Abstract
Objective
To estimate the prevalence of hypertension, diabetes and chronic kidney disease and their risk factors in a rural and urban region of Haiti.
Setting and participants
Community health workers enumerated 2648 households (909 rural and 1739 urban) via a multistage cluster random sampling method from July 2015 to May 2016, completed 705 rural and 1419 urban assessments for adults aged 25–65 years.
Outcome measures
We performed a WHO STEPS based questionnaire, measured two blood pressure values, weight, height, abdominal circumference and point of care test finger stick blood sample for haemoglobin A1c, creatinine and cholesterol (total, high density lipoprotein (HDL) and triglycerides).
Results
After adjusting for age and sex, the overall prevalence rates of hypertension, diabetes and chronic kidney disease were 15.6% (±2.93%), 19.7% (±1.57%) and 12.3% (±2.72%), respectively. Of the three non-communicable diseases (NCDs), only diabetes showed a significant difference between rural and urban sites (p=0.000), with the rural site (23.1%) having a higher prevalence than the urban site (16.4%). When comparing male and female participants, females were significantly more likely than males to have an NCD (p≤0.011). Females had a higher prevalence of most of the risk factors when compared with males. The urban location had a higher prevalence than the rural location for four risk factors that showed a significant difference between location (p≤0.037).
Conclusions
Women in Haiti had significantly higher prevalence rates of most NCDs and risk factors than men, and urban populations frequently, but not always, had higher rates of NCDs risk factors than the rural population. Further, it was shown that using point of care blood tests combined with community health workers, it is feasible to screen for NCDs and risk factors in remote areas which otherwise have limited access to healthcare.
Keywords: chronic disease, general diabetes, hypertension, haiti, chronic renal failure, non-communicable diseases
Strengths and limitations of this study.
One of the only studies conducted on non-communicable diseases and their risk factors of this scale in Haiti.
Use of point of care blood tests, including haemoglobin A1c for diabetes, to diagnose non-communicable diseases in Haiti.
Random selection of participants with high response rate.
Possible selection bias as women are over-represented in comparison with men.
Limited generalisability as sample size is representative of the two regions included in the study rather than the country as a whole.
Introduction
Non-communicable diseases (NCDs) are the leading cause of death worldwide1 and placing the greatest burden on low-income and middle-income populations and leading to earlier mortality. Few studies have been conducted in Haiti, the poorest country in the Western hemisphere, to determine NCDs and risk factor prevalence. WHO has cited a substantial lack of information on hypertension and other NCD prevalence in Haiti impeding progress in NCD interventions.2
The primary objective of this study was to estimate the prevalence of hypertension, diabetes and chronic kidney disease (CKD) among Haitians aged 25–65 years, located in urban and rural Haiti. In a comprehensive literature search of low-income country (LIC) populations with sub-Saharan African or black descent, the prevalence of hypertension ranged from 27.9% in Benin3 to 53% in Malawi,4 with many countries, such as Tanzania and Nigeria,5 6 demonstrating a prevalence of 30%–40%. Previous studies showed ranges from 36.6% in a rural area of Haiti7 to 85% in urban specialty clinics serving Haitian-Americans.8 A rural specialty hospital in Haiti reported a diabetes prevalence of 36.3% and CKD prevalence of 27% among its patients.9 Other LICs of black or African descent, such as Malawi (18%),4 Tanzania (5.7%)10 and Benin (1.8%–3.3%),4 have reported much lower rates of diabetes. Quality prevalence data on CKD data is very limited for LICs.
In addition to NCDs, we sought to estimate the prevalence of risk factors, including obesity, dyslipidaemia, metabolic syndrome and smoking. In Burkina Faso, the prevalence of low HDL cholesterol was 30%11 and in Benin 13%.12 Prevalence of obesity in some LICs was as much as one-fifth the population. For example, a 2008 study in Benin found obesity prevalence at 18%12 and in Southern Nigeria it was 10.6%, with a prevalence of overweight people of 21.8%.6 We hypothesised the prevalence of NCDs and their risk factors in Haiti would be similar to those found in other LICs whose populations were of black or of African descent.
We also sought to compare the prevalence of these NCDs and risk factors between men and women, as well as between urban and rural populations. Based on studies in other LICs, we hypothesised that urban populations would have higher rates of NCDs than rural populations13–15 and we expected men to have higher rates of NCDs and risk factors than women.16–19
Finally, we sought to demonstrate these NCDs could be diagnosed in the community by community health workers (CHWs) using point-of-care testing (POCT), in cooperation with the Haitian non-profit organisation Innovating Health International. POCT refers to medical testing that is preformed outside a laboratory setting, in close proximity to the patient, often by non-professional personnel. In this study, POCT where used to collect information on cholesterol, haemoglobin A1c (HbA1c) and creatinine levels in order to diagnose hypertension, diabetes and CKD, respectively. Few studies, if any, have had such a comprehensive approach to estimating NCD prevalence in LICs. We hope that the use of CHWs and point-of-care blood tests could set a new standard for prevalence studies in LICs, offering the possibility for improved community-based disease diagnosis and management.
