Abstract
Objectives
To study the association between maternal caffeine intake during pregnancy and the child’s weight gain and overweight risk up to 8 years.
Design
Prospective nationwide pregnancy cohort.
Setting
The Norwegian Mother and Child Cohort Study.
Participants
A total of 50 943 mothers recruited from 2002 to 2008 and their children, after singleton pregnancies, with information about average caffeine intake assessed at mid-pregnancy.
Outcome measure
Child’s body size information at 11 age points from 6 weeks to 8 years. We defined excess growth in infancy as a WHO weight gain z-score of >0.67 from birth to age 1 year, and overweight according to the International Obesity Task Force. We used a growth model to assess individual growth trajectories.
Results
Compared with pregnant women with low caffeine intake (<50 mg/day, 46%), women with average (50–199 mg/day, 44%), high (≥200–299 mg/day, 7%) and very high (≥300 mg/day, 3%) caffeine intakes had an increased risk of their child experiencing excess growth in infancy, after adjustment for confounders (OR=1.15, 95% CI 1.09 to 1.22, OR=1.30, 95% CI 1.16 to 1.45, OR=1.66, 95% CI 1.42 to 1.93, respectively). In utero exposure to any caffeine was associated with higher risk of overweight at age 3 years and 5 years, while the association persisted at 8 years, only for very high exposures. Any caffeine intake was associated with increased body mass index from infancy to childhood. Children prenatally exposed to caffeine intake >200 mg/day had consistently higher weight. Very high caffeine exposures were associated with higher weight gain velocity from infancy to age 8 years.
Conclusion
Any caffeine consumption during pregnancy is associated with a higher risk of excess infant growth and of childhood overweight, mainly at preschool ages. Maternal caffeine intake may modify the overall weight growth trajectory of the child from birth to 8 years. This study adds supporting evidence for the current advice to reduce caffeine intake during pregnancy.
Keywords: epidemiology, preventive medicine, public health, social medicine
Strengths and limitations of this study.
A strength of this study is the large sample size.
Maternal caffeine intake was estimated from all possible food sources.
This is the first study investigating the association between maternal caffeine intake and excess infant growth and growth velocity.
Missing data from body size measurements were handled with a growth model.
Limitations include self-reported dietary data and parental-reported measurements of height and weight after 2 years.
Introduction
Caffeine is the world’s most widely consumed central nervous system stimulant. It occurs naturally or is added to foods and beverages, with coffee and tea as the most common and major sources.1 After ingestion, caffeine is readily absorbed into the bloodstream and distributed to the tissues. It is metabolised in the liver by the microsomal cytochrome P450.2 During pregnancy, elimination of caffeine is prolonged and it rapidly passes all biological membranes, including the blood-brain and placenta barriers, resulting in exposure of the fetus.3 A maximum intake level of caffeine for pregnant women has been stipulated by several authorities, most of which agree that it should not exceed 200 mg/day, based on the evidence of its adverse effects on miscarriage rates and fetal growth restriction.1 4 The negative effects of caffeine consumption during pregnancy on fetal growth have been well documented in epidemiological studies, including the Norwegian Mother and Child Cohort Study (MoBa).5 In a recent meta-analysis, the highest, compared with the lowest, maternal caffeine intake level was associated with a 38% increased risk of low birth weight (<2.5 kg).6
Fetal growth and growth in infancy are important determinants for the development of obesity and for long-term cardiometabolic health.7–9 Excess infant growth programmes later obesity, fat mass and risk of adult disease, independent of intrauterine growth.10–15 The prevalence of metabolic disorders, including obesity, cardiovascular disease and type 2 diabetes is rapidly growing across the globe, with the number of obese people risen worldwide from 105 million in 1975 to 641 million in 2014.16 This trend indicates that the probability of reaching the WHO global obesity target, of no rise in obesity by 2025, is close to zero.16 There is compelling human and animal evidence supporting the ‘fetal programming’ hypothesis, according to which in utero exposures permanently alter an organism’s physiology and metabolism, leading to susceptibility to subsequent disease, including obesity and metabolic disorders, with transgenerational effects.17 18
In utero exposure to caffeine has been related to an increased risk of overweight and higher body fat in childhood, in two previous epidemiological studies.19 20 However, the link between in utero caffeine exposure and excess growth in infancy is yet to be studied, even though excess infant growth is an established risk factor in the aetiology of obesity and cardiometabolic disease.13 15 21 22
Based on our previous findings on the association of prenatal caffeine exposure with fetal growth restriction5 and the fetal programming hypothesis,23 we hypothesised that prenatal caffeine exposure might affect postnatal growth. Thus, the objective of this study was to investigate the associations between maternal caffeine intake in pregnancy and child growth and risk of overweight up to age 8 years in a large prospective population-based cohort.
