Abstract
Introduction
Substantial resources are required to provide lifelong postoperative care to people with cochlear implants. Most patients visit the clinic annually. We introduced a person-centred remote follow-up pathway, giving patients telemedicine tools to use at home so they would only visit the centre when intervention was required.
Objectives
To assess the feasibility of comparing a remote care pathway with the standard pathway in adults using cochlear implants.
Design
Two-arm randomised controlled trial. Randomisation used a minimisation approach, controlling for potential confounding factors. Participant blinding was not possible, but baseline measures occurred before allocation.
Setting
University of Southampton Auditory Implant Service: provider of National Health Service care.
Participants
60 adults who had used cochlear implants for at least 6 months.
Interventions
Control group (n=30) followed usual care pathway.
Remote care group (n=30) received care remotely for 6 months incorporating: home hearing in noise test, online support tool and self-adjustment of device (only 10 had compatible equipment).
Main outcome measures
Primary: change in patient activation; measured using the Patient Activation Measure.
Secondary: change in hearing and quality of life; qualitative feedback from patients and clinicians.
Results
One participant in the remote care group dropped out. The remote care group showed a greater increase in patient activation than the control group. Changes in hearing differed between the groups. The remote care group improved on the Triple Digit Test hearing test; the control group perceived their hearing was worse on the Speech, Spatial and Qualities of Hearing Scale questionnaire. Quality of life remained unchanged in both groups. Patients and clinicians were generally positive about remote care tools and wanted to continue.
Conclusions
Adults with cochlear implants were willing to be randomised and complied with the protocol. Personalised remote care for long-term follow-up is feasible and acceptable, leading to more empowered patients.
Trial registration number
Keywords: cochlear implants, cochlear implantation, telemedicine, patient-centred care, hearing
Strengths and limitations of this study.
This is the first randomised controlled trial of a triple approach to remote care for adults using cochlear implants.
Six months of follow-up may be insufficient to highlight benefits and limitations of remote care.
People who volunteered to take part in a trial of remote care may not be representative of the broader population of people with cochlear implants.
The Patient Activation Measure may not be very sensitive to changes in empowerment in people using cochlear implants due to its medical perspective.
Introduction
Cochlear implants provide hearing to people mainly with severe to profound deafness. Around 14 000 people in the UK use cochlear implants, increasing by approximately 1200 new patients each year.1 There are 18 cochlear implant centres in the UK, providing surgery, an acute phase of adjustment and rehabilitation, and then clinic-led long-term follow-up services. Accessing services can involve considerable time commitment, travel expense, missed work and family disruption. Most clinics provide regular annual follow-up, whether or not further intervention is needed. This may result in resources being used to provide appointments that provide little if any benefit to the patient. However, routine appointments do provide opportunities for the clinician to detect deterioration in a patient’s hearing or speech recognition. Deterioration usually occurs gradually, and without tools to test at home, the patient may not notice a change and may spend several months listening with poorer hearing. The following tasks are usually completed in routine follow-up appointments: hearing and speech recognition testing, device adjustment, rehabilitation, equipment check and troubleshooting, and provision of replacement or upgraded equipment. We designed, implemented and evaluated remote care tools that the patient could use at home to manage these tasks. If effective, these tools could mean that people using cochlear implants could only attend the clinic when intervention is required, eliminating the need for routine clinic-led appointments.
Potential benefits for the patient from the use of these remote care tools are:
increased confidence to manage one’s own hearing;
more stable hearing as problems could be identified and resolved quicker;
more appropriate adjustments of the cochlear implant processor in real-life situations as they provide an ability to fine-tune when away from clinic;
convenience of not travelling to routine appointments;
reduction of travel cost and time, time off work and disruption to family life;
greater equality in service delivery as routine support can be accessed regardless of distance from clinic.
Patients who are empowered and involved in their care tend to have better outcomes,2 3 and those using self-management tools show a significant improvement in outcomes.4 Clinics may be able to improve use of resources through reducing the need for follow-up appointments.
Methods
Trial design and setting
This was a 6-month two-arm randomised controlled trial (RCT) involving 60 adults with at least 6 months of cochlear implant experience; the protocol was previously published.5 The trial was conducted at the University of Southampton Auditory Implant Service (USAIS), a tertiary treatment centre mostly funded by National Health Service referrals. We followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs.
Recruitment
The project was advertised on the USAIS website (www.ais.southampton.ac.uk), a link was tweeted from @UoS_AIS and @CIRemoteCare. The study was also publicised by the National Cochlear Implant Users Association. The primary investigator (PI) accessed the USAIS clinical database and contacted all patients who fulfilled the inclusion criteria, except those who had indicated that they did not want to receive research invitations. Sample sizes between 30 and 50 are suggested for a feasibility trial.6 7 We chose 60 to allow a range of different patients and to account for attrition.
Inclusion criteria
person using one or two cochlear implants (any make(s)/model(s)) for at least 6 months;
living in the UK;
aged 18 years or more;
able to give informed consent;
sufficient English to understand study documentation and participate in testing;
access to a computer or device with internet access.
Exclusion criteria
Those who did not fulfil the inclusion criteria plus any medical condition or known disability that would limit their capacity to use the remote care tools.
Staff change management assessment
Moving to remote care represents a significant change to staff at cochlear implant centres. The protocol indicated that formal staff interviews would be conducted at 3-month intervals over the 6-month follow-up period; that is, at baseline, 3 months and 6 months. However, early interviews suggested that most staff did not feel affected by the introduction of remote care as only 30 out of the total case load of well over 1000 patients were on a remote pathway. A formal staff change management assessment was therefore not conducted and a smaller number of interviews with audiologists, rehabilitationists and clinical support staff were carried out to capture qualitative staff feedback.
