Abstract
Introduction
Bronchiolitis is the most common reason for hospitalisation in infants in developed countries. The main focus of hospital care is on supportive care, such as monitoring for hypoxia and supplemental oxygen administration, as active therapies lack effectiveness. Pulse oximetry is used to monitor hypoxia in hospitalised infants and is used either intermittently or continuously. Observational studies have suggested that continuous pulse oximetry use leads to a longer length of hospital stay in stable infants. The use of continuous pulse oximetry may lead to unnecessary clinical intervention due to readings that are of little clinical significance, false-positive readings and less reliance on the clinical status. There is a lack of high-quality evidence to guide which pulse oximetry monitoring strategy, intermittent or continuous, is superior in infants hospitalised with bronchiolitis with respect to patient and policy-relevant outcomes.
Methods and analysis
This is a multicentre, pragmatic randomised controlled trial comparing two strategies for pulse oximetry monitoring in infants hospitalised for bronchiolitis. Infants aged 1 month to 2 years presenting to Canadian tertiary and community hospitals will be randomised after stabilisation to receive either intermittent or continuous oxygen saturation monitoring on the inpatient unit until discharge. The primary outcome is length of hospital stay. Secondary outcomes include additional measures of effectiveness, acceptability, safety and cost. We will need to enrol 210 infants in order to detect a 12-hour difference in length of stay with a type 1 error rate of 5% and a power of 90%.
Ethics and dissemination
Research ethics approval has been obtained for this trial. This trial will provide data to guide hospitals and clinicians on the optimal pulse oximetry monitoring strategy in infants hospitalised with bronchiolitis. We will disseminate the findings of this study through peer-reviewed publication, professional societies and meetings.
Trial registration number
Keywords: bronchiolitis, pulse oximetry, randomized controlled trial
Strengths and limitations of this study.
This pragmatic trial is addressing how to best use pulse oximetry for bronchiolitis, a common hospital condition in children.
The trial is recruiting patients in both community and specialised children’s hospitals and measuring outcomes relevant to patients, clinicians and the health system so that the finds are meaningful to the real-world setting.
Clinicians and patients are not blinded to the interventions as we are interested in knowing if knowledge of the treatment arm affects behaviour and management decisions.
Introduction
Bronchiolitis is the most common acute lower respiratory tract infection that affects infants and young children less than 2 years of age.1 2 It presents with a viral upper respiratory prodrome followed by tachypnoea, chest retractions and diffuse crackles, wheeze or both. It is a leading cause of infant hospitalisation and is cumulatively expensive for the healthcare system.3 4 Although the illness is self-limited, some infants require hospitalisation for fast and laboured breathing, hypoxia and feeding difficulties. Systematic reviews of a large body of evidence have shown minimal effectiveness for a range of active medical treatments, specifically drug therapies including steroids and inhaled bronchodilators.5–9 Thus, the focus of inpatient management is on supportive care, which includes monitoring vital signs, oxygen supplementation for hypoxia, and nutritional and/or fluid supplementation.
Over the past two decades, non-invasive oxygen saturation monitoring or pulse oximetry has been widely available for identifying hypoxia.10 Pulse oximetry can be used intermittently, such as every 4 hours, or continuously in hospitalised infants with bronchiolitis. Although pulse oximetry was introduced into bronchiolitis hospital management without health technology assessment, it has become common clinical practice to use continuous oxygen saturation monitoring at many centres.