Methods
Study population
Our study was conducted within the Norwegian Mother and Child Cohort Study (MoBa), a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health.24 Pregnant women from all over Norway were recruited during 1999–2008 and 40.6% of the invited women consented to participate. The cohort now includes 114 500 children, 95 200 mothers and 75 200 fathers. Follow-up of the participants, after delivery, have been conducted at 6 months, 18 months, 36 months, 5 years, 7 years and 8 years. Data used in this study are based on V.8 of the quality-assured data files, released for research in February 2014, with linkage to the Medical Birth Registry of Norway. The data collection in MoBa was licensed by the Norwegian Data Inspectorate. All MoBA participants have provided a signed consent form.
After exclusion of multiple gestations, stillbirths, malformations and chromosomal abnormalities, 96 875 live-born singletons remained. Of these, 78 819 pregnant women had answered the Food Frequency Questionnaire (FFQ) developed and validated for MoBa and in use from 2002 and onwards. The eligible study population, with available information on maternal caffeine intake and all relevant covariates, constituted 62 034 mother-child pairs. Our final study population consisted of 50 943 mother-child pairs with additional information on small for gestational age (SGA) and at least one postnatal measurement of weight or length/height. The cohort retention is presented in online supplementary table 1. After 5 years, approximately 40% of the study population returned the questionnaire and had information on weight and height, while the distribution of mothers by caffeine intake level did not differ by follow-up age, meaning that loss to follow-up was not related to maternal caffeine intake in pregnancy.
bmjopen-2017-018895supp001.pdf (403.3KB, pdf)
Maternal caffeine intake during pregnancy
Maternal caffeine intake estimation in MoBa has been described in detail previously by Sengpiel et al.5 In brief, self-reported intake of 255 dietary items was assessed at pregnancy week 22 with a FFQ developed and validated for MoBa.25 This is a semiquantitative FFQ designed to record dietary habits during the first 4–5 months of gestation. Average, daily caffeine intake was calculated as the aggregated intake (in mg/day) from all available sources, including several types of coffee, black tea, caffeinated soft drinks, energy drinks, chocolate, chocolate milk, and sandwich spread, desserts, cakes and sweets containing cocoa. Online supplementary table 2 includes more details on the estimation of maternal caffeine intake. The median (25th−75th centiles) caffeine intake was 57 mg/day (23–120 mg/day) for the included population and 64 mg/day (25–129 mg/day) for the non-included population with available caffeine information (n=11 091 mothers) (P<0.001 for Mann-Whitney test). We categorised caffeine intake, based on the calculated median as well as national and international recommendations for caffeine consumption during pregnancy, in four levels of caffeine intake: low (0–49 mg/day), average (50–199 mg/day), high (200–299 mg/day) or very high (≥300 mg/day).
Child postnatal growth and overweight
Anthropometric data
Weight and length/height measurements at eleven age points (6 weeks, 3 months, 6 months and 8 months and 1 year, 1.5 years, 2 years, 3 years, 5 years, 7 years and 8 years) were reported. Up to 18 months the reported measurements were as documented in the child’s health card, while for measurements from 2 years to 8 years no specification was provided. Implausible anthropometrics were identified and excluded by separately implementing three different methods: (1) by comparing with the WHO Growth Standards, as a weight-for-age or height-for-age z-score <6 SD below or >6 SD (5 SD for weight) above the mean,26 (2) by identifying measured values with a >|5SD| difference from the predicted value as derived from the Jenss-Bayley growth curve model, and (3) by the conditional growth centiles.27 After exclusion of implausible values, 464 343 and 452 980 measurements of weight and height/length were reported for our study population. Seven repeated measurements per child were available on average, for both anthropometrics. More details on anthropometric measurements are presented in online supplementary table 1.