Interventions
Control group: standard clinical care pathway
Participants in the control group continued with their usual clinic-based care pathway, without access to the remote care tools. This involved either clinic-led or patient-instigated appointments at the centre for device programming, monitoring outcomes, rehabilitation support or equipment maintenance.8 Appointments are frequent in the first year and less often subsequently. They attended two trial-related visits: one at baseline and another at exit.
Intervention group: remote care
Those randomised into the treatment group (remote care group) received cochlear implant care remotely for 6 months. Participants were told that clinic appointments would still be arranged if requested by the patient or if a clinician deemed it necessary. They were also informed that they needed to adhere to any medical check-ups with the cochlear implant surgeon. However, no routine follow-up appointments were scheduled for the trial duration. Participants were able to access the remote care tools as often as they wished, but had protocol requirements in order to assess compliance. Remote care comprised:
Remote and self-monitoring
Participants in the remote care arm accessed a password-protected online hearing in noise test based on the Triple Digit Test (TDT); each participant had an individual log-in. The customised site was provided by the charity Action on Hearing Loss. Each time a participant completed the test, the results were emailed to the research team and included a code to identify the participant. Participants listened to sets of three digits in background noise and typed in the numbers they heard. If they entered all three digits correctly, the noise level increased. An incorrect response caused a decrease in noise. Full methodological details of the test have been published elsewhere.9 Participants were advised that although a direct connection between the sound processor and the computer eliminates the effects of background noise, testing with the computer speakers allows assessment of the whole hearing pathway including the microphone—a part that commonly deteriorates due to dust, humidity or damage. The protocol required participants to complete the hearing test a minimum of two times: month 1 and month 6 of the trial. However, participants could complete the test at any other time during the 6 months. Participants were encouraged to experiment with different processor settings and programs and redo the test whenever they wanted. They were advised to keep the test parameters the same, as change in result (rather than the absolute value) was the variable of interest. Those participants using a Cochlear CP810 or CP910 sound processor (n=12) were also loaned an iPad with a calibrated TDT installed.
Self-adjustment of device (Remote Assistant Fitting)
Only those people using newer cochlear implant devices manufactured by Cochlear (CI500 series, CI422 or CI24RE implants using CP800 or CP900 series processors) were able to participate in the trial of device self-adjustment. The other manufacturers of cochlear implant systems did not have equivalent tools available at the time of the trial. Remote Assistant Fitting allows adjustment of bass, treble and master volume in addition to the usual sensitivity and volume settings.10 All participants with compatible processors were given brief group instruction on the use of Remote Assistant Fitting at the baseline visit. A reset facility allowed participants to return their device to the original settings established at the clinic. The protocol required participants to perform a self-adjustment at least twice (in months 1 and 6), but additional adjustments could be done at any time. It was not possible to receive logs of the changes made, so this was measured by self-report using a written question to ascertain usage at study exit.
Online support tool
The research team designed a new online support tool for adults with cochlear implants using LifeGuide.11 LifeGuide is an open-source software platform that allows the development and trialling of interactive, tailored web-based interventions. This was a codesign process incorporating feedback from service users at all stages, including focus groups. The online support tool (Cochlear Implant Remote Care, CIRCA) contained personalised equipment help and information, troubleshooting, rehabilitation, goal-setting, help with music and telephone use and a method of ordering replacement equipment. The site was designed to be accessible to people who may be inexperienced internet users; training occurred at the baseline visit. It also stored the TDT hearing in noise test result entered by the participant and provided appropriate feedback (‘no significant change’ or ‘significantly worse: contact the centre’). Participants were given a unique user name to log in to this tool and they could access it at any time; the protocol only required site registration so they could choose how often they used it. They had the option to include a mobile phone number to receive reminder text messages for speech processor maintenance and study information. The site also collected usage and flow data from participants’ interactions with the system, and welcomed comments about the site. These data are informing design of the next version.
In the UK, speech processors are generally upgraded every 5 years.8 Those patients in the trial who were eligible for a processor upgrade received the new equipment at home rather than coming into the clinic. Clinicians set up the required maps; these were downloaded onto the processors by the cochlear implant company who sent the equipment directly to the patient.
Outcome measures
The following outcomes were measured at baseline and at study exit (ie, at 6 months):
patient activation measured using Patient Activation Measure (PAM);
speech recognition assessed using Bamford-Kowal-Bench (BKB) sentences in quiet and noise, and also assessed using the TDT;
listening ability measured using the Speech, Spatial and Qualities of Hearing Scale (SSQ) questionnaire;
health-related quality of life measured using the Health Utilities Index (HUI) Mark 3.
The PAM is a well-validated generic measure of patient activation that evaluates the knowledge, skills, beliefs and behaviours that patients have for self-management of their long-term condition.12 13 It is a one-page questionnaire comprising 13 statements about health. The subjects are asked to indicate how much they agree or disagree with each statement on a 4-point Likert-type scale.12
BKB sentences14 are the standard clinical speech recognition test used in the UK. Testing was conducted in quiet and in background noise. Full details of the test method are published elsewhere.15 For the test in quiet, the outcome measure was the percentage of keywords identified correctly. If a participant repeated 70% or more keywords correctly, an adaptive presentation of noise was also included. The dependent variable was the speech reception threshold (SRT) in dB. A lower score indicates that the listener can cope with a more challenging signal-to-noise ratio.