Observational studies have suggested that the use of continuous oxygen saturation monitoring in stable hospitalised infants with bronchiolitis may actually unnecessarily prolong hospital stay.11–13 It has been proposed that continuous monitoring leads to ‘over monitoring’ in stable infants. This leads to greater false-positive readings, clinicians reacting to low readings that are not clinically important and less reliance on the clinical status of the infant in decision-making around management and disposition. This then results in a longer duration of oxygen supplementation and/or prolonged observation in hospital. A randomised controlled trial (RCT) conducted in the emergency department demonstrated clinician over-reliance on oxygen saturation monitoring in the management of infants with bronchiolitis.14 Experts concluded, “the art of medicine and clinical assessment should not be trumped by over-reliance on a single-physiologic parameter.”15
Current clinical practice guidelines (CPGs) from the American Academy of Paediatrics (AAP) have recommended that clinicians ‘may not choose to use continuous pulse oximetry or administer supplemental oxygen if the saturation exceeds 90%’.1 Their recommendations are graded as evidence level D (expert opinion, case reports, reasoning from first principles). Subsequent to the guideline publication, the first trial comparing intermittent versus continuous pulse oximetry monitoring was reported.16 All infants were randomised on admission to hospital. Infants randomised to intermittent monitoring were switched after the infants were non-hypoxaemic. Length of stay (LOS) was measured from the time of admission (not from the time of implementation of the intervention) and did not differ based on the oxygen saturation monitoring strategy (48.9 hours for continuous monitoring vs 46.2 hours for intermittent monitoring; p=0.77). Some limitations of this trial include: only inclusion of non-hypoxaemic infants for intermittent monitoring; powered to detect only an 18-hour difference in LOS (ie, underpowered) and initiating measurement of the primary and some secondary outcomes before implementation of the monitoring intervention. An expert commentary highlighted the need for further trials.17
Two broad concerns around healthcare delivery have emerged that make this trial especially relevant. One is a concern of the widespread overuse of physiological monitoring devices and alarms in hospital care, the resulting alarm fatigue of staff, and the potential to compromise patient safety.18–20 Second is a concern around overdiagnosis, the detection of an abnormality that does not benefit the patient, and how it may be harming children.21 A recent review on overdiagnosis highlighted the detection of clinically insignificant desaturations using continuous oxygen monitoring in bronchiolitis as an example of overdiagnosis in children.21 Given these broad concerns around overuse of physiological monitoring and the evidence gap around the most effective oxygen monitoring strategy for such a common condition as bronchiolitis, high-quality evidence is needed to guide best practices and healthy policy.
Methods
Trial design
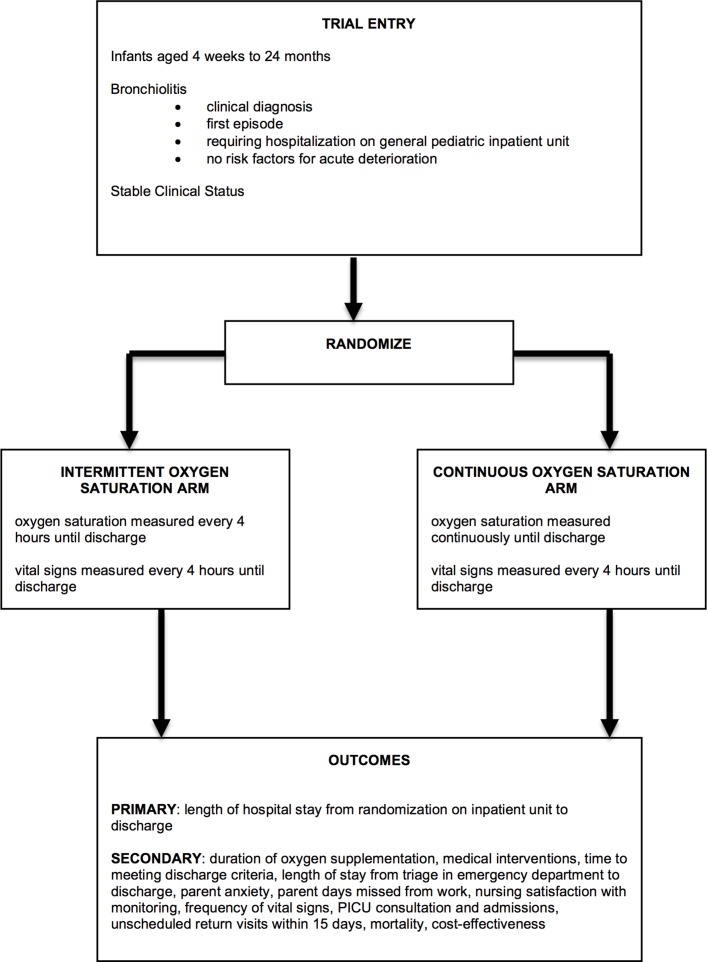
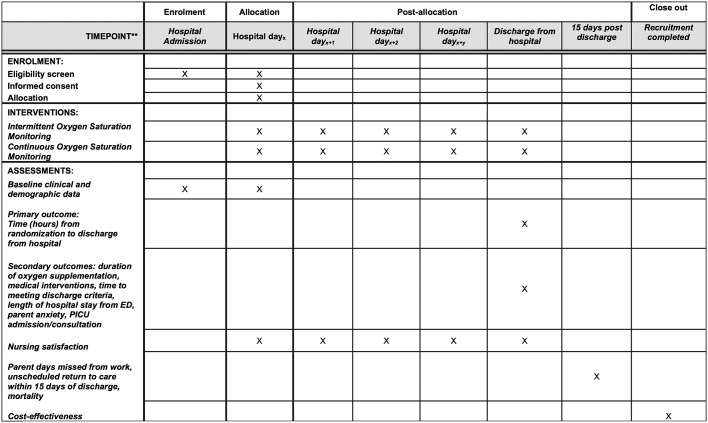
This is a six centre, pragmatic randomised controlled superiority trial designed with two parallel groups with a 1:1 allocation ratio with enrolment occurring over bronchiolitis seasons (each season from November to May) (see figure 1 for trial schemata). Trial recruitment commenced November 2016. This protocol follows Standard Protocol Items: Recommendations for Interventional Trials guidelines (see figure 2 for schedule of enrolment, interventions and assessment).22
Figure 1.