Outcomes
First, we assessed excess infant weight gain by calculating the difference in gender-adjusted WHO weight-for-age z-scores between birth and age 1 year, using reported weights.26 A z-score gain of >0.67 represents an upward crossing of the centile line,28 referred to as excess growth.29
Second, we determined childhood overweight, including obesity, at two preschool-age (3 years and 5 years) and one school-age (8 years) time points, using the International Obesity Task Force criteria.30 Used body mass index (BMI) cut-offs and overweight prevalences are presented in online supplementary table 3.
BMI was derived by growth models. Individual growth trajectories for weight and length/height were obtained by modelling the overall growth from age 1 month to age 8 years, using the Jenss-Bayley growth curve model, a structural growth model based on a basic functional form of growth. This four-parameter, non-linear model is suitable for describing growth of both weight and length/height during infancy and early childhood, up to age 8 years,31 before growth starts to accelerate again at puberty. To assess individual growth trajectories, we applied a mixed-effect approach using the stochastic approximation of expectation-maximisation (SAEM) algorithm.32 33 We then calculated weight and length/height, BMI (weight (kg) divided by squared height (m)), as well as weight and height gain velocities at 14 age points (1 month, 2 months, 3 months, 6 months, 9 months, 12 months and 18 months and 2 years, 3 years, 4 years, 5 years, 6 years, 7 years and 8 years), using the growth model derivatives. These predicted anthropometrics were also assessed as outcomes.
As including birth weight in the model may influence the estimated trajectories, and in order to assess the effect of caffeine on early growth independently of its effect on birth size,5 we did not include birth weight and length in the growth models.
Statistical analysis
We used logistic regression models to examine associations between maternal caffeine intake in categories and excess growth in infancy and childhood overweight. Low caffeine intake (0–49 mg/day) was the reference group. Similar analysis was performed after modelling caffeine by restricted cubic splines with four knots at centiles 5, 35, 65 and 95, as recommended by Harrell,34 and corresponding to caffeine intakes of 6 mg/day, 34 mg/day, 91 mg/day and 253 mg/day, respectively. The reference level of caffeine intake was set at 50 mg/day, corresponding to the median intake in our study population. The associations were described graphically. Finally, we used mixed-effect linear regression models with random intercept by child and a random slope for age to analyse associations between predicted weight, length/height, BMI, weight and height gain velocities from ages 1 month to 8 years (14 age points: 1 month, 2 months, 3 months, 6 months, 9 months, 12 months and 18 months and 2 years, 3 years, 4 years, 5 years, 6 years, 7 years and 8 years). Covariates’ effects have been models as fixed in the mixed-effect models. All regression models were adjusted for random effects of sibling clusters since some mothers participated with more than one pregnancy.
Logistic and linear mixed models were adjusted for variables related to both maternal caffeine intake and excess growth by bivariate analysis: maternal age, maternal education, parity, prepregnancy BMI, paternal BMI, maternal and paternal smoking during pregnancy, maternal energy intake and nausea/vomiting during pregnancy. Gestational age and child’s gender were also included in the models as a priori covariates (online supplementary table 4). Maternal height, paternal weight, paternal alcohol consumption and gestational diabetes (yes/no) were also considered but not included in the final models as they did not meet the criteria. Our main analysis consists of complete case analysis of 38 338 mother-child pairs for the risk of excess growth and of 50 943 mother-child pairs for all other growth outcomes. The cohort attrition due to loss to follow-up was addressed by the use of predicted anthropometric measurements. The correlation between measured and predicted anthropometrics ranged from 0.85 to 0.99 for weight and from 0.95 to 0.98 for length/height (data not shown).
