Abstract
Background
Advanced practice providers (APPs) are important members of stroke teams. Stroke code simulations offer valuable experience in the evaluation and treatment of stroke patients without compromising patient care. We hypothesized that simulation training would increase APP confidence, comfort level, and preparedness in leading a stroke code similar to neurology residents.
Methods
This is a prospective quasi-experimental, pretest/posttest study. Nine APPs and 9 neurology residents participated in 3 standardized simulated cases to determine need for IV thrombolysis, thrombectomy, and blood pressure management for intracerebral hemorrhage. Emergency medicine physicians and neurologists were preceptors. APPs and residents completed a survey before and after the simulation. Generalized mixed modeling assuming a binomial distribution was used to evaluate change.
Results
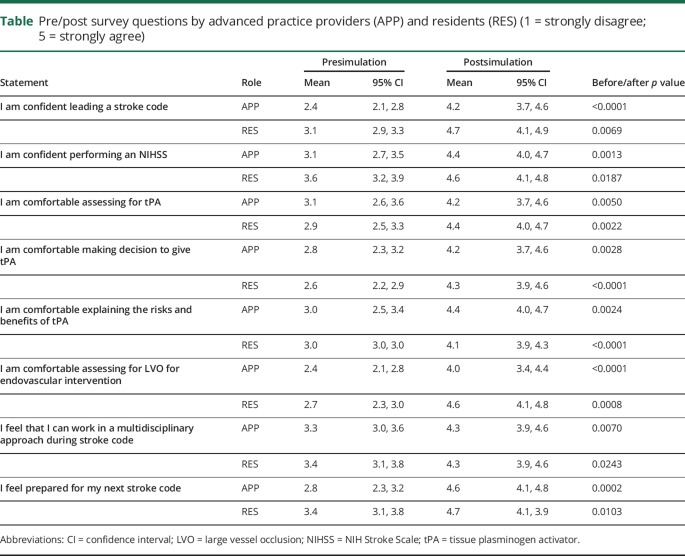
On a 5-point Likert scale (1 = strongly disagree and 5 = strongly agree), confidence in leading a stroke code increased from 2.4 to 4.2 (p < 0.05) among APPs. APPs reported improved comfort level in rapidly assessing a stroke patient for thrombolytics (3.1–4.2; p < 0.05), making the decision to give thrombolytics (2.8 vs 4.2; p < 0.05), and assessing a patient for embolectomy (2.4–4.0; p < 0.05). There was no difference in the improvement observed in all the survey questions as compared to neurology residents.
Conclusion
Simulation training is a beneficial part of medical education for APPs and should be considered in addition to traditional didactics and clinical training. Further research is needed to determine whether simulation education of APPs results in improved treatment times and outcomes of acute stroke patients.
Recent advances in acute stroke care with thrombolysis and thrombectomy have significantly reduced morbidity and mortality.1–3 However, a significant number of patients do not receive thrombolysis or thrombectomy.4,5 Lack of physician experience and confidence with tissue plasminogen activator (tPA) contributes to the underutilization of these treatment options.6 In academic centers, neurology residents are the first responders to stroke codes in the emergency department (ED). Therefore, it is important to provide adequate stroke code training to residents to improve confidence and efficiency.7 An emerging practice is incorporation of advanced practice providers (APPs) into stroke response teams, a model that has been successful in delivering thrombolytic therapy to patients with ST elevation myocardial infarction.8–11 We tested the hypothesis that stroke simulation training would improve confidence levels in both APPs and neurology residents.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review board (IRB) of Spectrum Health (Grand Rapids, MI). We obtained an IRB waiver for permission to use the participants' responses without their explicit consent.
This is a prospective quasi-experimental, pretest/posttest study without a control group. Stroke code simulations were performed at the Graduate Medical Education Simulation Center. Each room was equipped with a hospital bed and chairs for family and faculty preceptors, and had video recording capability. A presimulation survey was completed by all residents and APPs. A presimulation briefing covered the logistics of the simulation and comprehensive stroke center protocols for acute stroke management.
All neurology residents and APPs were required to complete formal NIH Stroke Scale training prior to the program. Simulation cases consisted of 3 patient scenarios: (1) a patient presenting within 2 hours of last known normal with a large vessel occlusion, (2) a patient presenting within 6 hours of last known normal and a large vessel occlusion, and (3) a patient presenting with intracerebral hemorrhage. Stroke unit and ED nurses acted as standardized patients and family members. Each case had a neurology and emergency medicine faculty member assigned to precept, and all the interactions were videotaped. All neurology residents and APPs rotated through each of the cases. Feedback was provided by the faculty preceptors at the conclusion of each case and a debrief session was conducted at the conclusion of the simulation exercise led by the director of the comprehensive stroke center.
