Abstract
Background
Fingolimod is a daily oral medication used to treat relapsing multiple sclerosis (MS). Clinicians often adopt less frequent dosing for patients with profound drug-induced lymphopenia or other adverse events. Data on the effectiveness of alternate dose fingolimod are limited.
Methods
We conducted a multicenter, retrospective, observational study at 14 sites and identified 170 patients with MS taking alternate doses of fingolimod for ≥1 month. Clinical and radiologic outcomes were collected and compared during daily and alternate fingolimod dosing.
Results
Profound lymphopenia (77%), liver function abnormalities (9%), and infections (7%) were the most common reasons for patients to switch to alternate fingolimod dosing. The median follow-up was 12 months on daily dose and 14 months on alternate dose. Most patients (64%) took fingolimod every other day during alternate dosing. Disease activity was similar on alternate dose compared to daily dose: annualized relapse rate was 0.1 on daily dose vs 0.2 on alternate dose (p = 0.25); proportion of patients with contrast-enhancing MRI lesions was 7.6% on daily vs 9.4% on alternate (p = 0.55); proportion of patients with cumulative MS activity (clinical and radiologic disease) was 13.5% on daily vs 18.2% on alternate (p = 0.337). Patients who developed contrast-enhancing lesions while on daily dose were at higher risk for breakthrough disease while on alternate dose fingolimod (odds ratio 11.4, p < 0.001).
Conclusions
These data support the clinical strategy of alternate dosing of fingolimod in patients with good disease control but profound lymphopenia or other adverse events while on daily dose.
Classification of Evidence
This study provides Class IV evidence that for patients with MS on daily dose fingolimod with adverse events, alternate dose fingolimod is associated with disease activity similar to daily dose fingolimod.
Daily fingolimod is an effective therapy for relapsing multiple sclerosis (MS). It prevents egress of lymphocytes from lymph nodes and thereby reduces lymphocyte counts in peripheral circulation.1,2 Because profound lymphopenia may be a risk factor for infection, patients who developed sustained, grade 4 lymphopenia (<200 cells/mm3) were excluded from continuing in the clinical trials.
In clinical practice, patients who develop profound lymphopenia or other adverse events, such as liver enzyme elevation and opportunistic infections,3,4 during daily fingolimod treatment present a management dilemma. Rather than discontinue fingolimod, many clinicians switch such patients to nonapproved, less frequent dosing regimens, especially in patients who have achieved good disease control on the daily dose. This strategy is biologically plausible because fingolimod has a half-life of 6–9 days, suggesting that its therapeutic effect may be maintained with less-than-daily dosing.5 As current data are limited on the effectiveness of an alternate dose approach, we conducted a multicenter, retrospective, observational study of clinical and radiographic outcomes for patients treated with alternate dose fingolimod to address the question of whether fingolimod effectiveness is maintained with reduction in dosing frequency. We hypothesized that alternate dose fingolimod for patients with lymphopenia and other adverse events would be similar to standard dose fingolimod for controlling inflammatory MS activity.
Methods
Study design
This was a multicenter, retrospective observational study conducted at 12 centers in the United States, 1 in Lebanon, and 1 in Spain (see appendix e-1, links.lww.com/CPJ/A14, for a complete listing of centers). Participating sites were recruited through the Medical Partnership 4 MS (MP4MS) listserv, a group comprising over 1,300 neurologists and allied health professionals dedicated to the treatment and management of MS.
Patient population
Patients were included in the study if they were ≥18 years old, had a neurologist-confirmed diagnosis of MS, were followed at one of the participating centers, and had taken an alternate (nondaily) dose of fingolimod for ≥1 month. Participating centers were asked to submit data for all patients followed at their sites who met the inclusion criteria. Data were retrospectively collected based on chart reviews performed at each site. Included patients initiated fingolimod between January 6, 2007, and January 11, 2016. Follow-up data were collected through January 4, 2017.
Standard protocol approvals, registrations, and patient consents
This study was approved by the local institutional review boards at each participating center. Waivers of informed consent were obtained given the retrospective, minimally invasive nature of the study.