The TDT was conducted in clinic at baseline and at exit using the same Action on Hearing Loss online test that participants in the remote care arm used at home. The dependent variable was the SRT in dB. Testing was done using an Anker mini speaker (A7910) to present the stimuli at a comfortable level; those using two (bilateral) implants were tested with both together. These two speech recognition tests were chosen because BKB sentences are used clinically in the UK, and the TDT was the test used at home in the project.
The SSQ is a 49-item questionnaire measuring self-reported hearing disability over three domains: difficulties understanding speech in different situations, localising and tracking sounds, and ease of listening and naturalness of sound.16 The HUI Mark 3 (HUI3) is a multiattribute health status classification system evaluating eight domains of vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain.17 Participants’ responses were used to derive ‘utility values’ for their health states based on the preferences of a sample of the Canadian public.18
Demographic data were collected including highest formal educational qualification based on categories used in the Office for National Statistics 2011 census. Initially, a log of clinic contacts was kept in order to evaluate the workload in the two groups. However, it became difficult to separate study-related contact from clinical care contact.
Participants in the remote care group attended a focus group at study exit to explore and clarify views on remote care using structured questions.
Primary outcome measure
Change (from day of study entry to 6 months follow-up) in patient activation measured using the PAM.
Secondary outcome measures
stability of hearing measured by change (from day of study entry to 6 months of follow-up) in speech recognition measured in clinic using BKB sentences, the TDT and the SSQ;
stability of quality of life measured by change (from day of study entry to 6 months of follow-up) in quality of life measured using the HUI3;
patient preference for and experience of remote care in treatment arm reported qualitatively from feedback in online support tool and focus groups at study exit;
clinician preference for and experience of remote care measured qualitatively from interviews with staff.
Feasibility outcomes
recruitment (number of eligible and willing participants)
attrition (dropout) and bias
adherence to protocol
acceptability of randomisation to service users
willingness to use and ability to access remote care tools (compliance with minimum use).
Hypotheses
Primary
The remote care group will show a greater increase in patient activation over the 6-month remote care trial period than the control group, measured using the PAM.
Secondary
There will be no more deterioration in hearing in the remote care group compared with the control group, measured using speech recognition (BKB, TDT) and the SSQ questionnaire.
There will be no more deterioration in quality of life in the remote care group compared with the control group, measured using the HUI3.
Service users (patients) will feel positive about remote care, measured qualitatively from feedback in online support tool and in focus groups.
Clinicians will feel positive about remote care, measured qualitatively from interviews with clinical staff.
Randomisation
Participants were allocated to groups at the baseline visit after consenting to participate and after baseline measures had been obtained. Allocation to the remote care pathway or to the standard care pathway was done by the PI (HC) using minimisation software.19 Minimisation seeks to achieve a balance across the arms of a trial on one or more predefined patient characteristics.20 21 The minimisation balanced the following factors:
cochlear implant user less than a year or more than a year
gender
distance from the clinic (local or non-local, defined as within 20 miles or more than 20 miles away, respectively)
device (Cochlear or not)
use of equipment compatible with Cochlear Remote Assistant Fitting (or not).
Gender was included because men are more likely to use the internet than women, especially in the older age group.22 Biased coin minimisation was used with a base probability of 0.7. Group imbalance was quantified using the marginal balance method.23