Methods
Study population and design
The Rivière Froide, Carrefour and Cabral region of Thomonde, Central Plateau were selected as the urban and rural sites of the study, respectively. Both study sites were chosen due to past experience working in these areas and access to facilities to work out for the purposes of this study.
The study used a multistage random cluster sampling method to choose which areas within the study sites would be sampled. The rural and urban sites were divided into sections with roughly equivalent populations and the clusters to be included in the study were chosen using a random number table. Within the clusters, the household to be the starting point for enumeration was selected using community structure anchor points (eg, schools, churches, police stations). For even numbered clusters, enumeration began from the left side of the anchor point and for odd-numbered clusters, the right side of the anchor point. Households to be interviewed were selected based on a random interval number that was chosen daily from preprepared sealed envelopes. One individual within the household was then randomly selected to be interviewed using a Kish table.20 Men and women between 25 and 65 years of age, residing in Rivière Froide, Carrefour and Cabral of Thomonde, Central Plateau were eligible to participate in the study. Men and women who were cognitively impaired or women who were pregnant were excluded. If the selected individual was not home or not free to participate in the survey on first approach, at least two additional attempts to conduct the interview were made. See figure 1 for accrual numbers.
Figure 1.
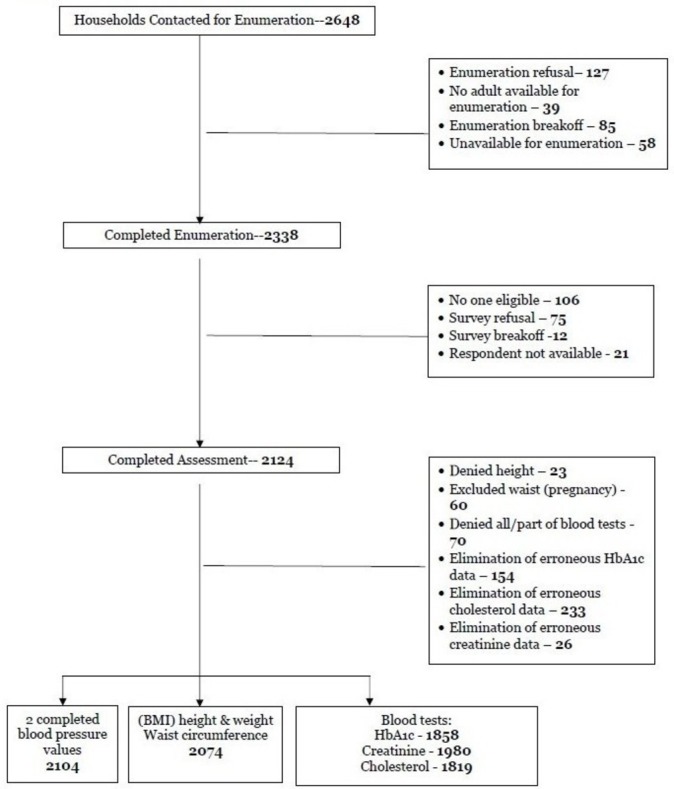
Accrual of participants (see attached). BMI, body mass index; HbA1c, haemoglobin A1c.
The estimated population of the urban site of Rivière Froide, Carrefour as of the 2012 census was 193 694.21 The average household size was 4.2 members per household and the total number of households was 46 118. The urban sampling area was divided into eight clusters with each cluster estimated to have 5765 households. Using the same census data, the estimated population of the rural site of Cabral was 13 292, with an average household size of 4.9 members/per household and 4799 households.21 Section Cabral was divided into 20 clusters with each cluster having about 240 households.
Data collection
Data were collected between July 2015 and May 2016 by Haitian CHWs who participated in a week-long training programme. Eligible participants were identified and consent was obtained in accordance with human research standards. Accrual of sample occurred as outlined in figure 1.