All participants completed a postsimulation survey. The presimulation and postsimulation surveys contained questions based on a Likert scale.12 Preevaluation and postevaluation was examined by APP and neurology resident status using generalized mixed modeling with sandwich estimation assuming a binomial distribution (1–5), where observations were nested within subjects. Models were fitted using the GLIMMIX procedure with SAS software 9.4 (SAS Inc., Cary, NC). Multiple comparisons were adjusted using the Tukey-Kramer correction. Alpha was established a priori at the 0.05 level and all interval estimates were calculated for 95% confidence.
Results
Nine neurology residents (3 third year residents, 6 second year residents) and 9 APPs (6 physician assistants and 3 nurse practitioners) participated in the simulation. None of the APPs had any familiarity with managing acute stroke codes. Both APPs and neurology residents demonstrated improved confidence in managing stroke codes after the simulation training (table). The magnitude of this change was not different between APPs and residents for any question (p = 0.5126, 0.7804, 0.2666, 0.4309, 0.1991, 0.1427, 0.8250, 0.6848, respectively). No differences were observed between APPs and residents at baseline on all statements (p = 0.3250, 0.8907, 0.9999, 0.7669, 0.9611, 0.0997), except for confidence to lead a stroke code: APPs had lower confidence than residents at baseline (p = 0.0172). In addition, no differences were observed between APPs and residents at follow-up on any statement (p = 0.4136, 0.9649, 0.8358, 0.9725, 0.4590, 0.2656, 0.999, 0.9625).
Table.
Pre/post survey questions by advanced practice providers (APP) and residents (RES) (1 = strongly disagree; 5 = strongly agree)
Discussion
Medical education via simulation training continues to be an area of growth and interest. Our study demonstrates that simulation-based acute stroke training of neurology residents and APPs improves confidence in leading a stroke code to a similar degree. We also show that a simulation-based approach for acute stroke is feasible.
Acute stroke is a medical emergency with high mortality and morbidity that requires rapid evaluation and effective decision-making for optimal treatment. Previous research has demonstrated improvement in door to needle times for tPA administration following stroke simulation training for neurology residents.7 Academic medical centers are able to achieve prompt response to acute stroke by utilizing neurology residents to respond to stroke codes, rapidly evaluate patients, and facilitate decisions for neuroimaging and treatment.13 However, duty hour regulations have restricted on-site coverage by neurology residents.14
This gap has been addressed by the addition of well-trained APPs to the treatment team. Moreover, not all stroke centers have neurology residents who can respond to stroke codes. The addition of APPs to neurology teams is further supported by inadequate numbers of neurologic experts available to take care of patients with neurologic disorders.15,16 APPs are currently providing neurologic services in the inpatient as well as outpatient setting.17,18 Therefore, there is a considerable need for training them effectively to take care of neurologic patients.
We found that although APPs had lower comfort and confidence level in taking care of an acute stroke patient prior to simulation as compared to neurology residents, they achieved similar levels of confidence after the simulation. This finding highlights the need for continued education of APPs with structured didactics and simulation training not only for acute stroke but other aspects of neurologic care. Neurologic residency for APPs has already been proposed as a mechanism to accomplish this.19 Simulation may play an important role in such a curriculum.
Our study has limitations. We were unable to control for prior exposure to stroke codes for both residents and APPs on presimulation survey; nonetheless, no differences at baseline were observed between residents and APPs, except for confidence to lead a stroke code. Moreover, this study provides an assessment of perceived confidence among the participants, which does not necessarily reflect comparable degrees or competence on real-world patient care or patient outcomes. Since the confidence assessment occurred immediately following training, we also do not have data regarding the sustainability of confidence levels among residents and APPs over time. Finally, our results may not be generalizable to other centers without similar simulation facilities, though simulation may be practiced in a variety of settings.
Further research is needed to determine whether simulation education of APPs results in improved treatment times of acute stroke patients, similar to improvement seen with neurology resident simulation teaching. Further studies are needed to evaluate if simulation training can be utilized to train for stroke mimics and complex cases requiring difficult decision-making in the future.