Blinding
Due to the nature of the intervention, participant blinding was not possible. Baseline measures were therefore obtained before allocation. When exit measures were obtained, clinicians did not know which group the patient had been in, although it is possible that the patient could have volunteered this information.
Statistical analysis
IBM SPSS Statistics V.22 was used. One-tailed p values were used in each case that the hypothesis was directional. We tested data for normality using the Shapiro-Wilk test and used non-parametric statistics when assumptions were violated.
Data handling
Data were managed according to the University of Southampton Research Data Management Policy.
Results
Demographics
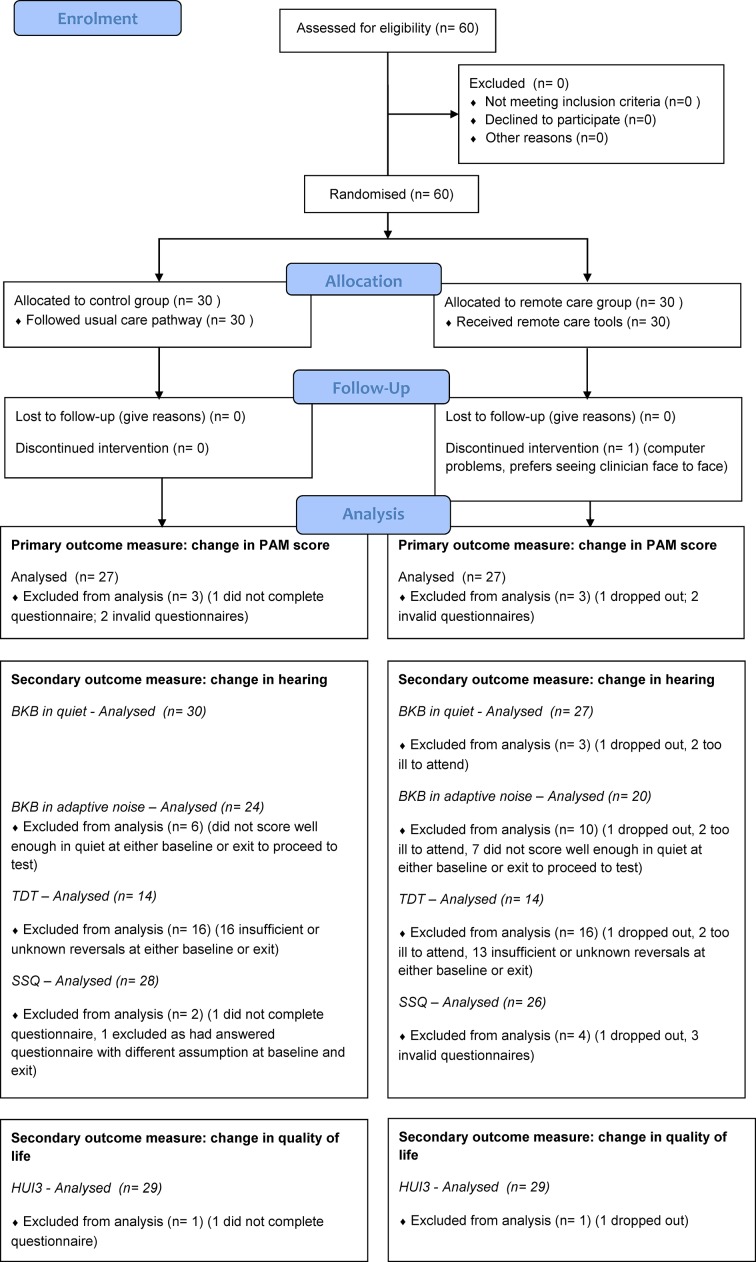
Figure 1 shows the CONSORT flow chart for the trial. Table 1 shows the demographic characteristics of the participants in each group.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow chart of project participants. BKB, Bamford-Kowal-Bench; HUI3, HUI Mark 3; PAM, Patient Activation Measure; SSQ, Speech, Spatial and Qualities of Hearing Scale; TDT, Triple Digit Test.
Table 1.
Demographics of 60 trial participants
| Measure | Control group (n=30) | Remote care group (n=30) |
| Age (years) | ||
| Mean | 63 | 64 |
| SD | 12 | 14 |
| Median | 68 | 69 |
| Range | 35–80 | 20–83 |
| Gender | ||
| Female | 17 | 19 |
| Male | 13 | 11 |
| Distance from home to clinic (miles) | ||
| Mean | 39 | 46 |
| SD | 25 | 36 |
| Median | 34 | 39 |
| Range | 9–106 | 5–156 |
| Cochlear implant manufacturer | ||
| Advanced Bionics (AB) | 9 | 4 |
| Cochlear | 14 | 12 |
| Cochlear in one ear, AB in the other ear | 1 | 0 |
| MED-EL | 4 | 13 |
| Neurelec/Oticon Medical | 2 | 1 |
| Speech processor | ||
| AB Harmony | 1 | 1 |
| AB Naida | 9 | 4 |
| Cochlear CP810 | 3 | 9 |
| Cochlear CP910 | 10 | 4 |
| Cochlear Freedom | 2 | 1 |
| MED-EL Opus 2 | 4 | 9 |
| MED-EL Rondo | 1 | 2 |
| MED-EL Sonnet | 0 | 2 |
| Neurelec/Oticon Medical Saphyr | 2 | 1 |
| Unilateral or bilateral implant | ||
| Unilateral | 28 | 27 |
| Bilateral | 2 | 3 |
| Qualification | ||
| No qualifications | 1 | 3 |
| Level 1 (1–4 GCSEs, Scottish Standard Grade or equivalent qualifications) | 3 | 1 |
| Level 2 (5 or more GCSEs, Scottish Higher, Scottish Advanced Higher or equivalent qualifications) | 4 | 3 |
| Apprenticeship | 0 | 2 |
| Level 3 (2 or more A-levels, HNC, HND, SVQ level 4 or equivalent qualifications) | 6 | 2 |
| Level 4+ (first or higher degree, professional qualifications or other equivalent higher education qualifications) | 15 | 18 |
| Other qualifications | 1 | 0 |
| Cochlear implant clinic | ||
| Birmingham | 1 | 0 |
| Cambridge | 1 | 1 |
| Oxford | 3 | 0 |
| Royal National Throat Nose and Ear, London | 1 | 2 |
| Southampton | 24 | 27 |
GCSE, General Certificate of Secondary Education; HNC, Higher National Certificate; HND, Higher National Diploma; SVQ, Scottish Vocational Qualification.
Evaluation outcomes
Baselines measures occurred from 9 to 29 January 2016; exit measures occurred from 11 July to 10 August 2016. One subject (in the remote care group) withdrew from the project because she was having problems with the computer and decided she preferred face-to-face interactions with a clinician and therefore no exit measures were obtained. Two further subjects in the remote care group were too ill to attend the exit appointment so did not complete the clinic-based hearing tests, but completed exit questionnaires at home.
Primary outcome measure: change in patient activation
Figure 1 shows the number available for analysis in each group. Ten out of 13 questions need to be answered in order to obtain a valid overall score; answering ‘Not applicable’ to more than three questions would make the questionnaire invalid. The PAM was scored using a spreadsheet supplied by Insignia which sums and normalises the items to a 100-point scale, with a higher score reflecting a greater level of activation.
PAM scores were normally distributed in both groups at baseline and exit. The baseline PAM score was not related to age (Pearson correlation r=−0.068, p=0.615, n=57) but those participants with a higher level of qualification tended to have a higher baseline PAM score (Jonckheere-Terpstra test=3.126, p=0.002, n=57).
