes.
Notes: (A) Fluorescence intensity from the popliteal nodes. (B and C) Iron amount evaluated by SQUID measurements. ICG and ICG′ were used with distilled water and saline as the solvent, respectively.
Abbreviations: ICG, indocyanine green; SQUID, superconducting quantum interference device.
Iron quantification
Groups 1–6 and 1′ demonstrated iron uptake by SQUID magnetometry in popliteal nodes only, with a mean iron content (g) of 7.36×10−7 (SD 3.25×10−7), 13.0×10−7 (SD 8.11×10−7), 20.3×10−7 (SD 12.2×10−7), 11.3×10−7 (SD 10.3×10−7), 7.44×10−7 (SD 5.13×10−7), 11.4×10−7 (SD 5.78×10−7), and 14.1×10−7 (SD 5.76×10−7), respectively (P=0.48 in groups 1 vs 5, P=0.25 in groups 2 vs 6, P=0.005 in groups 3 vs 5, P=0.49 in groups 4 vs 6, P=0.003 in groups 1′ vs 5, and P=0.07 in groups 1′ vs 1), as shown in Figure 3B and C. There was no uptake of iron in the inguinal lymph nodes, except for a single node using Sienna+ and ICG (Group 2). Detailed results are shown in Tables 1 and 2. Iron content in the popliteal nodes was ~1% of the injection amount (139 μg).
Table 1.
Injection tracer(s), mean iron content, and fluorescent intensity in each group
| Group | Tracer(s) | Iron content (10−7 g) | Fluorescence intensity (au) |
|---|---|---|---|
| 1 | Resovist (5 μL) and ICG (5 μL) | 7.36 | 29,430 |
| 2 | Sienna+ (5 μL) and ICG (5 μL) | 13.0 | 36,869 |
| 3 | Resovist (5 μL) and saline (5 μL) | 20.3 | – |
| 4 | Sienna+ (5 μL) and saline (5 μL) | 11.3 | – |
| 5 | Resovist (5 μL) | 7.44 | – |
| 6 | Sienna + (5 μL) | 11.4 | – |
| 7 | ICG (5 μL) | – | 23,987 |
| 1′ | Resovist (5 μL) and ICG′ (5 μL)a | 14.1 | 33,354 |
Notes:
ICG′ was used with saline as the solvent. Bold font represents the co-localization groups.
Abbreviation: ICG, indocyanine green.
Table 2.
P-values calculated from unpaired t-tests
| Groups | P-value |
|---|---|
| Resovist, ICG/Resovist | 0.48a |
| Sienna+, ICG/Sienna+ | 0.25a |
| Resovist/Sienna+ | 0.03*,a |
| Diluted Resovist/Resovist | 0.005*,a |
| Diluted Sienna+/Sienna+ | 0.49a |
| Resovist, ICG/ICG | 0.25b |
| Sienna+, ICG/ICG | 0.13b |
| Resovist, ICG′/ICG | 0.11b |
| Resovist, ICG′/Resovist | 0.003**,a |
| Resovist, ICG′/diluted Resovist | 0.07a |
Notes: P-values of
iron content and
fluorescence intensity. Significant difference,
P<0.05,
P<0.005. ICG′ was used with saline as the solvent.
Abbreviation: ICG, indocyanine green.
Relationship between fluorescence intensity from the node and uptake of magnetic tracers in the node
Groups 1 and 2 demonstrated a relationship between fluorescence intensity and iron uptake, with the correlation factor R being ~0.40 (Figure 4).
Figure 4.
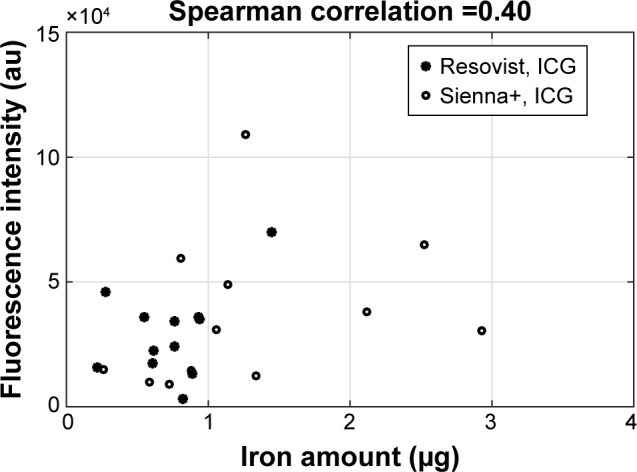
Correlation between uptake of magnetic tracers in the nodes and fluorescence intensity from the node.