An adapted version of WHO STEPS instrument was used for the main survey of the study.22 CHWs obtained blood pressure values two times—once at the beginning of the survey and a second time at the end, approximately 30–45 min afterwards. This was done using the Omron Series 7 electronic wrist blood pressure device as per instructed in the user manual.23 In addition, weight, height and abdominal circumference were measured with portable electronic scales and tape measures as per WHO STEPwise Surveillance Manual.24 A POCT finger stick blood sample was obtained to provide a HbA1c value to diagnosis diabetes (A1c Now+, Polymer Technology Systems), a creatinine value was obtained for CKD (StatSensor Creatinine Express device, Nova Biomedical) and cholesterol (total, HDL and triglycerides (TGs)) for dyslipidaemias (CardioChek Lipid Test, Polymer Technology Systems).25–27
Hypertension was defined as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg for both of the two separate occasions during the survey or alternatively, those taking blood pressure medication. Diabetes was diagnosed using HbA1c with normal defined as <5.7%, prediabetes as defined by HbA1c ≥5.7%–<6.4%, and diabetes as defined by HbA1c ≥6.5% or on diabetes treatment. CKD was categorised by glomerular filtration rates defined by stage 1 as ≥90 mL/min, stage 2 as 60–89 mL/min, stage 3 as 30–59 mL/min, stage 4 as 15–29 mL/min, stage 5 as <15 mL/min. Metabolic syndrome was defined using Adult Treatment Panel III criteria which is defined by three of the following: HbA1c >5.0 mg/dL, waist circumference ≥40 inches (men) or ≥35 inches (women), blood pressure ≥130/85 mm Hg, TG level ≥150 mg/dL, HDL cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women).28 Large waist circumference was defined as >40 inches for men and >35 inches for women.29 Overweight was defined as body mass index ≥25 kg/m2 and obesity was defined as body mass index over 30 kg/m2. Desirable total cholesterol profiles were defined as <200 mg/dL, borderline high as 200–239 mg/dL and high as ≥240 mg/dL. A normal TG profile was defined as <150 mg/dL, borderline high as ≥150<200 mg/dL and high as ≥200 mg/dL. Favourable HDL is ≥50 mg/dL in women and ≥40 mg/dL in men. For both genders, low density lipoprotein (LDL) <130 mg/dL was considered desirable, LDL ≥130–159.9 mg/dL borderline high and LDL ≥160 mg/dL high. After completion of the survey, if the participant had an abnormal or dangerous test value, the CHWs would refer them to the closest local clinic.
Data quality control occurred at several steps in the data flow process. Before the CHWs leave the household, they reviewed the study documents for completeness, legibility and logic. Following this, study staff performed a same-day review of all completed surveys, with any clarifications or corrections completed the next work day. Reviewed and corrected paper surveys were then entered into a secure REDCap database, with range and consistency checks performed daily.30
Statistical analysis
Other studies have shown a 4% difference in prevalence between the urban and rural groups in terms of hypertension, a 5% difference in obesity, a 3.5% difference in diabetes. Using the one with the smallest predicted difference of 3.5%, diabetes, the minimum sample size needed was estimated to be 1798, at a 5% significance level and power of 80%. To this, we added a 10% buffer in case some participants opted out of providing measurement data and then rounded the sample size to 2000 people.
Primary outcomes were prevalence of each NCD (hypertension, diabetes, CKD) and risk factor (smoking, obesity, waist circumference, dyslipidaemia, metabolic syndrome). The LDL cholesterol, body mass index and glomerular filtration rate (Cockroft-Gault) were calculated values. Each variable prevalence was calculated based on the number of complete values for that variable with missing observations assumed to be missing at random and therefore removed from the analysis.
To estimate diabetes prevalence, discussed later, both Stata and R31 were used. To determine if the presence of haemoglobin abnormalities, estimated at 13.97% prevalence, presented a bias, we used bootstrap randomisation algorithm. The first algorithm resamples and excludes 13.97% of the observations randomly from the data, and recalculates the new prevalence. Resampling was done for 10 000 iterations. The second algorithm splits the data into portions that represent 13.97% of the total number of observations and excludes each cluster once, then recalculates the prevalence of diabetes, replaces the excluded cluster and repeats the process.
Data are presented as crude and age–sex direct standardised prevalence for each variable, disaggregated by sex and location. Additionally, Poisson regression models were fitted with the response being the counts of number of observations with hypertension adjusting for age and gender, and with SEs estimated using the robust sandwich variance estimator to account for heterogeneity in the data. Both the deviance goodness-of-fit and Pearson goodness-of-fit χ2 tests were used to assess if the model fit the data. Data were analysed using Stata statistical software.32
Results
In total, 2648 households (909 rural and 1739 urban) were contacted for enumeration with 705 assessments completed from the rural area of Thomonde commune in the Central Plateau and 1419 from an urban neighbourhood on the edge of Port-au-Prince called Carrefour (table 1). The response rate (defined as eligible participants who underwent the survey over the total number of households contacted minus those who were determined to be ineligible secondary to age) was 89.6%. Non-responders were generally those who did not consent to two finger sticks or who had something urgent arise during the hour-long interview and could not be contacted after two attempts.