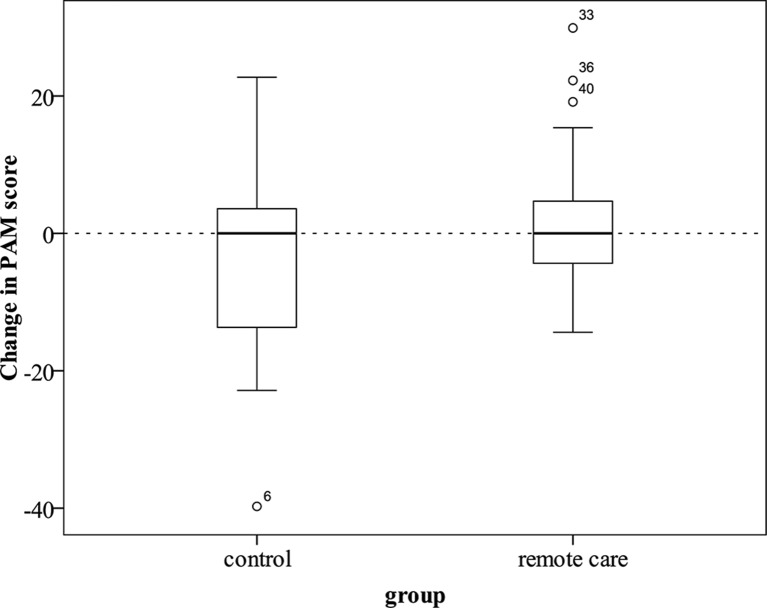
As hypothesised, patient activation increased more in the remote care arm (mean PAM change score=2.38, SD=10.16) compared with the usual care arm (mean PAM change score=−3.44, SD=14.59) (one-way analysis of variance (ANOVA) F(1,52)=2.89, one-tailed p=0.048) (figure 2). The effect size was medium (Cohen’s d=0.5). The primary hypothesis was retained: the remote care group showed a greater increase in patient activation than the control group.
Figure 2.
Change in Patient Activation Measure (PAM) score from baseline to study exit in control (n=27) and remote care (n=27) groups. Outliers (more than 1.5 box lengths above or below the box) are shown as circles. The numbers by the markers represent individual case identifiers.
Secondary outcome measures
Stability of hearing
BKB sentences
The CONSORT flow chart shows number of participants. The dependent variable was change in score. For BKB sentence testing in quiet and adaptive noise, the remote care group did not deteriorate more than the control group (quiet: Mann-Whitney U test, one-tailed p=0.475; adaptive noise: Mann-Whitney U test, one-tailed p=0.266).
Triple Digit Test
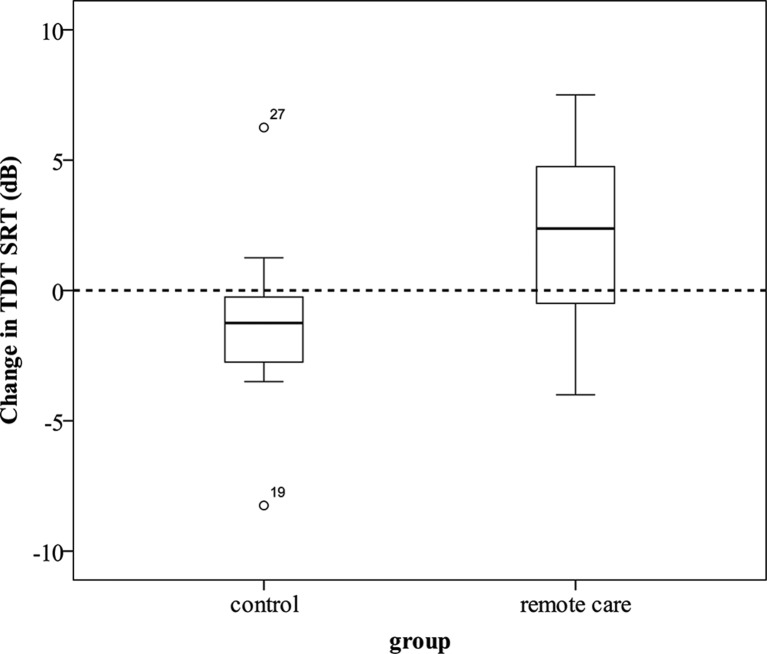
Figure 1 shows the number of participants included. In its current iteration, the test was too difficult for some people—they were only able to identify the correct set of three digits a few times or not at all, even at the most favourable signal-to-noise ratio. In line with the procedure recommended by Wetherill and Levitt,24 those stimulus runs exhibiting less than six reversals on the adaptive staircase were excluded from analysis. The number of reversals for each test was calculated by analysing the follow-up email sent automatically to the researcher after each test. This email was not sent in 13 tests at baseline and four at exit, although the final SRT was noted on paper by the clinician at the time. It is unclear whether this was a website error or clinician user error. In those cases where the number of reversals was not known, the SRT result was not included. The change in TDT SRT was calculated by subtracting the exit SRT from the baseline SRT. This produced a positive result if the participant scored better at the exit of the study than at the baseline because a lower score is better on the SRT measure.
Only 14 participants in each group were known to have obtained six or more reversals at both the baseline and exit measure. Contrary to our expectations, the control group deteriorated significantly more than the remote care group (one-way ANOVA F(1,26)=8.641, one-tailed p=0.004). While the remote care group showed an improvement in the TDT after the project (mean change=2.32 dB, SD=3.38 dB), the control group showed a slight deterioration (mean change=−1.29 dB, SD=3.12 dB). The effect size was large (Cohen’s d=1). Figure 3 shows the change in TDT SRT in the control and remote care groups. The secondary hypothesis related to hearing deterioration as measured with the TDT is therefore rejected.