Trial schematic. PICU, paediatric intensive care unit.
Figure 2.
Schedule of enrolment, interventions and assessments. Patients who are eligible are approached once they meet clinical stability criteria during the hospitalisation. This maybe on the first day of hospitalisation or subsequent days. The intervention is applied until discharge and follow-up occurs after 15 days postdischarge. *ED, emergency department. PICU, paediatric intensive care unit.
Rationale for choice of methods
Pragmatic randomised trials seek to answer the question ‘Does this intervention work under usual conditions?’ and guides trial design decisions in 10 domains.23 A pragmatic design will strengthen the generalisability and relevance of the study findings to the practice setting for which it is intended. We will include patients from both tertiary and community hospital settings; medical management will be consistent with usual clinical care and we will be measuring outcomes that are important to patients and healthcare decision-makers including cost. This study is embedded within the environment of the knowledge users who will promote uptake of the intervention and study findings; a study conducted in several settings of different types (community regional hospital as well as free-standing children’s hospital) over more than one bronchiolitis season will also enhance generalisability and knowledge transfer.
A pilot study was conducted at one site (The Hospital for Sick Children, Toronto; ClinicalTrials.gov NCT01646606). The pilot study demonstrated feasibility of the trial processes (ie, number of eligible subjects, recruitment rate, inclusion/exclusion procedures, the acceptability of the intervention and willingness to randomise for clinicians, adherence to interventions, rates of completion of follow-up data) and provided data for sample size determination for this multicentre trial.
Study setting
This study will occur at three Ontario children’s hospitals (The Hospital for Sick Children, Toronto (SickKids), McMaster Children’s Hospital, Hamilton and Children’s Hospital of Eastern Ontario (CHEO), Ottawa) and three Ontario community paediatric centre (Trillium Health Partners, Mississauga, North York General Hospital, Toronto and Lakeridge Health, Oshawa) on the general paediatric inpatient unit (GPIU). Children with bronchiolitis are admitted to the GPIU following initial stabilisation and will be eligible for the study. Children with severe bronchiolitis are admitted to the paediatric intensive care unit (PICU) and will not be eligible for the study.
Eligibility criteria
Our eligibility criteria reflects our intention of only including infants who are in a stable phase of their hospitalisation and not at higher risk of deterioration.
Inclusion criteria
Age: 4 weeks to 24 months old. Infants less than 4 weeks are at high risk for requiring care in the PICU; infants greater than 24 months do not meet the standard definitions for bronchiolitis.
First episode of acute bronchiolitis. Infants with recurrent episodes may have an alternate diagnosis such as asthma.
Clinical diagnosis of bronchiolitis by the attending physician as a constellation of clinical findings on history and physical examination; clinical findings include: a preceding viral upper respiratory infection, presence of wheeze on chest auscultation and increased respiratory rate.1
- Stable clinical status:
- For infants receiving oxygen, clinical status must be stable for 6 hours on the GPIU as defined by all: Stable or decreasing requirement for supplemental oxygen and a stable or decreasing respiratory rate on at least two measurements; respiratory rate <70 breaths/minute; heart rate <180 beats/minute; oxygen supplementation <40% fractional inspired oxygen or <2 L/min by nasal prongs; not on heated high flow oxygen at time of enrolment.
- For infants in room air (ie, no supplemental oxygen), clinical status must be stable (as defined above) for 6 hours and can be assessed from the first vital signs measured in the emergency department.
Exclusion criteria
- The exclusion criteria are based on known risk factors for acute clinical deterioration:
- Chronic medical condition: congenital heart disease, that is, cyanotic, haemodynamically significant requiring diuretics and/or with pulmonary hypertension; chronic lung disease with home oxygen requirement and/or pulmonary hypertension; neuromuscular disease; immunodeficiency; haemoglobinopathy.
- Premature birth (<35 weeks).
- History of apnoea.
- Weight <4 kg.
- Receiving morphine infusions.