Table 1.
Description of study participants by location, Rivière Froide, Carrefour and Cabral region of Thomonde, Central Plateau, Haiti, 2015
| Variable | Rural (n=706) |
Urban (n=1425) |
Total (n=2131) |
|||
| n | (%) | n | (%) | n | (%) | |
| Sex | ||||||
| Male | 258 | (36.5) | 571 | (40.1) | 829 | (38.9) |
| Female | 448 | (63.4) | 854 | (59.9) | 1303 | (61.1) |
| Mean age (SD) (years) | 42.4 | (SD 11.7) | 39.9 | (SD 11.7) | 40.8 | (SD11.8) |
| Monthly income (US$) | ||||||
| <25 | 211 | (30.1) | 206 | (14.7) | 417 | (19.8) |
| 25–50 | 89 | (12.7) | 144 | (10.2) | 233 | (11.1) |
| 51–250 | 194 | (27.7) | 357 | (25.5) | 551 | (26.2) |
| 251–500 | 177 | (25.2) | 544 | (38.8) | 721 | (34.5) |
| >500 | 29 | (4.1) | 148 | (10.5) | 177 | (8.4) |
| Education | ||||||
| No formal schooling | 559 | (85.3) | 715 | (56.9) | 1274 | (66.6) |
| Less than primary school | 67 | (10.2) | 332 | (26.4) | 399 | (20.8) |
| Primary school | 19 | (2.9) | 173 | (13.7) | 192 | (10.5) |
| Secondary school | 5 | (0.7) | 25 | (1.9) | 30 | (1.5) |
| University | 5 | (0.7) | 11 | (0.8) | 16 | (0.8) |
Demographics
The overall mean age was 40.8 years old, with minimal difference by sex (male=40.6 years, female=40.8 years) but age varied by location, with rural participants older (42.4 years) than the urban participants (39.9 years) (p=0.000) (table 1). Among the participants, 829 were male (38.9%) and 1303 were female (61.1%), with similar participation rates for the rural and urban sites.
Prevalence of NCDs and their risk factors
As seen in table 2, there was a significant difference in prevalence between male and female participants when it came to the following risk factors: smoking, overweight and obese, large waist circumference, high total cholesterol, high TGs, high LDL and metabolic syndrome. Females had a higher prevalence of all of these risk factors when compared with males, except for smoking.
Table 2.
Crude and age–sex standardised prevalence of non-communicable diseases and risk factors among study participants, Rivière Froide, Carrefour and Cabral region of Thomonde, Central Plateau, Haiti, 2015
| Prevalence by sex | Prevalence by location | Overall prevalence | ||||||
| Males | Females | P values*
(sex) |
Urban | Rural | P values† (location) | Standardised‡ | SE | |
| Risk factors | ||||||||
| Smoking (n=2124) | 13.7 | 5.0 | 0.000 | 8.9 | 9.8 | 0.666 | 9.3 | 1.50 |
| Overweight and Obese§ (n=2074) | 14.3 | 34.3 | 0.000 | 30.4 | 18.2 | 0.000 | 24.3 | 2.98 |
| Large waist circumference (n=2074) | 4.2 | 34.4 | 0.000 | 21.4 | 17.1 | 0.000 | 19.3 | 3.70 |
| High total cholesterol (n=1819) | 0.4 | 4.0 | 0.000 | 2.7 | 1.8 | 0.037 | 2.3 | 0.64 |
| High triglycerides (n=1819) | 5.9 | 9.0 | 0.001 | 7.1 | 7.8 | 0.521 | 7.4 | 0.78 |
| High LDL (n=1819) | 0.8 | 4.5 | 0.000 | 3.3 | 2.1 | 0.203 | 2.7 | 0.69 |
| Low HDL (n=1819) | 35.8 | 33.6 | 0.100 | 32.3 | 36.2 | 0.094 | 34.7 | 1.49 |
| Metabolic syndrome (n=2074) | 15.1 | 37.5 | 0.000 | 30.1 | 22.5 | 0.000 | 26.3 | 3.27 |
| Non-communicable diseases | ||||||||
| Hypertension (n=2104) | 11.0 | 20.2 | 0.000 | 17.1 | 14.1 | 0.185 | 15.6 | 2.93 |
| Diabetes (n=1858) | 18.6 | 20.8 | 0.001 | 16.4 | 23.1 | 0.000 | 19.7 | 1.57 |
| Chronic kidney disease¶ (n=1980) | 8.8 | 15.8 | 0.000 | 14.2 | 10.5 | 0.086 | 12.3 | 2.72 |
*Comparing male and female prevalence.
†Comparing urban and rural prevalence.
‡Age–sex standardised.
§BMI defined as ≥25 kg/m2.