Clinical and radiologic assessment
Clinical relapses were defined as new neurologic symptoms lasting more than 24 hours that were judged by the treating neurologist to represent new MS activity. Annualized relapse rates (ARR) were calculated based on the number of relapses divided by the length of follow-up, measured in years. Disability at time of initiating daily fingolimod was estimated using the Patient-Determined Disease Steps (PDDS), which is scored as follows: 0 = normal, 1 = mild disability, mild symptoms/signs, 2 = moderate disability, visible abnormality of gait, 3 = early cane, intermittent use, 4 = late cane, cane-dependent, 5 = bilateral support, and 6 = confined to wheelchair.6,7 PDDS has been shown to correlate closely with Expanded Disability Status Scale (EDSS) scores.6 Because baseline MRIs were obtained at variable intervals from the start of fingolimod therapy, the determination of which new T2 lesions emerged while on therapy was problematic. We therefore considered only contrast-enhancing lesions (CELs) as radiologic evidence of new disease activity for the analyses reported below. In clinical practice, follow-up MRIs are almost always obtained >2 months—usually 6–12 months—after switching disease-modifying therapies (e.g., to standard or alternate dose fingolimod). Because contrast enhancement typically persists <2 months, CELs were interpreted to represent disease activity on the current dosing regimen. Overall disease activity refers to either a clinical relapse or a new CEL during treatment. As confirmatory secondary analyses, we also analyzed the data including both new T2 and CELs as evidence for radiologic activity.
Statistics
Demographic and clinical characteristics were summarized using descriptive statistics. Paired t tests were used to compare ARR during daily dose vs alternate dose fingolimod. McNemar test was used to compare MS disease activity between standard and alternate dose fingolimod. Study site, age, sex, body mass index (BMI), years of disease, PDDS, natalizumab use prior to starting fingolimod, disease activity prior to fingolimod, CELs on daily dose fingolimod, and ARR on daily dose fingolimod were evaluated in univariate logistic regression models with MS disease activity on alternate dose fingolimod as the dependent variable. We also performed multivariate logistic regression including age, sex, BMI, years of disease, and presence of CELs during daily dose fingolimod as predictors of disease activity during alternate dose fingolimod. All statistical analyses were performed using SPSS (version 24; IBM, Armonk, NY).
Classification of level of evidence