Figure 3.
Change in Triple Digit Test (TDT) speech reception threshold (SRT; dB) from baseline to exit for control (n=14) and remote care (n=14) groups.
SSQ questionnaire
The SSQ was analysed by obtaining an overall average score. Thirty-nine out of 49 questions were required to be answered in order to obtain a score; resulting in the exclusion of one baseline result and two exit results. One participant was excluded from the comparison because they had made an assumption that they should answer the questionnaires thinking about their ability to hear without lip-reading at the exit point but not at the baseline.
Change in hearing was measured by subtracting the baseline overall SSQ score from the overall exit score. The mean change in SSQ was −0.35 in the control group and 0.17 in the remote care group. There was more deterioration in perceived hearing in the control group than in the remote care group (one-way ANOVA F(1,52)=6.391, one-tailed p=0.008).
Overall the secondary hypothesis 1 that there will be no more deterioration in hearing in the remote care group compared with the control group was retained. The control group deteriorated more than the remote care group in the TDT hearing test and in the SSQ questionnaire.
Stability of quality of life
Change in the HUI3 utility value was calculated by subtracting the baseline measure from the exit measure. There was no significant difference in the quality of life change between the two groups (one-way ANOVA F(1,56)=0.304, one-tailed p=0.292). Secondary hypothesis 2 was therefore retained as there was no more deterioration in quality of life in the remote care group compared with the control group, measured using the HUI3.
Patient preference for and experience of remote care
This was reported qualitatively from feedback by users of remote care in focus groups. The home hearing test was reported to be the best feature. Although there were only 30 people using the remote care tools, in a 6-month period results from 554 home hearing tests were received. It is likely that many more tests were done as participants told us that they did a lot more tests than they submitted through the online support tool. The number of home hearing tests logged per participant ranged from 0 to 121, with a median of 9 (mean=18, SD=27).
Clinician preference for and experience of remote care
Generally, staff felt positive about remote care and patients being given choice, and felt that more tools for patients to use at home represented an improvement over the current standard care pathway. The main concern raised was that the remote care pathway would not be suitable for all patients, and that a more extensive roll-out should be on an opt-in basis. Some staff felt that a move to remote care would have implications for their clinical role, in that they would see a higher proportion of more complex patients in clinic, which would have a greater emotional load for them and may provide a skewed view of how well people can hear with a cochlear implant.
Feasibility outcomes
Recruitment (number of eligible and willing participants)
No difficulties were experienced in recruiting 60 participants. Many more people with implants had contacted the PI to say they were interested in taking part than the required sample size.
Adherence to protocol
Participants in the remote care group were asked to access the three remote care tools a minimum number of times as follows, but were given no other guidance or requirements.
Remote and self-monitoring: minimum of two home hearing tests—one in the first and one in the last month. Twenty-eight out of 30 people (93%) complied. One person did only one home hearing test; one person withdrew from the study before they had done a home hearing test. Twenty-six out of 30 (87%) did a home hearing test in the first month; 24 out of 30 (80%) did a test in the last month.
Self-adjustment of device: do self-adjustment at least twice. From self-report, seven out of nine people (78%) used self-adjustment at some point. Although one of these did not show evidence of use on their sound processor, so may have been mistaken. One person did not answer the question. Compliance may have been six out of nine (67%).
Online support tool: register with the site and use it as they wished. One hundred per cent compliance.
Acceptability of randomisation to service users
All participants agreed to proceed once the randomisation was explained to them. Some people were disappointed to be in the control group. An information sheet was provided to them explaining why the control group was important.
Willingness and ability to use remote care tools
All 30 remote care participants signed up for the online support tool at the start of the project, although some reported initial log-in problems. The number of separate log-ins per participant ranged from 1 to 98 with a median of 12 (mean=18, SD=22). The total number of log-ins during the 6-month trial period was 537.
All participants in the remote care arm were asked to answer some questions about the online support tool. Responses were received from 27 out of 30 participants. One person dropped out, and two people fell ill during the trial. Sixty-seven per cent of people found the CIRCA website useful, and 64% of people would recommend it to other people with cochlear implants. We also received a lot of constructive feedback about what people would want in the next version.
Ten people were shown how to change their speech processor programs using Remote Assistant Fitting. Remote Assistant Fitting can only be used with one specific device; all eligible participants in the remote care arm were provided with the means to use it. Nine of the 10 eligible participants answered a question at study exit about how much they had used Remote Assistant Fitting. As expected for such a new tool, feedback was variable. Almost half of the respondents (44%) (n=4) used Remote Assistant Fitting ‘all the time’ or ‘often’; 22% (n=2) ‘never used it’. One-third (n=3) used it ‘once or twice’. One person reported that they had used Remote Assistant Fitting ‘all the time’, however it seemed from the speech processor settings that it had not been used so possibly this participant misunderstood what they were being asked.
Five participants in the remote care arm were due to receive an upgraded speech processor during the study period. These were all users of Cochlear devices. One participant had a clinic visit scheduled as she was experiencing some hearing problems, so received the upgraded equipment on that day. The other four participants received their new speech processors at home preprogrammed with their settings. They were sent an introductory email before receiving the processor, with links to videos to get used to their equipment, unpacking instructions and details of which programs were in the speech processor. Two out of four were happy with their upgrades and did not attend clinic further. One participant wanted some changes made to the programs, which was done by post without seeing the patient. One participant attended clinic after the clinical trial as he felt he was not hearing so well with the new processor.