¶Chronic kidney disease stages 3–5.
BMI, body mass index.
Between the urban and rural sites of the study, a significant difference in prevalence was seen for the following risk factors: overweight and obese, large waist circumference, high total cholesterol and metabolic syndrome. The urban location had a higher prevalence of all of these risk factors when compared with the rural location. The rural location had a significantly higher rate of diabetes.
Other observations of note include that urban women were significantly more likely to have metabolic syndrome than rural women, but there was no difference in men. Also, there was no significant difference in prevalence of large waist circumference in urban men and urban women than rural men and rural women, respectively.
Among all study participants, hypertension prevalence rates were estimated to be 15.6% (±2.93%) and there was a significant difference between men (11.9%) and women (20.2%). Of the 1858 participants evaluated for diabetes, 37% were prediabetic and 19.7% (±1.57%) had an HbA1c diagnostic of diabetes, with a significantly higher rate in women (20.8%) than men (18.6%) and rural (23.1%) location than urban (16.4%). The overall prevalence of CKD stages 3–5 combined was 12.3% (±2.72), with women (15.8%) suffering significantly higher rates than men (8.8%).
Discussion
This study found that, after adjusting for age and sex, the overall prevalence rates of hypertension, diabetes and CKD were 15.6% (±2.93%), 19.7% (±1.57%) and 12.3% (±2.72%), respectively. Of the three NCDs, only diabetes showed a significant difference between rural and urban sites (p=0.000), with the rural site (23.1%) having a higher prevalence than the urban site (16.4%). When comparing male and female participants, females were significantly more likely than males to have an NCD (p≤0.011). With respect to NCD risk factors, females had a higher prevalence of most of the risk factors when compared with males. For the four risk factors that showed a significant difference between location (p≤0.037), the urban location had a higher prevalence than the rural location.
The hypertension prevalence was lower than that noted in many other LICs in Africa and among populations of black descent, including those reported previously in Haiti (20%–70%).33–36 Most published Haitian studies were performed among clinic populations using manual blood pressure cuffs with large interoperator variability. Another possible explanation for the comparatively low level of hypertension in this study includes the absence of ‘white coat’ hypertension seen in clinics. We used a handheld electronic device to eliminate interoperator variability for CHWs, whose skills with manual cuff may not have been that of more trained clinicians. The wrist location was chosen for ease of use compared with upper arm monitors that would require multiple-sized cuffs. However, the device we used has been well-validated against traditional means for blood pressure measurement,37 although others elsewhere have cited some inaccuracies in their use depending on the brand.38
The prevalence of prediabetes and diabetes is much lower than rates reported in a previous study in Haiti (36.3%),9 and higher than those reported in other nations including Malawi (18%),4 Tanzania (5.7%)10 and Benin (1.8%–3.3%)4. Notably, our findings were similar to those found in US blacks.39 The consistency with US rates may have been due to the methods we used to assess these conditions. Instead of blood glucose, we used HbA1c to diagnose prediabetes and diabetes to eliminate inaccuracies due to ingestion of food or drink and the inability to control for last oral intake. This method may be especially important to use in cultures like Haiti’s, where drinks, even high sugar ones, are often not considered food or oral intake when asked.
According to previous studies, approximately 13.97% of Haitian adults have a haemoglobin abnormality (range of 12.7%–15.3%),40–42 compared with 12% in blacks in the USA.43 Previous research has shown variations of HbA1c in those with abnormal haemoglobin, with the results depending on the type of abnormal haemoglobin, the amount of elevation of the HbA1c with those farther from the mean having higher error rates, and the test itself that is used. Most errors have been shown to be small (less than 0.5% increase or decrease in HbA1c), but others, including the machine that we used, HbA1c Now+, has previously been reported to overestimate HbA1c by 1.17%–1.73% depending on haemoglobin type. To determine if this possible overestimation presented a bias in our study, we performed a bootstrap randomisation algorithm in two different ways described above. All statistical analysis methods yielded similar results to that which was observed without any correction (16.1% prevalence urban and 23.5% rural).
A limitation of this study is the under-representation of men among our sample: 39.5% of the data came from men, and 60.5% from women. Since the study was conducted during the day and at homes, men may have been at work and thus less likely to have completed the study if they were selected. This has been cited as a limitation in other surveillance work in Haiti.34 In our findings, the men had lower rates of many of the risk factors and diseases than the women. Since working men may be healthier than men who stay at home, our estimated prevalence rates may have been somewhat biased; however, crude and age–sex standardised NCD prevalence values were generally similar.