The primary research question was whether MS activity (clinical and radiologic) increased on alternate dose compared to daily dose fingolimod in patients with adverse events on daily dose. This study provides Class IV evidence that for patients with MS with adverse events on daily dose fingolimod, alternate dose fingolimod is associated with disease activity similar to daily dose fingolimod.
Results
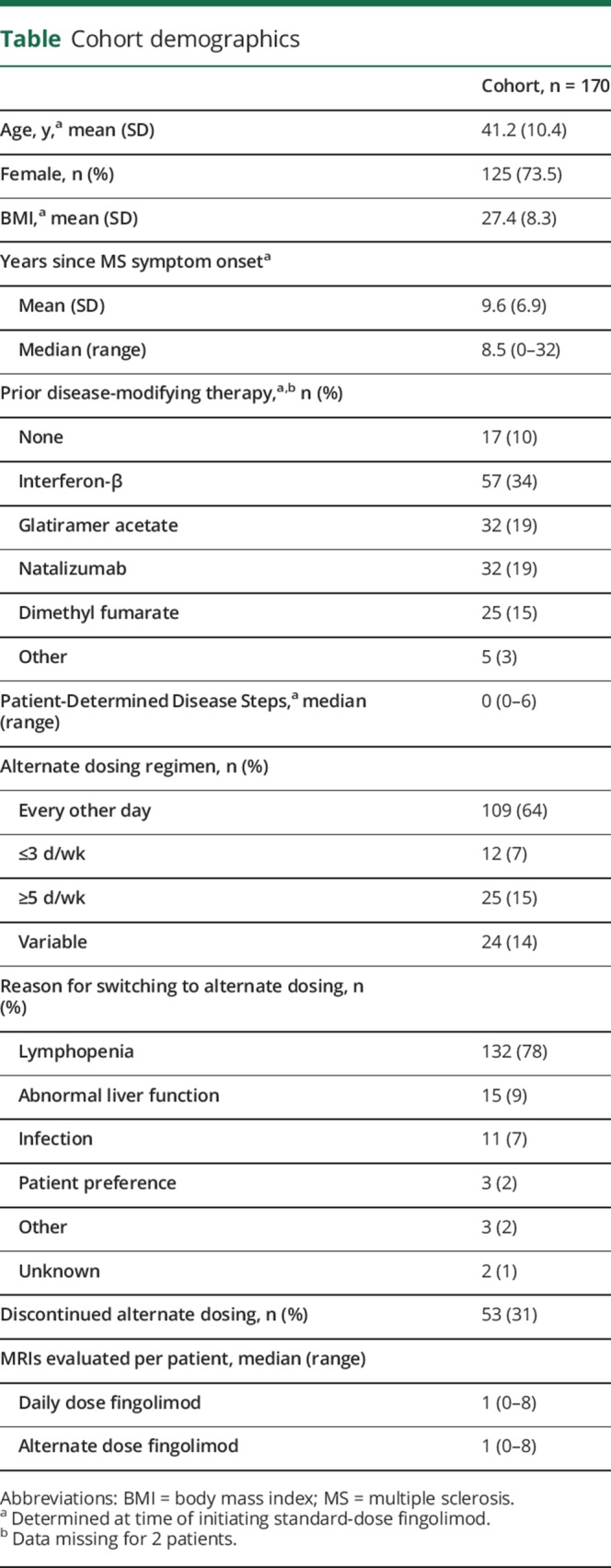
A total of 170 patients with MS fulfilled our inclusion criteria. Cohort demographics are reported in the table. By the time of fingolimod initiation, most patients had longstanding disease (average 9.6 years) and 90% had previously received other disease-modifying therapies (DMT). Patients were started on daily dose fingolimod first and were then switched to an alternate dosing regimen in response to what the treating clinician deemed an unacceptably low peripheral lymphocyte count (77% of switches), or less commonly, liver enzyme elevation (9%) or other adverse events (e.g., recurrent shingles, persistent bradycardia, headaches). Seventy-five percent of patients were on standard dose fingolimod for ≥6 months prior to alternate dosing. The most common alternate dosing schedule was every other day (64%), followed by 5 d/wk (15%), 2 d/wk (7%), and some combination of these schedules (14%). The median time on daily fingolimod was 12 months (range 1–102 months), and the median time on alternate dose was 14 months (range 1–70 months). Eighty-four percent of patients were treated with alternate dose fingolimod for ≥6 months.
Table.
Cohort demographics
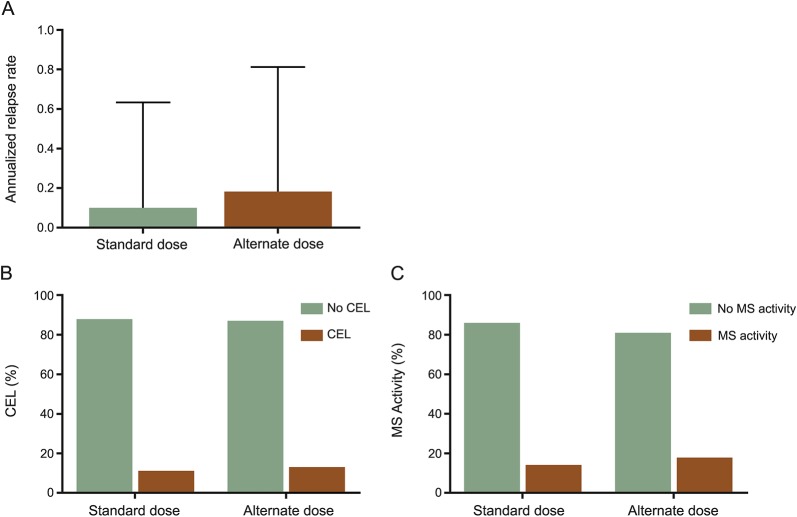
ARR were similarly low during the daily dose phase and alternate dose fingolimod phase (0.1 vs 0.2, p = 0.249) (figure, A), as were the proportion of patients with enhancing lesions (7.6% during daily dose vs 9.4% during alternate dose, p = 0.549; figure, B). Proportion of patients with either clinical or radiologic disease activity was also similar during the 2 phases of treatment—13.5% during daily dose vs 18.2% during alternate dose phase, p = 0.337 (figure, C). Of the 23 patients who had either clinical or radiologic disease activity while on daily dose fingolimod, 7 (30.4%) also had disease activity while on alternate dose fingolimod.
Figure. Multiple sclerosis (MS) disease activity on daily and alternate dose fingolimod.
(A) Annualized relapse rate was calculated as number of clinical relapses divided by time on fingolimod; n = 169. (B) The proportion of patients developing contrast-enhancing lesions (CEL) on fingolimod; n = 110 (daily dose) or 123 (alternate dose). (C) Overall MS activity was defined as having either a clinical relapse or CEL; n = 169. There were no statistically different differences between daily dose and alternate dose fingolimod.