Adverse events
Six adverse events were logged during the clinical trial. Four were unrelated to the treatment (two adverse events related to breach of confidentiality, two hospitalisations). Two adverse reactions were related to the treatment. One participant suffered a headache and nausea after the long day of baseline measures. During exit measures, one participant and their spouse became upset. Both adverse reactions were considered mild and were resolved. Appropriate action was taken in all cases in terms of reporting to the sponsor and Research Ethics Committee.
Discussion
This is the first RCT of a triple approach to remote care for adults using cochlear implants. The remote care group showed a greater increase in patient activation than the control group after the 6-month clinical trial. The remote care group improved on the TDT hearing test; the control group perceived their hearing was worse on the SSQ questionnaire. Quality of life was unchanged in both groups. Patients and clinicians were generally positive about remote care tools and wanted to continue. Therefore, the provision of remote care is feasible and acceptable in adults using cochlear implants, and an RCT is possible.
We used the data on the PAM change score to derive an estimate of effect size. The observed effect size (difference in size of change in PAM between the two groups) was 0.463 SD. To detect an effect of that size with power of 80%, alpha of 0.05 and 1:1 allocation treatment to control would require 59 patients in each group. We would recommend a subsequent fully powered RCT has this number of participants.
Limitations
Participants who volunteered to take part in a trial of remote care are unlikely to be representative of the UK population of people using cochlear implants—in terms of activation, interest in telemedicine and access to and familiarity with technology. Entrants into the study were self-selecting. In addition, this study’s participants generally had a higher level of educational qualification than the UK population.
Although not statistically significant, there appeared to be a trend towards the control group being less empowered at the end of the follow-up period that the current sample size may have been too small to detect. If present, such a change may have related to some participants feeling disgruntled or disempowered because they were allocated to the control group. While patients were blinded to their allocation at baseline, they knew whether they were in the control or remote care group once allocated. This may have introduced bias.
Out of 118 completed PAM questionnaires (60 at baseline and 56 at exit), four were not valid due to ‘N/A’ being answered for more than three questions. On examining these responses, the question that was answered ‘N/A’ the most (27 out of 118 times) was question 4, ‘I know what each of my prescribed medications do.’ The questions with the second and third most ‘N/A’ responses were question 9, ‘I know what treatments are available for my health problems’ (n=17) and question 8, ‘I understand my health problems and what causes them’ (n=12). Aside from these three questions, the response ‘N/A’ was provided just zero to three times per question. It is apparent that people using cochlear implants are different from patients receiving a medical treatment for a health condition. They are simply using a technological solution to deafness, and may have no health problems and take no medication, and do not view their hearing loss as related to ill health. The PAM questionnaire and other similar scales, for example, the revised Partners in Health Scale,25 may therefore provide an overmedicalised model of activation in people using cochlear implants and may not be the most valid measure of empowerment. A measure of beliefs, skills and knowledge specific to users of a hearing device may be even more sensitive to changes in activation related to the use of remote care tools and is under development by the authors.26
The remote care group improved slightly on the TDT although their BKB sentence test scores were unchanged. During the trial, remote care participants used the TDT at home so their improvement may have been a result of familiarity and/or a training effect. An alternative explanation could be that the TDT may be more sensitive to subtle hearing change than the traditional BKB clinic test. The control group on average reported their hearing to be worse, although this was not backed up by objective data. This may also be related to the fact that the control group were not blinded to their allocation at exit, and may have felt that they were ‘missing out’ by not having access to the remote care tools. The TDT is not suitable for wider roll-out in its current form as it was too difficult for some people to complete and there were problems with receipt of results.
Conclusions
Adults with cochlear implants were willing to be randomised and comply with the study protocol. Personalised remote care for long-term follow-up appears to be feasible and acceptable to both patients and clinicians, leading to more empowered patients. We are not recommending that all adults with cochlear implants should follow a telemedicine pathway; instead we suggest personalised stratification of care. This should involve a careful process of shared decision-making with the decision about which pathway to follow being jointly made between the patient, their families and the clinician.27
Supplementary Material
Acknowledgments
The authors thank the people with cochlear implants who gave so freely of their time and experience. Marta Glowacka, Anna Weston and Jin Zhang provided expertise in LifeGuide. Thanks to Professor Nicholas Clarke for conducting the staff interviews. Thanks to Dean Parker from Action on Hearing Loss for providing the Triple Digit Test interface. Thanks to Mike Firn from Springfield Consultancy for his support. Many thanks to the funder (The Health Foundation) and sponsor (University of Southampton).
Footnotes
Contributors: HC, PK, MW and MMG made substantial contributions to the conception and design of the work, revised it critically for intellectual content and approved the final manuscript. HC led the work and takes overall responsibility for the manuscript. PK was involved in all aspects. MW worked especially on the LifeGuide online support tool parts. MMG contributed to analysis and interpretation. The authors agree to be accountable for their work.
Funding: This work was supported by The Health Foundation Innovating for Improvement Award (grant number 1959).