Patient on heated high flow oxygen at time of enrolment.
ICU admission on current admission requiring mechanical or non-invasive ventilation.
Recruitment strategy and baseline measurements
Research assistants (RAs) will assess children for eligibility 5 days in a week (Monday to Friday) between 8:00 and 18:00. Recruitment on Saturday and Sunday is permitted if feasible. We will implement the intervention during daytime hours to simulate anticipated practice. Baseline characteristics and covariates, including those known to be associated with the LOS will be collected prior to randomisation: age, sex, history of atopy, parental cigarette smoking, treatments prior to randomisation (antibiotics, salbutamol, nebulised epinephrine, steroids, intravenous fluids, nasogastric feeds), feeding adequacy, oxygen supplementation and respiratory rate at time of randomisation, and duration from hospital admission to randomisation.
Interventions
The target oxygen saturation for oxygen supplementation will be the same for both groups at sites—90%. Sites that also permit an acceptable oxygen saturation of greater than or equal to 88% while children are asleep (as indicated in their bronchiolitis CPG, order sets or usual practice) will continue with that practice, in keeping with a pragmatic trial. The target oxygen saturations are based on recommendations from local CPGs, society guidelines and a trial.1 24 Nurses will measure vital signs in every 4 hours.
Intermittent oxygen saturation monitoring group
Oxygen saturation and vital signs will be measured intermittently at a frequency of every 4 hours by the bedside nurse through the child’s hospital stay until discharge. Weaning of oxygen (ie, when to wean oxygen and by how much) is at the discretion of the attending physicians and nurses and will occur at the 4 hourly time interval. Weaning oxygen more frequently than at the 4 hours usual spot check is permitted. Nurses can perform an additional spot check following the oxygen wean.
Continuous oxygen saturation monitoring group
Oxygen saturation will be measured continuously through the child’s hospital stay until discharge. Weaning of oxygen will be as usual practice and will be left to the discretion of the attending physicians and nurses.
Criteria and procedures for discontinuing or modifying allocated intervention
In our pilot RCT, no modifications to the allocated intervention occurred. However, the following criteria will be available for converting the group allocation of intermittent monitoring to continuous monitoring: severe tachypnoea, tachycardia, apnoea and clinical deterioration as assessed by the attending medical team. The infant will be converted back to intermittent monitoring when deemed clinically stable by the attending medical team.
Strategies to improve adherence
A multifaceted approach will be taken to support implementation of the trial and adherence to the allocated arms. Leadership support for the trial will be obtained from nursing and physician leaders and communicated to the clinical staff. Tailored education for nurses and physicians, including resident physicians, will occur before and during the trial using a variety of methods (eg, small group sessions, distribution of reference material including pocket cards). Key local opinion leaders for nurses and physicians were engaged in the trial concept and design and will provide support at sessions. RA and nurse educators will provide one-on-one support for nurses and physicians participating in the trial.
Concomitant care
In keeping with a pragmatic trial design, all infants will receive standard care for bronchiolitis. A care map has been adapted from the site CPGs and order sets which were based on the AAP guidelines and recent systematic reviews.
Outcomes
Study outcomes include measures of effectiveness, acceptability of the interventions, safety and cost.
Primary outcome
Length of hospital Stay from randomisation on the inpatient unit to discharge from hospital (hours). Length of hospital stay was chosen as the primary outcome as it represents a clinically meaningful outcome in the context of this acute illness for families and clinicians.2 It is important to hospital administrators and the healthcare system as hospital stay accounts for a major portion of the large costs associated with bronchiolitis.25 It has also been used as the primary outcome in other trials in inpatient management of bronchiolitis.16 26 27
Secondary outcomes
Duration of oxygen supplementation from randomisation to discontinuation of supplementation (hours) will be measured from the medical record.
Medical interventions
performed from time of randomisation to discharge: (1) Chest X-ray (yes/no) (2) Number of blood samples drawn and blood tests (3) Nasopharyngeal tests for viruses (yes/no) (4) Blood culture (yes/no) (5) Number of bronchodilator treatments used (6) steroid administration (yes/no) (7) Number of times the nasal passage (or deeper) was suctioned (8) Intervention fluids initiated (yes/no) and duration (9) nasogastric feeds initiated (yes/no) and duration.
Time from randomisation to meeting discharge criteria (hours)
This will be assessed two times per day (9:00 hours and 16:00 hours) by a RA and defined as: no fever (temperature <38°C), no supplemental oxygen, normal respiratory rate for age (using WHO age-specific criteria (<50 breaths/min for 2–12 months, <40 breaths/min for 1–5 years)) and adequate feeding (defined as a feeding adequacy score of ≥7 on a 10 cm Visual Analogue Scale (VAS) feeding adequacy scale).