CELs while on daily dose fingolimod were a predictor of MS disease activity on alternate dose fingolimod before (odds ratio [OR] 11.4, 95% confidence interval [CI] 3.1–41.4, p < 0.001) and after controlling for age, sex, BMI, and years of disease (OR 11.1, CI 3.0–41.2, p < 0.001). None of the other demographic or clinical variables predicted disease activity. When new T2 and CELs were both included as radiologic evidence of MS, no new predictive variables emerged and CEL during daily dose phase remained the only variable associated with MS activity (clinical or radiologic) on alternate dose fingolimod (OR 5.5, 95% CI 1.6–18.4, p = 0.006).
At the end of the follow-up period, 31% of patients (n = 53) had discontinued alternate dose fingolimod. The most common reasons for patients to discontinue alternate dose fingolimod were breakthrough MS activity (n = 24, 49% of those who stopped), adverse events (n = 14, 29%), and, less commonly, drug intolerance, disease progression in the absence of relapse, and patient preference. Only 3 patients (5%) stopped alternate dosing due to continued lymphopenia, illustrating that alternate dosing successfully raised circulating lymphocyte counts. Of the 53 patients who discontinued alternate dose fingolimod, 18 patients (34%) resumed daily dose fingolimod, 18 (34%) initiated infusible therapies (natalizumab, rituximab, or alemtuzumab), 11 (21%) switched to an alternative oral therapy (dimethyl fumarate or teriflunomide), 2 (4%) were switched to an injectable DMT, and 3 (6%) did not reinstitute DMT.
Discussion
In clinical practice, neurologists may decrease the frequency of fingolimod dosing rather than discontinue it outright for patients with MS with profound lymphopenia or adverse events while on daily dose. Our study provides empiric support for this clinical strategy. Patients who were well-controlled on daily dose fingolimod but experienced profound lymphopenia or another adverse event did not have an apparent increase in rates of relapses or new contrast-enhancing MRI lesions after switching from daily to alternate dose. In contrast, patients with active disease on MRI while on daily dose fingolimod were at 11-fold higher risk for experiencing clinical or radiographic breakthrough disease while on an alternate dose.
It is important to emphasize that our study represents real-world experience with a selected patient population and should not be extrapolated to imply that alternate dosing and daily dosing of fingolimod are equivalent. Although no significant differences were observed between standard and alternate dose fingolimod, increased ARR, more enhancing MRI lesions, and increased overall MS activity during alternate dose fingolimod were observed. It is possible that despite the relatively large size of our patient cohort (n = 170), our negative results are due to insufficient power. It will be informative to compare our results to those from the randomized, dose-blinded ASSESS clinical trial, which included full-dose (0.5 mg daily) and reduced dose (0.25 mg daily) fingolimod arms. Results from this trial are anticipated in 2018. Alternatively, the apparent equivalence in effectiveness observed in our study may be due to an above average reduction in circulating lymphocyte counts on daily dose in the majority of our alternate dose patients (77%); such patients may be able to achieve an adequate degree of lymphocyte suppression even on alternate dose. The retrospective nature of our data collection precluded systematic evaluation of lymphocyte counts and its relation to drug effectiveness. Our assessment of radiologic MS activity was also limited by nonstandard intervals between MRIs. Since the interval between MRIs often included time on both daily and alternate dose fingolimod, we were unable to interpret the significance of new T2 lesions and therefore did not include these in our primary analysis.