Competing interests: The primary investigator, HC, performed occasional private consultancy work for the cochlear implant company Cochlear Europe. HC reports grants from The Health Foundation during the conduct of the study; other from Cochlear Europe, other from Advanced Bionics, grant from British Society of Audiology, grants from Healthcare Quality Improvement Partnership, other from MED-EL, grants from Oticon Medical, personal fees from Maney publishers, grant from Ida Institute, outside the submitted work. PK reports grants from The Health Foundation and British Society of Audiology during the conduct of this study related to the submitted work; also grant from Cochlear Europe, grant from Phonak, grant from Action on Hearing Loss, grant from National Institute for Health Research, grant from Ida Institute outside the submitted work.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: North West—Greater Manchester South Research Ethics Committee (15/NW/0860) and the University of Southampton Research Governance Office (ERGO 15329)
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Due to ethical concerns, supporting data cannot be made openly available. Further information about the data is available from the University of Southampton repository: https://doi.org/10.5258/SOTON/D0252. Adults with cochlear implants are still rare in the general population (approximately 0.01% of the UK population, or approximately 1 in 10 000 people). This makes anonymity more challenging. In addition, sensitive personal data were collected in this project by virtue of the fact that data relate to a physical condition (deafness). The study protocol is published.
References
- 1. BCIG. Annual update 2015-2016. 2016. http://www.bcig.org.uk/wp-content/uploads/2016/12/CI-activity-2016.pdf.
- 2. Hibbard JH, Greene J, Shi Y, et al. Taking the long view: how well do patient activation scores predict outcomes four years later? Med Care Res Rev 2015;72:324–37. 10.1177/1077558715573871 [DOI] [PubMed] [Google Scholar]
- 3. Mosen DM, Schmittdiel J, Hibbard J, et al. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007;30:21–9. 10.1097/00004479-200701000-00005 [DOI] [PubMed] [Google Scholar]
- 4. Panagioti M, Richardson G, Small N, et al. Self-management support interventions to reduce health care utilisation without compromising outcomes: a systematic review and meta-analysis. BMC Health Serv Res 2014;14:356 10.1186/1472-6963-14-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cullington H, Kitterick P, DeBold L, et al. Personalised long-term follow-up of cochlear implant patients using remote care, compared with those on the standard care pathway: study protocol for a feasibility randomised controlled trial. BMJ Open 2016;6:e011342 10.1136/bmjopen-2016-011342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301–8. 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 7. Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933–40. 10.1002/sim.4780141709 [DOI] [PubMed] [Google Scholar]
- 8. British Cochlear Implant Group. Quality Standards. Cochlear implant services for children and adults. Birmingham: British Cochlear Implant Group, 2016. [Google Scholar]
- 9. Cullington HE, Agyemang-Prempeh A. Person-centred cochlear implant care: Assessing the need for clinic intervention in adults with cochlear implants using a dual approach of an online speech recognition test and a questionnaire. Cochlear Implants Int 2017;18:76–88. 10.1080/14670100.2017.1279728 [DOI] [PubMed] [Google Scholar]
- 10. Vroegop JL, Dingemanse JG, van der Schroeff MP, et al. Self-Adjustment of Upper Electrical Stimulation Levels in CI Programming and the Effect on Auditory Functioning. Ear Hear 2017;38:e232–40. 10.1097/AUD.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 11. The Society for the Study of Artificial Intelligence and Simulation of Behaviour. Introduction to the LifeGuide: software facilitating the development of interactive behaviour change internet interventions. Edinburgh: The Society for the Study of Artificial Intelligence and Simulation of Behaviour, 2009. [Google Scholar]
- 12. Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40(6 Pt 1):1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39(4 Pt 1):1005–26. 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bench J, Kowal A, Bamford J. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. Br J Audiol 1979;13:108–12. 10.3109/03005367909078884 [DOI] [PubMed] [Google Scholar]
- 15. Cullington HE, Aidi T. Is the digit triplet test an effective and acceptable way to assess speech recognition in adults using cochlear implants in a home environment? Cochlear Implants Int 2017;18:97–105. 10.1080/14670100.2016.1273435 [DOI] [PubMed] [Google Scholar]
- 16. Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol 2004;43:85–99. 10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feeny D, Furlong W, Boyle M, et al. Multi-attribute health status classification systems. Health Utilities Index. Pharmacoeconomics 1995;7:490–502. [DOI] [PubMed] [Google Scholar]
- 18. Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care 2002;40:113–28. 10.1097/00005650-200202000-00006 [DOI] [PubMed] [Google Scholar]
- 19. Saghaei M, Saghaei S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomed Sci Eng 2011;04:734–9. 10.4236/jbise.2011.411090 [DOI] [Google Scholar]
- 20. Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974;15:443–53. 10.1002/cpt1974155443 [DOI] [PubMed] [Google Scholar]
- 21. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 22. Office for National Statistics. Statistical bulletin: Internet users in the UK. London: Office for National Statistics, 2017. [Google Scholar]
- 23. Han B, Enas NH, McEntegart D. Randomization by minimization for unbalanced treatment allocation. Stat Med 2009;28:3329–46. 10.1002/sim.3710 [DOI] [PubMed] [Google Scholar]
- 24. Wetherill GB, Levitt H. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol 1965;18:1–10. 10.1111/j.2044-8317.1965.tb00689.x [DOI] [PubMed] [Google Scholar]
- 25. Smith D, Harvey P, Lawn S, et al. Measuring chronic condition self-management in an Australian community: factor structure of the revised Partners in Health (PIH) scale. Qual Life Res 2017;26:149–59. 10.1007/s11136-016-1368-5 [DOI] [PubMed] [Google Scholar]
- 26. Kitterick PT, Fackrell K, Cullington HE. Measuring empowerment in adult cochlear implant users - The development of the CI-EMP questionnaire [poster]. London: British Cochlear Implant Group Meeting, 2016. [Google Scholar]
- 27. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361–7. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.