Length of hospital stay from triage in the emergency department
This will be defined as the length of time (measured in hours) from triage in the emergency department to discharge from hospital. This has been chosen as a secondary outcome and not a primary outcome as the length of time from triage to transfer to the GPIU will not be influenced by the intervention.
Parent anxiety
Parents will be asked to rate their level of anxiety at the current time (state anxiety) and generally (trait anxiety) every 24 hours, using two questions abstracted from the adult State Trait Anxiety Inventory28: ‘I feel at ease’ (state, right now); ‘I am a steady person (trait, generally). Response options are: not at all (1); somewhat (2); moderately so (3); very much so (4).
Number of parent work days missed from randomisation to 15 days after discharge
The RA will conduct telephone follow-up with the parent.
Nursing satisfaction
The attending nurse will be asked to complete a 10 mm VAS to measure their satisfaction with the quality of monitoring for each participant two times per day (one by the day nurse and one by the night nurse).
PICU admission and consultation after randomisation
Unscheduled return to care within 15 days of discharge
Parents will be phoned after discharge to record the number of unscheduled visits to an emergency department, physician’s office or admission to hospital within 15 days of discharge. Fifteen days after discharge represents approximately 23 days from onset of symptoms and will capture the range of duration of symptoms for bronchiolitis.29 The electronic medical record will also be reviewed to determine any emergency department visits and any admissions to hospital and the reasons for the visit.
Mortality
We will include mortality from any cause during the hospitalisation and up to 15 days from discharge.
Cost-effectiveness
We will perform a cost-effectiveness analysis to determine the incremental costs (or savings) of intermittent compared with continuous oxygen saturation monitoring per change in hospital LOS (in hours). We will take both a healthcare system and societal perspective. As there is no anticipated difference in long-term clinical outcomes from this condition or the intervention, our time horizon will be from admission to 15 days postdischarge.29 All costs, parameter estimates and ranges will be derived from study data. Standardised methods for the conduct of health economic evaluations will be followed.
Adherence to assigned intervention group
Adherence rate (proportion) and reasons for modifications will be reported for each group.
Assignment of interventions
Allocation
The allocation sequence will be generated using computer-generated random numbers by the trial biostatistician. Randomisation will be stratified by centre. An allocation ratio of 1:1 with random permuted blocks of varying size will be used within centre. Allocation concealment will be achieved by using a central randomisation system using the Research Electronic Data Capture Software (REDCap) randomisation module. The site RA will confirm eligibility and obtain consent; then they will obtain the participant group assignment through the REDCap application.
Blinding
Statisticians and investigators will be blinded to the group allocation during the data analysis. Parents, attending nurses, physicians and research personnel involved with data collection will not be blinded to the group allocation. It is important that the clinicians receive the allocated monitoring strategy with fidelity (eg, are aware that monitoring is intermittent and that they will not receive saturation readings more frequently) as we are interested in determining if the oxygen monitoring strategy affects their behaviour and management decisions. By taking this pragmatic approach, our estimates of effectiveness will be more applicable to usual care settings.30 31
Data collection methods
The RAs will be embedded in each inpatient unit and will collect data.
Health service utilisation and cost data
At the end of the trial, decision support at each of the study sites will provide individual case costing for each participant’s hospitalisation for the index admission. Direct out-of-pocket costs of caregivers/parents and productivity losses will be obtained directly from caregivers. A custom data collection form has been developed to measure these costs and losses on discharge. It will be administered to participants in both arms of the trial and can be self-administered or collected via interview with the RA. Any additional healthcare utilisation, out-of-pocket expenses and productivity losses incurred in the 15 days after discharge will be obtained by the RA at the follow-up call.
Data management
The Ontario Child Health Support Unit at SickKids and CHEO (oschu.ca) will serve as the trials and data management centre. REDCap software will be used for data management.
Data monitoring
A Data Monitoring Committee was deemed not to be necessary by Research Ethics Board (REB). There will be no interim analysis or plans for early trial termination.
Statistical methods
Sample size
Sample size and recruitment duration
The primary outcome is length of hospital stay from time of randomisation on the GPIU to discharge. Assuming a median length of hospital stay from randomisation to discharge of 36 hours (from pilot data, published trials), a type 1 error rate of 0.05 (two sided), power (1-β) of 90%, 105 subjects per group is needed to detect a clinically significant difference of 12 hours. There will be no adjustment due to loss to follow-up as this outcome is assessed in hospital. We believe that a 12-hour difference between treatment groups is a clinically meaningful difference, based on consensus with our research team, hospital administrators and clinical experts.