This study cannot be generalised to all of Haiti as it represents one urban and one rural region of Haiti. However, this is the largest study conducted on NCDs and their risk factors in Haiti. Other studies looking at NCDs in Haiti are limited and those that have been completed are hospital based with small sample sizes, making the observations highly prone to selection bias.9 33–36 They also do not measure as many NCDs or risk factors. As such, this study is one of the best resources available to provide an indication about what NCD prevalence may be for the country as a whole.
One of the strengths of this study is that although many of the communities included in the sampled clusters were difficult to access, with limited access by road, requiring long distances and difficult terrain to be traversed on foot, using CHWs and POCT blood tests enabled the data to be captured. Our methods may create a new standard for chronic disease diagnosis and monitoring in countries with remote and difficult to access locations. The success of the CHWs can be attributed to their persistence, commitment and dedication to the public health of their communities. Knowing that local buy-in was of tantamount importance, prior to initiation of the study existing CHWs announced to local community leaders (priests, government officials, etc) the study aims and sought their input, and acceptance. Community buy-in was facilitated by the fact that all participants were educated on NCDs and given a ‘Know your numbers’ card that documented their study results for their own use. Thus, the research attempted to give back to the communities; CHWs were pleased to offer this information to their community members. The CHWs had daily contact with study staff who performed long distance quality control, as done in previous studies.44
Though at first it was difficult to use, POCT was a major strength of this study. POCT for HbA1c,45 46 creatinine47 48 and cholesterol (total, HDL, TGs)49 50 has been well validated as comparable to that in laboratory settings. POCT has been shown to increase the testing of specific diseases when available, for example, when rapid HIV tests were made accessible.51 In order to have drawn, transported and tested vials of blood instead of using POCT, significantly more infrastructure would have been required including freezers, centrifuges and a network of transportation via motorcycles through the mountains. In order to carry out POCT, each CHW was equipped with a backpack that weighed over 25 pounds at the start of each day, including sharps and biohazard disposal, a weight scale, three POCT machines, ice packs as needed and paper surveys. Without the paper surveys, the backpacks weighed significantly less and make POCT in remote places even more practical as an actual clinical tool. The method of POCT for determining prevalence of numerous NCDs offers a dramatic alternative to otherwise logistically difficult data collection which has impeded disease management in LICs.
Some small adjustments were made to use POCT in difficult conditions. As per recommendations from each POCT manufacturer, the creatinine test could be added to either of the finger sticks for HbA1c or cholesterol without changing the result and was determined as good practice to decrease burden on the participants. Each POCT device required certain temperature ranges and had programmed error codes when temperatures were out of range. The collection period varied between 7 and 10 hours per day in temperatures consistently exceeding 35°Celsius, which necessitated carrying the POCT devices in bags packed with ice so as to assure their proper temperature. Through careful packing and periodic cooling periods, these error codes were completely avoided after the first 2 weeks in each location.
Conclusions
In Rivière Froide, Carrefour and Cabral region of Thomonde, Central Plateau, Haiti it was found that the prevalence of NCDs was higher in women than men. It was also shown that the prevalence of NCDs was similar between the urban and rural sites, except for diabetes which was higher in the rural location. The use of CHWs and POCTs has shown to be a promising, cost-effective way to determine NCD prevalence and should be considered in other LIC and remote settings. While our study may give an indication about the prevalence of NCDs and their risk factors in Haiti, further research is required to accurately make observations about the country as a whole.
Supplementary Material
Footnotes
Contributors: VD: designed study, wrote paper, analysis, all criteria below. SM, LCr: implementation of research, analysis, all below. KV: designed study, wrote paper, analysis, all below. LKM: wrote paper, performed analysis, all below. CS: designed study, wrote paper, analysis, all below. LCo: provided funding, designed study, wrote paper, analysis, all below. KT: provided research, designed study, wrote paper, all below. RL: provided funding, designed study, wrote paper, analysis, all below. **Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work and drafting the work or revising it critically for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: University of Florida Gatorade Trust, University of Florida Public Health and Health Professions, Innovating Health International.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: IRB approval was obtained through the University of Florida College of Medicine and the Haitian Medical Association National Bioethics Committe.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Technical appendix, statistical code and dataset available on request from primary author.
Correction notice: This article has been corrected since it first published. ’Jr' has been added to the end of author Vincent DeGennaro’s name.