Note: Correlation factor R is ~0.40.
Abbreviation: ICG, indocyanine green.
Particle size and zeta potential of injected tracers
Groups 1–6 and 1′ demonstrated fairly similar particle size and zeta potential of the injected tracer. Detailed results are shown in Table 3.
Table 3.
Particle size and zeta potential of the injection tracer(s) in each group
| Group | Tracer(s) | Particle size (nm) | Zeta potential (mV) |
|---|---|---|---|
| 1 | Resovist (5 μL) and ICG (5 μL) | 78.8 | −51.8 |
| 2 | Sienna+ (5 μL) and ICG (5 μL) | 77.9 | −43.5 |
| 3 | Resovist (5 μL) and saline (5 μL) | 78.0 | −44.6 |
| 4 | Sienna+ (5 μL) and saline (5 μL) | 68.5 | −36.0 |
| 5 | Resovist (5 μL) | 73.5 | −44.9 |
| 6 | Sienna+ (5 μL) | 72.6 | −39.6 |
| 7 | ICG (5 μL) | N/A | N/A |
| 1′ | Resovist (5 μL) and ICG′ (5 μL)a | 75.7 | −47.6 |
Notes:
ICG′ was used with saline as the solvent. These parameters were not measured for ICG because its particle size corresponds to molecular size.
Abbreviations: ICG, indocyanine green; N/A, not available.
Discussion
SNB is the gold standard for axillary staging in breast cancer patients with suspected low axillary burden. The combined use of radioisotope and blue dye has been demonstrated by meta-analysis to yield the highest sensitivity and lowest false-negative rate compared to either technique used alone.1 Novel techniques have emerged as alternatives for SNB, which are radioisotope independent and therefore allow easier access and utilization in isotope-replete areas. This study was conducted to assess the feasibility of combining two radioisotope-independent techniques to form a feasible “combined technique” for SNB. The magnetic and fluorescent (ICG) techniques have been the most extensively evaluated of these novel procedures. A technical limitation of the magnetic technique has been the inability to identify a transcutaneous signal (hotspot) to precisely define the incision point preoperatively. This results in the surgeon being more dependent upon signal identification after the skin incision is made. On the contrary, the fluorescent technique allows transcutaneous visualization of sentinel nodes using a PDE camera and precise positioning of a surgical incision. Therefore, by combining these two techniques, it would be considered possible to attain the benefits of transcutaneous fluorescent marking and precise, real-time intraoperative guidance using the handheld magnetometer. However, such an approach is only feasible if there is co-localization of sentinel nodes by the two techniques, otherwise it would result in unnecessary nodal retrieval.
This study has been able to demonstrate that uptake of both tracers was limited to the primary draining lymph node of the murine lower limb (popliteal node)12 and did not spread to other locations. This is highly suggestive of an adequate dose of tracer being administered to prevent saturation of the primary draining node and passage to higher echelon nodes. In all cases, co-localization of the two tracers occurred without any evidence of drainage to higher echelon nodes, except for one single case. In terms of the fluorescence evaluations, there was no significant difference among groups, indicating that ICG uptakes into the lymph nodes without serious particle interactions. No suggestions of deleterious effects from combining the two tracers were demonstrated, with no significant difference in fluorescence intensity or iron uptake between groups 1, 2, 5, 6, and 7. This result indicates that the dilutional effect and interaction of the two separate tracers did not result in any adverse effect on nodal uptake. Furthermore, the correlation factor of the relationship between fluorescence intensity and iron uptake is ~0.40, suggesting the presence of a moderate correlation. In terms of the dilution group, we could not find significant differences in the experiment with Sienna+ (P=0.49). In contrast, a significant difference was found in the diluted Resovist group (P=0.005). This difference between the two magnetic tracers and dilutional effect is likely the result of their differing osmolalities. Resovist possesses an osmolality of 333 mOsm/kg, whereas Sienna+ has a value of 80–160 mOsm/kg. The osmolality of interstitial fluid is in the range of 275–295 mOsm/kg. The lower osmolality of Sienna+ encourages fluid shifts from the interstitium toward the lymphatics, as was demonstrated by its significantly greater nodal deposition of iron using neat Sienna+ compared to Resovist (P=0.03). This