Based on administrative data, there are approximately 415 bronchiolitis admissions per year in total at the six sites. Approximately, 40% will not meet the eligibility criteria and of these 30% will not be recruited due to off-season presentation (May to November) or missed, leaving 174 admissions. Assuming a conservative recruitment rate of 70% (based on pilot study), we expect approximately 120 recruited patients per season. Thus, two 6 month seasons, each from mid-November to mid-May, will be needed to recruit the 210 subjects. This seasonal definition of November to May will capture the peak months of respiratory viral infections responsible for bronchiolitis.32
Statistical analysis
Primary outcome
Data will be analysed according to intention-to-treat principles for the primary outcome. Given that the primary and most secondary outcomes are obtained during hospitalisation, and mortality is rare, it is anticipated that there will be no missing data. For the outcomes measured after discharge (readmissions and parental work days missed), outcomes with the available data and lost to follow will be reported.
The primary outcome, length of hospital stay (hours) from randomisation on the inpatient unit to discharge, will be described as the ratio of the two medians with the 95% CIs. Kaplan-Meier-type survival curves will be graphed for both treatment arms. Since no censoring is anticipated, the arms will be compared using a Wilcoxon rank-sum test. Since each site will follow one of two oxygen saturation targets for all their patients, as per their usual practice (≥90% awake and asleep OR ≥90% awake and 88% asleep), a treatment by target interaction will be tested to see if the treatment effect differs between targets.
Secondary outcomes
To control for multiple testing, the statistical level for significance for the secondary outcomes will be set to 0.005, two sided. For the time-to-event outcomes (oxygen supplementation, discharge criteria) a Wilcoxon rank-sum test will be applied. For count data (interventions), a Poisson model will be applied. For continuous data (parent anxiety, nursing satisfaction), a normal model for repeated observations will be applied. For binary data (PICU admission, unscheduled readmission, mortality, adherence), a Fisher’s exact test will be applied.
Cost-effectiveness analysis
For the cost-effective analysis costs will be adjusted for inflation and reported in Canadian dollars. Cost-effectiveness will be expressed as an incremental cost-effectiveness ratio (ICER), calculated by dividing the incremental costs between intermittent and continuous oxygen saturation monitoring by the incremental difference in hospital LOS.33 34 Extensive sensitivity analyses will be performed to evaluate the robustness of the results and evaluate uncertainty in assumptions. Deterministic one-way sensitivity analysis will be performed with all variables using ranges obtained from the 95% CIs generated directly from study data. Probabilistic sensitivity analysis will also be performed to establish a point estimate and 95% CI around the ICER.
Patient and public involvement
Patients and the public were not directly involved in the development of the study (ie, research question, outcomes choice, study design, recruitment, assessment of burden of interventions). Outcomes chosen include those reported as a priority to patients as noted in the literature.2 35 Furthermore, we conducted a pilot study to ensure that trial processes were feasible and acceptable from a patient perspective. Study results will be disseminated to the public through social media.
Ethical and dissemination
We received approval from the REB at all sites. Written informed consent will be obtained from each participant by the site research staff. Identifiable personal health information will not be uploaded to the REDCap database. Protocol amendments will be approved by REB prior to implementation of protocol changes. All study investigators will have access to the final trial dataset. The International Committee of Medical Journal Editors authorship eligibility guidelines will be used for publications. End of study dissemination activities will be conducted locally to clinical groups and incorporated into site CPGs; findings will be presented through webinars and society meetings (eg, the Paediatric Academic Society, AAP Paediatric Hospital Medicine meetings, Canadian Paediatric Society), and through social media. We anticipate publication of findings in a general medical or paediatric journal. We will work with knowledge users to incorporate the study findings into professional society practice guidelines.
Discussion
Bronchiolitis is one of the most common reasons for hospitalisation in infants in the developed world and accounts for significant healthcare costs. The use of pulse oximetry has become common practice in hospitalised infants, however, there is no RCT evidence on how to best use this technology in this practice context. The overall goal of our pragmatic RCT is to determine whether intermittent versus continuous pulse oximetry results in a shorter length of hospital stay in infants with a stable clinical status hospitalised with bronchiolitis. Secondary outcomes include nursing satisfaction with monitoring, parental anxiety and days missed from work, and outcomes related to safety (ICU consultation and admission, revisits after discharge, and mortality).