References
- 1. World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization, 2011. [Google Scholar]
- 2. World Health Organization. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Houehanou YC, Lacroix P, Mizehoun GC, et al. Magnitude of cardiovascular risk factors in rural and urban areas in Benin: findings from a nationwide steps survey. PLoS One 2015;10:e0126441 10.1371/journal.pone.0126441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manjomo RC, Mwagomba B, Ade S, et al. Managing and monitoring chronic non-communicable diseases in a primary health care clinic, Lilongwe, Malawi. Public Health Action 2016;6:60–5. 10.5588/pha.16.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zack RM, Irema K, Kazonda P, et al. Determinants of high blood pressure and barriers to diagnosis and treatment in Dar es Salaam, Tanzania. J Hypertens 2016;34:2353–64. 10.1097/HJH.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isara AR, Okundia PO. The burden of hypertension and diabetes mellitus in rural communities in southern Nigeria. Pan Afr Med J 2015;20 10.11604/pamj.2015.20.103.5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanon MA, Mohammed SA, McCullagh MC. Definition and management of hypertension among Haitian immigrants: a qualitative study. J Health Care Poor Underserved 2014;25:1067–78. 10.1353/hpu.2014.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierce L, Shannon A, Sonnenfeld J, et al. Hypertension prevalence and knowledge assessment in rural Haiti. Ethn Dis 2014;24:213–9. [PubMed] [Google Scholar]
- 9. Burkhalter F, Sannon H, Mayr M, et al. Prevalence and risk factors for chronic kidney disease in a rural region of Haiti. Swiss Med Wkly 2014;144 10.4414/smw.2014.14067 [DOI] [PubMed] [Google Scholar]
- 10. Stanifer JW, Cleland CR, Makuka GJ, et al. Prevalence, Risk Factors, and Complications of Diabetes in the Kilimanjaro Region: A Population-Based Study from Tanzania. PLoS One 2016;11:e0164428 10.1371/journal.pone.0164428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeba AN, Delisle HF, Renier G, et al. The double burden of malnutrition and cardiometabolic risk widens the gender and socio-economic health gap: a study among adults in Burkina Faso (West Africa). Public Health Nutr 2012;15:2210–9. 10.1017/S1368980012000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sodjinou R, Agueh V, Fayomi B, et al. Obesity and cardio-metabolic risk factors in urban adults of Benin: relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health 2008;8:84 10.1186/1471-2458-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Msyamboza KP, Ngwira B, Dzowela T, et al. The burden of selected chronic non-communicable diseases and their risk factors in Malawi: nationwide STEPS survey. PLoS One 2011;6:e20316 https://doi.org/ 10.1371/journal.pone.0020316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oyebode O, Pape UJ, Laverty AA, et al. Rural, urban and migrant differences in non-communicable disease risk-factors in middle income countries: a cross-sectional study of WHO-SAGE data. PLoS One 2015;10:e0122747 10.1371/journal.pone.0122747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens 2014;32:1170 10.1097/HJH.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. 10.1056/NEJMoa0908292 [DOI] [PubMed] [Google Scholar]
- 17. Gao Y, Chen G, Tian H, et al. Prevalence of hypertension in china: a cross-sectional study. PLoS One 2013;8:e65938 10.1371/journal.pone.0065938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens A, Schmidt MI, Duncan BB. Gender inequalities in non communicable disease mortality in Brazil. Cien Saude Colet 2012;17:2627–34. [DOI] [PubMed] [Google Scholar]
- 19. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kish L. A Procedure for Objective Respondent Selection within the Household. J Am Stat Assoc 1949;44:380–7. 10.1080/01621459.1949.10483314 [DOI] [Google Scholar]
- 21. Finances, H. M. o. E. a. Total Population, 18 years and older. Port-au-Prince, Haiti: Haitian Institute of Statistics and Information (IHSI), 2015. [Google Scholar]
- 22. World Health Organization. STEPS instruments for NCD risk factors (core and expanded version 1.4): the WHO STEPwise approach to Surveillance of noncommunicable diseases (STEPS). Geneva: World Health Organization. [Google Scholar]
- 23. Omron 7 Series Wrist Blood Pressure Monitor (BP652). Omron Healthcare. 2018. https://www.omronhealthcare.ca/products/7-series-wrist-blood-pressure-monitor-bp652/ (cited 3 Jan 2018).
- 24. World Health Organization. WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. Geneva: World Health Organization. [Google Scholar]
- 25. A1c Now+, Polymer Technology Systems. Ptsdiagnostics-apps.com. 2018. http://ptsdiagnostics-apps.com/product_labeling/a1cnow_professional/user_guides/A1CNOW_Pro_UG_PN-9708.pdf (cited 3 Jan 2018).
- 26. Nova Biomedical: World Leader in Biosensor Technology. Novabio.us. 2018. http://www.novabio.us/statstrip-creatinine/ (cited 3 Jan 2018).
- 27. CardioChek Lipid Test, Polymer Technology Systems. Ptsdiagnostics.com. 2018. http://www.ptsdiagnostics.com/uploads/2/6/2/8/26289179/ps-002461_en_rev._4_user_guide_cardiochek_pa.pdf (cited 3 Jan 2018).