Our results differ from the recent Italian-Swiss study8 that compared 60 patients with MS treated with every-other-day fingolimod with 60 randomly selected daily-dose treated fingolimod patients. The Italian-Swiss study reported an increased risk of MS activity among patients on alternate dose fingolimod. The apparent discrepancy may relate to differences between methodologies and study populations. Both studies included middle-aged patients (average 41.2 years in our study and 42.3 years in the Italian-Swiss study) with longstanding disease (average 9.6 and 12.5 years), relatively low disability (median PDSS 0 and median EDSS 2.5), and similar rates of prior exposure to DMT (90% and 97%). Such demographics portend a cohort with relatively little inflammatory MS activity.9 Nevertheless, patients in the Italian-Swiss study had a relatively high ARR at the start of treatment (0.75), while most of our patients had no clinical or radiologic evidence of MS activity within the year prior to starting fingolimod (table). During fingolimod treatment, the ARR in the daily dose arm of the Italian-Swiss study was 0.04, which is substantially lower than that observed in either the pivotal trials (ARR 0.16–0.181,2) or in our daily dose patients (ARR 0.10), and may have skewed the results in favor of the daily treated group. Our patients took daily dose fingolimod for a much longer period prior to initiating alternate dosing—average of 1.5 vs 0.8 years in the Italian-Swiss cohort. Thus, our patients may have had better control of the disease on daily dose at the time when they entered the alternate dose phase. The longer duration of lead-in daily dose phase may also help explain why prior natalizumab use did not predict relapses on alternate dose in our patients in contrast to what was observed in Italian-Swiss study. In our study, only the presence of gadolinium enhancement while on daily fingolimod was predictive of disease activity on alternate dose. Finally, our study evaluated the effectiveness of daily and alternate dose among the same group of patients, while the Italian-Swiss study compared 2 distinct patient groups using a cross-sectional approach.
Our study is the largest to date to assess the effectiveness of alternate dose fingolimod dosing for MS, but it may still have insufficient power to detect small differences in effectiveness between daily and alternate dosing (type 2 error). Other limitations include heterogeneity of clinical and radiologic follow-up, lack of blood lymphocyte counts for patients, and heterogeneity of alternate dosing schemes employed in clinical practice. Notwithstanding these limitations, our multicenter observational study with relatively long follow-up provides empiric support for the clinical strategy of alternate dosing of fingolimod for patients who achieved good disease control on the daily dose, but experienced a higher than expected degree of lymphopenia or other adverse events. A pragmatic prospective clinical trial of daily vs alternate dose fingolimod is warranted to more definitively examine whether alternate dosing can achieve comparable effectiveness and reduce the adverse events (and costs) associated with this therapy.
Footnotes
Class of Evidence: Criteria for rating therapeutic and diagnostic Studies NPub.org/coe
Author contributions
E.E.L., T.E.B., and I.K. were involved with design/conceptualization, data analysis/interpretation, and drafting the manuscript for important scientific concept. D.K., S.P., M.J.B., G.v.G., S.C., A.H.C., B.J.P., M.R., S.J.K., B.Y., M.Z., S.R.-G., A.C.-R., K.E., E.L., D.V., W.M., R.B., and L.G. were involved with data collection, data interpretation, and drafting the manuscript for important scientific content.
Study funding
No targeted funding reported.
Disclosure
E.E. Longbrake serves on scientific advisory boards and/or as a consultant for Sanofi, Genzyme, Teva, Genentech, EMD Serono, and Biogen; has received speaker honoraria from Genzyme and Biogen; and receives support from NIH and National MS Society. D. Kantor serves on a scientific advisory board for Corrona MS; has served as a consultant for, on the speakers' bureaus for, and/or received speaker honoraria from Avanir, Bayer, Biogen, Mylan, Novartis, EMD Serono, AbbVie, and Sanofi Genzyme; and receives research support from Genzyme. S. Pawate serves on a scientific advisory board for and receives research support from Biogen. M. J. Bradshaw serves on the editorial board of Continuum: Lifelong Learning in Neurology. G. von Geldern has received research support from Novartis. S. Chahin serves on the speakers' bureau for Teva and receives research support from PhRMA Foundation. A.H. Cross serves on scientific advisory boards for Roche/Genentech; has received speaker honoraria from EMD Serono and AbbVie; has served as an Associate Editor for Annals Clinical Translational Neurology and serves on the editorial boards of Brain Pathology and Journal of Neuroimmunology; is an author on US patent 15060-630 “Methods for simultaneous