Several aspects of this trial are important to highlight. First, our inclusion criteria were specifically designed to include infants who are in the stable phase of their illness during hospitalisation and exclude infants at higher risk of deterioration. We took this conservative approach to maximise safety and promote acceptance of clinicians to the intermittent monitoring intervention. Second, infants who are on supplemental oxygen and have a stable clinical status are eligible for randomisation. Third, we are using the same target oxygen saturation in both groups. Fourth, it is important to take a multifaceted approach to supporting this practice change to ensure adherence to the allocated arm and success of the trial. We have obtained support from clinical leadership, including nursing, physicians, respiratory therapists and hospital administrators. We will also target groups using opinion leaders using small group sessions and support front line clinicians.
We took the approach of not blinding clinicians and parents to the allocated monitoring strategy in this trial for several reasons. First, it is important to simulate the monitoring strategy intended with fidelity. The act of continuous or intermittent monitoring of oxygen saturation may alter the clinical assessments of treating nurses and physicians and their decisions regarding oxygen use and need for additional days of hospitalisation as well as parental perceptions of their child’s health. For example, previous researchers have suggested that continuous oxygen saturation monitoring results in over-reliance in technology and under-reliance of clinical assessment, which leads to over use of oxygen and longer hospital stay. Thus, we are interested in understanding if knowledge of treatment arm affects clinician behaviour and decisions around oxygen use and LOS, assuming the same target oxygen saturation of 90% in both groups. By taking this approach, our estimates of effectiveness will be more applicable to usual care settings. In pragmatic trials, it has been suggested that non-blinded treatment and assessment of clinical outcomes may be important for the preservation of the ‘ecology of care’, since blinding may have a significant effect on patients’ experience.30 31 Further, the inclusion of objective outcome measures may reduce the potential for bias resulting from patients’ expectations about the effectiveness of each treatment. Our primary outcome measure is an objective measure of length of hospital stay. Second, although methods are available to blind group assignment in monitoring trials (eg, providing a non-true continuous reading in between intermittent oximetry spot checks), this would ostensibly result in comparing two continuous monitoring arms. Third, as we are also measuring discharge readiness as a secondary outcome (defined by the child’s clinical status), we will be able to assess differences between both arms in discharge readiness and total LOS.
Supplementary Material
Footnotes
Contributors: SM conceived and designed the study and drafted the first version of the manuscript. GW conceived the study and participated in the design and manuscript revisions. PCP conceived and designed the study and revised the manuscript. LG, CP, RK, AB, MR, MS, NK, KB-R, ML, LP, MEM, ARW and SS participated in the design of the study and manuscript revisions. All authors read and revised the manuscript critically for important intellectual content and approved the final manuscript.
Funding: This pilot study was funded by a peer-reviewed grant from the Paediatric Consultants Partnership, Hospital for Sick Children. It was also supported by the Paediatric Outcomes Research Team, Hospital for Sick Children, which receives funding from the SickKids Foundation, Toronto. The multicentre trial is funded by a grant from the Canadian Institutes of Health Research (funding reference number PJT-148635).
Disclaimer: Funders were not involved in any way in the design, collection, analysis, interpretation of data, writing of the manuscript or the decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Research Ethics Board Hospital for Sick Children, Toronto, Canada.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134:e1474–e1502. 10.1542/peds.2014-2742 [DOI] [PubMed] [Google Scholar]
- 2. Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368:312–22. 10.1016/S0140-6736(06)69077-6 [DOI] [PubMed] [Google Scholar]
- 3. Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999;282:1440–6. 10.1001/jama.282.15.1440 [DOI] [PubMed] [Google Scholar]
- 4. Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med 2012;166:1155–64. 10.1001/archpediatrics.2012.1266 [DOI] [PubMed] [Google Scholar]
- 5. Spurling GK, Doust J, Del Mar CB, et al. Antibiotics for bronchiolitis in children. Cochrane Database Syst Rev 2011:CD005189 10.1002/14651858.CD005189.pub3 [DOI] [PubMed] [Google Scholar]