- 28. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 30. REDCap. Projectredcap.org. 2018. https://projectredcap.org/ (cited 3 Jan 2018).
- 31. RDevelopment CORE TEAM. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2010. ISBN 3–900051–07–0. [Google Scholar]
- 32. Stata Corp LP. Stata Statistical Software Release 14: Stata Press Publication, 2015. [Google Scholar]
- 33. Dickstein Y, Neuberger A, Golus M, et al. Epidemiologic profile of patients seen in primary care clinics in an urban and a rural setting in Haiti, 2010-11. Int Health 2014;6:258–62. 10.1093/inthealth/ihu033 [DOI] [PubMed] [Google Scholar]
- 34. Jean-Baptiste ED, Larco P, Charles-Larco N, et al. Glucose intolerance and other cardiovascular risk factors in Haiti (PREDIAH). Diabetes Metab 2006;32:443–51. 10.1016/S1262-3636(07)70302-6 [DOI] [PubMed] [Google Scholar]
- 35. Lluberas G, Parrish LA, Kling CM. Hypertension prevalence in a rural Haitian missionary clinic. Nurse Pract 2000;25:59–61. 10.1097/00006205-200025110-00005 [DOI] [PubMed] [Google Scholar]
- 36. Martin P. Describing the chief complaints in a mobile, primary care clinic in Haiti: Using data to enlighten the knowledge of the burden of non-communicable diseases. In141st APHA Annual Meeting (November 2-November 6, 2013), 2013. [Google Scholar]
- 37. Mourad A, Gillies A, Carney S. Inaccuracy of wrist-cuff oscillometric blood pressure devices: an arm position artefact? Blood Press Monit 2005;10:67–71. 10.1097/00126097-200504000-00003 [DOI] [PubMed] [Google Scholar]
- 38. Kikuya M, Chonan K, Imai Y, et al. Accuracy and reliability of wrist-cuff devices for self-measurement of blood pressure. J Hypertens 2002;20:629–38. 10.1097/00004872-200204000-00019 [DOI] [PubMed] [Google Scholar]
- 39. Tull ES, Roseman JM. Diabetes in African Americans. Diabetes in America 1995;2:613–30. [Google Scholar]
- 40. Rotz S, Arty G, Dall’Amico R, et al. Prevalence of sickle cell disease, hemoglobin S, and hemoglobin C among Haitian newborns. Am J Hematol 2013;88:827–8. 10.1002/ajh.23510 [DOI] [PubMed] [Google Scholar]
- 41. Randolph TR. Estimated prevalence of sickle cell in northern Haiti. Clin Lab Sci 2010;23:79. [PubMed] [Google Scholar]
- 42. Pegelow CH, Mack AK. Incidence of hemoglobins S and C in infants born in Miami to recent Haitian immigrants. Trop Geogr Med 1989;41:316–9. [PubMed] [Google Scholar]
- 43. Roseman JM. Diabetes in black Americans. Diabetes in America 1985;8:1–24. [Google Scholar]
- 44. Cottler LB, Ajinkya S, Goldberger BA, et al. Prevalence of drug and alcohol use in urban Afghanistan: epidemiological data from the Afghanistan National Urban Drug Use Study (ANUDUS). Lancet Glob Health 2014;2:e592–e600. 10.1016/S2214-109X(14)70290-6 [DOI] [PubMed] [Google Scholar]
- 45. Knaebel J, Irvin BR, Xie CZ. Accuracy and clinical utility of a point-of-care HbA1c testing device. Postgrad Med 2013;125:91–8. 10.3810/pgm.2013.05.2664 [DOI] [PubMed] [Google Scholar]
- 46. Leal S, Soto-Rowen M. Usefulness of point-of-care testing in the treatment of diabetes in an underserved population: SAGE Publications, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shephard MD. Point-of-Care Testing and Creatinine Measurement. Clin Biochem Rev 2011;32:109. [PMC free article] [PubMed] [Google Scholar]
- 48. Service, N.N.H. Point of Care Tests for the Measurement of Blood Creatinine (CEP10042), in NHS and Supply Agency. London, 2010. [Google Scholar]
- 49. Batki AD, Nayyar P, Thomason HL. Buyer’s guide: Point-of-care testing for cholesterol measurement. NHS Purchasing and Supply Agency: Center for Evidence-based Purchasing. CEP, 2009:9020. [Google Scholar]
- 50. Plüddemann A, Thompson M, Price CP, et al. Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update. Br J Gen Pract 2012;62:224–6. 10.3399/bjgp12X630241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Cock KM, Bunnell R, Mermin J. Unfinished business--expanding HIV testing in developing countries. N Engl J Med 2006;354:440–2. 10.1056/NEJMp058327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