superiority over Resovist may also be attributable to the physiological pH maintained by the presence of mannitol in its excipients, which influences the osmolality. The osmolality of Sienna+ appears to be the optimal value, as when diluted further, there is no improvement in iron uptake. By contrast, Resovist, with an osmolality of more than twice that of Sienna+, allows for a significant improvement (P=0.005) in nodal iron uptake when diluted, therefore approaching an osmolality closer to that of Sienna+. This suggests that the dilution of Resovist could optimize the tracer for clinical use, as compared to Sienna+, in which case dilution appears unnecessary. In addition, we report significant differences (P=0.003) between Group 1′, which used Resovist and ICG′ (solvent is saline), and Group 5 using Resovist alone. The uptake of magnetic tracers into the nodes was approximately double the amount with Resovist only. This result shows that the combined use of Resovist and ICG′ (solvent is saline) leads to significantly improved detection of sentinel lymph nodes. To investigate the factor that could promote the uptake of magnetic nanoparticles, we measured the particle size and zeta potential of the tracers, but could not identify any particle aggregation and potential changes that would impact the accumulation in the node. Therefore, we conclude that the osmolality change is the most important factor for tracer accumulation in the nodes.
Conclusion
This is the first in vivo study to assess the use of combining a magnetic and fluorescent tracer for the purposes of SNB. It demonstrates that the technique is feasible, co-localizes sentinel nodes, and avoids higher echelon node involvement. This method represents an alternative radioisotope-free “combined technique” for SNB, which warrants assessment within a clinical trial to determine if it translates to any benefit within the clinical setting.
Acknowledgments
This work was supported by the Development of Medical Devices and Systems for Advanced Medical Services of the Japan Agency for Medical Research and Development (AMED) and a grant from the Magnetic Health Science Foundation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Douek M. The role of magnetic nanoparticles in the localization and treatment of breast cancer. Biomed Res Int. 2013;2013:281230. doi: 10.1155/2013/281230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zada A, Peek MC, Ahmed M, et al. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br J Surg. 2016;103(11):1409–1419. doi: 10.1002/bjs.10283. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15(8):e351–e362. doi: 10.1016/S1470-2045(13)70590-4. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Anninga B, Pouw JJ, et al. Optimising magnetic sentinel lymph node biopsy in an in vivo porcine model. Nanomedicine. 2015;11(4):993–1002. doi: 10.1016/j.nano.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Anninga B, Ahmed M, Van Hemelrijck M, et al. Magnetic sentinel lymph node biopsy and localization properties of a magnetic tracer in an in vivo porcine model. Breast Cancer Res Treat. 2013;141(1):33–42. doi: 10.1007/s10549-013-2657-0. [DOI] [PubMed] [Google Scholar]
- 7.Pouw JJ, Ahmed M, Anninga B, et al. Comparison of three magnetic nanoparticle tracers for sentinel lymph node biopsy in an in vivo porcine model. Int J Nanomedicine. 2015;10:1235–1243. doi: 10.2147/IJN.S76962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio IT, Diaz-Botero S, Esgueva A, et al. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol. 2015;41(1):46–51. doi: 10.1016/j.ejso.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Thill M, Kurylcio A, Welter R, et al. The Central-European SentiMag study: Sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23(2):175–179. doi: 10.1016/j.breast.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Douek M, Klaase J, Monypenny I, et al. SentiMAG Trialists Group Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol. 2014;21(4):1237–1245. doi: 10.1245/s10434-013-3379-6. [DOI] [PubMed] [Google Scholar]
- 11.Huizing E, Anninga B, Young P, Monnypenny I, Hall-Craggs M, Douek M. Analysis of void artefacts in post-operative breast MRI due to residual SPIO after magnetic SLNB in SentiMAG Trial participants. Eur J Surg Oncol. 2015;41(6):S18. [Google Scholar]
- 12.Ahmed M, Woo T, Ohashi K, et al. Magnetic sentinel lymph node biopsy in a murine tumour model. Nanomedicine. 2016;12(4):1045–1052. doi: 10.1016/j.nano.2015.12.366. [DOI] [PubMed] [Google Scholar]