- 6. Hartling L, Bialy LM, Vandermeer B, et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev 2011:CD003123 10.1002/14651858.CD003123.pub3 [DOI] [PubMed] [Google Scholar]
- 7. Gadomski AM, Brower M. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2010:CD001266 10.1002/14651858.CD001266.pub2 [DOI] [PubMed] [Google Scholar]
- 8. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev 2010:CD004878 10.1002/14651858.CD004878.pub3 [DOI] [PubMed] [Google Scholar]
- 9. Hartling L, Fernandes RM, Bialy L, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ 2011;342:d1714 10.1136/bmj.d1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics 2011;128:740–52. 10.1542/peds.2011-0271 [DOI] [PubMed] [Google Scholar]
- 11. Schroeder AR, Marmor AK, Pantell RH, Pantell MD, et al. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med 2004;158:527–30. 10.1001/archpedi.158.6.527 [DOI] [PubMed] [Google Scholar]
- 12. Unger S, Cunningham S. Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis. Pediatrics 2008;121:470–5. 10.1542/peds.2007-1135 [DOI] [PubMed] [Google Scholar]
- 13. Mallory MD, Shay DK, Garrett J, et al. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics 2003;111:e45–e51. 10.1542/peds.111.1.e45 [DOI] [PubMed] [Google Scholar]
- 14. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA 2014;312:712–8. 10.1001/jama.2014.8637 [DOI] [PubMed] [Google Scholar]
- 15. Vinci R, Bauchner H. Bronchiolitis, deception in research, and clinical decision making. JAMA 2014;312:699–700. 10.1001/jama.2014.8638 [DOI] [PubMed] [Google Scholar]
- 16. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr 2015;169:898–904. 10.1001/jamapediatrics.2015.1746 [DOI] [PubMed] [Google Scholar]
- 17. Cunningham S. Intermittent monitoring of oxygen saturation in infants and children with acute bronchiolitis: peekaboo pediatrics or good clinical care? JAMA Pediatr 2015;169:891–2. 10.1001/jamapediatrics.2015.1971 [DOI] [PubMed] [Google Scholar]
- 18. Chopra V, McMahon LF. Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA 2014;311:1199–200. 10.1001/jama.2014.710 [DOI] [PubMed] [Google Scholar]
- 19. Cvach M. Monitor alarm fatigue: an integrative review: Biomedical Instrumentation and Technology, 2010:268–77. [DOI] [PubMed] [Google Scholar]
- 20. The Joint Commission. The joint commission announces 2014 national patient safety goal. http://www.jointcommission.org/assets/1/18/jcp0713_announce_new_nspg.pdf (accessed 20 Mar 2015). [PubMed]
- 21. Coon ER, Quinonez RA, Moyer VA, et al. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics 2014;134:1013–23. 10.1542/peds.2014-1778 [DOI] [PubMed] [Google Scholar]
- 22. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47–E57. 10.1503/cmaj.090523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet 2015;386:1041–8. 10.1016/S0140-6736(15)00163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langley JM, Wang EE, Law BJ, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a pediatric investigators collaborative network on infections in Canada (PICNIC) study. J Pediatr 1997;131:113–7. 10.1016/S0022-3476(97)70133-1 [DOI] [PubMed] [Google Scholar]
- 26. Patel H, Gouin S, Platt RW. Randomized, double-blind, placebo-controlled trial of oral albuterol in infants with mild-to-moderate acute viral bronchiolitis. J Pediatr 2003;142:509–14. 10.1067/mpd.2003.196 [DOI] [PubMed] [Google Scholar]
- 27. Kuzik BA, Al-Qadhi SA, Kent S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr 2007;151:266–70. 10.1016/j.jpeds.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 28. Spielberger CD, Gorssuch RL, Lushene PR, et al. Manual for the state-trait anxiety inventory: Consulting Psychologists Press, Inc, 1983. [Google Scholar]
- 29. Petruzella FD, Gorelick MH. Duration of illness in infants with bronchiolitis evaluated in the emergency department. Pediatrics 2010;126:285–290. 10.1542/peds.2009-2189 [DOI] [PubMed] [Google Scholar]
- 30. Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ware JH, Hamel MB. Pragmatic trials — guides to better patient care? N Engl J Med Overseas Ed 2011;364:1685–7. 10.1056/NEJMp1103502 [DOI] [PubMed] [Google Scholar]
- 32. Moineddin R, Nie JX, Domb G, et al. Seasonality of primary care utilization for respiratory diseases in Ontario: a time-series analysis. BMC Health Serv Res 2008;8:160 10.1186/1472-6963-8-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ontario Case Costing Initiative. Ontario ministry of health and long-term care. 2015. http://www.occp.com/mainPage.htm.
- 34. Health Data Branch Web Portal. OntaRio ministry of health and long-term care. 2015. https://hsimi.on.ca/hdbportal/.
- 35. Dyson MP, Shave K, Gates A, et al. Which outcomes are important to patients and families who have experienced paediatric acute respiratory illness? Findings from a mixed methods sequential exploratory study. BMJ Open 2017;7:e018199 10.1136/bmjopen-2017-018199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.