Author contributions
M. Khan: study concept and design, participation in simulation, data acquisition, statistical analysis, interpretation of data, drafting the article. G.L. Baird: statistical analysis, interpretation of data, drafting the article. T. Price: participation in simulation, critical revision of the manuscript for important intellectual content. T. Tubergen: critical revision of the manuscript for important intellectual content. O. Kaskar, M. De Jesus, J. Zachariah, A. Oostema, R. Scurek, R.R. Coleman, and W. Sherman: participation in simulation, critical revision of the manuscript for important intellectual content. C. Hingtgen, T. Abdelhak, and B. Smith: critical revision of the manuscript for important intellectual content. B. Silver: interpretation of data, critical revision of the manuscript for important intellectual content.
Study funding
No targeted funding reported.
Disclosure
M. Khan, G.L. Baird, T. Price, T. Tubergen, O. Kaskar, M. De Jesus, and J. Zachariah report no disclosures. A. Oostema serves as a consultant to the Michigan Department of Health and Human Services for the Michigan Ongoing Stroke Registry to Accelerate Improvement of Care, the Michigan branch of the Paul Coverdell National Acute Stroke Program. R. Scurek serves as Clinical Advisor of Emergency Neurology for Spectrum Health. R.R. Coleman received travel and accommodations for a principal investigator meeting from LaRoche Pharmaceuticals. W. Sherman serves on scientific advisory boards for AbbVie and Novocure. C. Hingtgen owns stock in Merck. T. Abdelhak reports no disclosures. B. Smith serves on a scientific advisory board/as a consultant for UCB. B. Silver serves on a scientific advisory board for NIH StrokeNet study section, Women's Health Initiative Stroke Committee, and University of San Francisco California stroke adjudication committee; has received funding for travel and/or speaker honoraria from University of Southern Illinois and Spectrum Health Care; is author on a patent re: Transcranial magnetic stimulation helmet; serves as a surveyor for the Joint Commission; and has conducted medicolegal expert review. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Systematic Reviews 2014:CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 4.Messe SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology 2016;87:1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rai AT, Seldon AE, Boo S, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg 2017;9:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA). Ann Neurol 2009;66:6–10. [DOI] [PubMed] [Google Scholar]
- 7.Ruff IM, Liberman AL, Caprio FZ, et al. A resident boot camp for reducing door-to-needle times at academic medical centers. Neurol Clin Practice 2017;7:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd G, Roberts A, Bashir I, Mumby M, Kamalvand K, Cooke R. An audit of clinical nurse practitioner led thrombolysis to improve the treatment of acute myocardial infarction. J Public Health Med 2000;22:462–465. [DOI] [PubMed] [Google Scholar]
- 9.Wilmshurst P, Purchase A, Webb C, Jowett C, Quinn T. Improving door to needle times with nurse initiated thrombolysis. Heart 2000;84:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qasim A, Malpass K, O'Gorman DJ, Heber ME. Safety and efficacy of nurse initiated thrombolysis in patients with acute myocardial infarction. BMJ 2002;324:1328–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath SM, Bain RJ, Andrews A, Chida S, Kitchen SI, Walters MI. Nurse initiated thrombolysis in the accident and emergency department: safe, accurate, and faster than fast track. Emerg Med J 2003;20:418–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards AL, Kenney KC. A comparison of the Thurstone and Likert techniques of attitude scale construction. J Appl Psychol 1946;30:72–83. [DOI] [PubMed] [Google Scholar]
- 13.Fridman V, Raser J, Brizzi K, Cucchiara B. Graduating US neurology residents' experience with tissue-type plasminogen activator for acute stroke: a 10-year comparison. Stroke 2011;42:2963–2965. [DOI] [PubMed] [Google Scholar]
- 14.Bradley WG, Daube J, Mendell JR, et al. Quality improvement in neurology residency programs: report of the quality improvement committee of the Association of University Professors of Neurology. Neurology 1997;49:1205–1207. [DOI] [PubMed] [Google Scholar]
- 15.Dall TM, Storm MV, Chakrabarti R, et al. Supply and demand analysis of the current and future US neurology workforce. Neurology 2013;81:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardeman P, Hough R. Integration of advanced practice clinicians in neurology practices. JAMA Neurol 2017;74:894–895. [DOI] [PubMed] [Google Scholar]
- 17.Lee JD. Advanced practice provider utilization in the neurocritical care unit. Continuum 2015;21:1451–1454. [DOI] [PubMed] [Google Scholar]
- 18.Barton C, Merrilees J, Ketelle R, Wilkins S, Miller B. Implementation of advanced practice nurse clinic for management of behavioral symptoms in dementia: a dyadic intervention (innovative practice). Dementia 2014;13:686–696. [DOI] [PubMed] [Google Scholar]
- 19.Ermak DM, Cox L, Ahmed A. Advanced practice clinician training for neurology. Cureus 2017;9:e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]