multi-angular relaxometry of tissue using magnetic resonance imaging”; has served as a consultant for Biogen, Sanofi Aventis/Genzyme, Novartis, Teva, Bayer, Gerson Lehrman Group, Guidepoint Global, LLC, EMD Serono, AbbVie, Genentech, and At Point of Care; has received honoraria for other activities from Projects in Knowledge, Prime Education, Inc., Race To ERASE MS, the Conrad N. Hilton Foundation, and WebMD; serves on the Board of Governors for Consortium of MS Centers; and receives research support from Roche, Teva Neuroscience, Biogen, NIH/NINDS, Barnes-Jewish Hospital Foundation, and Conrad N Hilton Foundation. B.J. Parks was employed by Washington University in St. Louis at the time of this research and is currently employed by Biogen as Associate Medical Director, Drug Safety; has served as a consultant for Optum Rx; has served on scientific advisory boards for Genentech, Novartis, and EMD Serono; and holds stock/stock options in Biogen, Illumina, Bioverativ (spun off from BGN), BMS, Express Scripts, Regeneron, Ionis, Vertex, and Rexahn. Her spouse receives research funding from NIH, the Edward P. Evans Foundation, the Taub Foundation, and the V. Foundation. M. Rice receives/has received research support from Biogen Idec and Sanofi-Genzyme. S.J. Khoury serves as a Section Editor for Clinical Immunology and for Current Treatment Options in Neurology, Associate Editor for Annals of Neurology, and on the editorial board of MS Journal; and receives research support from Novartis and CNRS-Lebanon. B. Yamout has received honoraria for speaking and consulting for Biogen, Novartis, Sanofi Genzyme, Roche, Merck, and Bayer; and receives research support from Novartis, Merck, Sanofi Genzyme, Bayer, Biogen, and Roche. M. Zeineddine has received speaker honoraria from Novartis, Biologix, and Merck. S. Russell-Giller and A. Caminero report no disclosures. K. Edwards has received funding for travel from Biogen and Genzyme; is employed as Director, MS Center of Northeastern NY; has served as a consultant for Biogen, Genzyme, and EMD Serono; has served on speakers' bureaus for Biogen and Genzyme; and receives research support from Biogen, Genentech/Roche, Genzyme, Novartis, and Eli Lilly. E. Lathi serves on speakers' bureaus for Genentech, Biogen, Teva, Sanofi Genzyme, and Novartis. D. Vanderkodde has received funding for travel and speaker honoraria from Biogen-Idec; served as a consultant for Sanofi Genzyme; and serves on the speakers' bureau for Biogen-Idec. W. Meador receives research support from Genzyme and MedImmune. R. Berkovich serves on scientific advisory boards for MSAA, NMSS, Biogen, Genentech, Novartis, Sanofi Genzyme, Bayer, Mallinckrodt, and Teva; has received funding for travel and speaker honoraria from Acorda, Avanir, Bayer, Biogen, Sanofi, Genentech, Mallinckrodt, Novartis, and Teva; serves as an Associate Editor for Journal of Medical Case Reports; and serves on speakers' bureaus for Acorda, Biogen, Sanofi, Novartis, and Teva. L. Ge reports no disclosures. T.E. Bacon served on a scientific advisory board for Genentech; is author on a patent re: Harnessing altered intestinal microbial compositions for diagnosis, prevention, and treatment of multiple sclerosis; and receives research support from Actelion, Alexion, Biogen, EMD Serono, Genzyme, Novartis, Teva Pharmaceuticals, Patient-Centered Outcomes Research Institute, NIH, and Guthy-Jackson Charitable Foundation. I. Kister serves on scientific advisory boards for Biogen Idec and Genentech; has served as a consultant for Biogen Idec; and has received research support from Biogen-Idec, Serono, Novartis, Genzyme, Guthy-Jackson Charitable Foundation, and National Multiple Sclerosis Society. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Cohen JA, Barkhof F, Comi G, et al. . Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Radue EW, O'Connor P, et al. . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 3.Dubey D, Cano CA, Stuve O. Update on monitoring and adverse effects of approved second-generation disease-modifying therapies in relapsing forms of multiple sclerosis. Curr Opin Neurol 2016;29:278–285. [DOI] [PubMed] [Google Scholar]
- 4.Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord 2016;9:158–162. [DOI] [PubMed] [Google Scholar]
- 5.David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet 2012;51:15–28. [DOI] [PubMed] [Google Scholar]
- 6.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient-Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995;45:251–255. [DOI] [PubMed] [Google Scholar]
- 8.Zecca C, Merlini A, Disanto G, et al. . Half-dose fingolimod for treating relapsing-remitting multiple sclerosis: observational study. Mult Scler 2018:24:167–174. [DOI] [PubMed] [Google Scholar]
- 9.Derfuss T, Ontaneda D, Nicholas J, Meng X, Hawker K. Relapse rates in patients with multiple sclerosis treated with fingolimod: subgroup analyses of pooled data from three phase 3 trials. Mult Scler Relat Disord 2016;8:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]