In separate sensitivity analyses, (1) we excluded SGA neonates (SGA was defined as birth weight below the 10th centile, according to population curves as described by Skjaerven et al 35), (2) we excluded smokers during pregnancy, (3) we adjusted for birth weight; only the overweight models and not the excess growth model, because birth weight is included in the excess growth calculation formula, (4) we explored caffeine intake by three main sources (ie, from black coffee, black tea and soda drinks), (5) we excluded very high caffeine consumers and (6) we assessed the association between maternal caffeine intake and childhood overweight, using the measured instead of predicted anthropometric data to define the outcome. Possible interactions with SGA and birth weight were tested with all studied outcomes. Since the associations between the outcomes and the interaction terms were not significant and the inclusion of the interaction term did not modify our results, we have not included these analyses in the manuscript.
Finally, we performed negative control analysis, using paternal caffeine intake as the negative control. Negative control analysis is a suggested method to test for the possibility of unmeasured confounding. We have assumed that there is no direct association between the father’s exposure during the pregnancy period and the child’s outcome, and that the shared confounders are equally associated with the mother’s and the father’s exposures.36 37 We have calculated the caffeine intake of the father using the caffeine concentrations and serving sizes as used for the mother’s calculations (online supplementary table 2) for five food items: filtered coffee, boiled coffee, espresso coffee, caffeinated soft-drink with sugar or artificially sweetened. Only 16 455 (32%) fathers had available information.
The main analyses were performed with the Stata V.14 statistical software (Stata Corporation, College Station, Texas, USA) and R V.3.2.238 was used for the growth models.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Lifestyle and sociodemographic characteristics related to maternal caffeine intake during pregnancy
In our study population, 7.13% (n=3633) and 3.21% (n=1634) of women reported caffeine intake higher than 200 mg/day and 300 mg/day, respectively. The distribution of women not included in the analysis, by caffeine intake level, was similar to the included (low: 43%, average: 46%, high: 8% and very high: 3%). The higher the caffeine intake, the higher the likelihood of a mother being older than 30 years, being multiparous, having a daily energy intake in the upper tertile, being a smoker during pregnancy and not suffering nausea and/or vomiting during pregnancy. Moreover, women with very high caffeine intake were more likely to have low education, have been obese before pregnancy and have partners who were obese and smokers, compared with those consuming less caffeine per day (online supplementary table 4).
Paternal median (5th−95th centiles) intake was 193 mg/day (0–493 mg/day), with caffeine from coffee being the main contributor (median: 187 mg/day). Fathers were consuming statistical significantly more caffeine than their partners (P<0.001 for Wilcoxon matched-pairs signed-ranks test). The spearman correlation coefficient between maternal and paternal caffeine intakes was 0.15 (P value <0.0001). However, paternal intake was increasing by increasing levels of maternal intake and 45% of mothers with very high intake were with partners in the highest quartile of caffeine intake (online supplementary table 4).
Prenatal caffeine exposure and excess growth in infancy
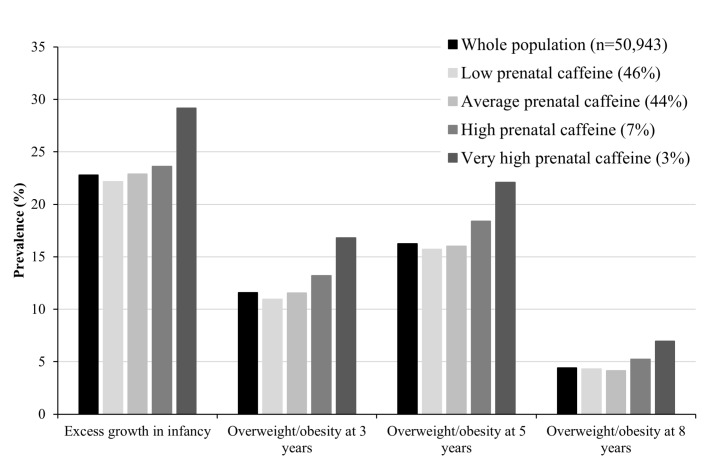
The prevalence of excess growth in infancy increased from 23% to 29% as prenatal caffeine intake increased from low to very high (figure 1). After adjustment for confounders, children born to average, high and very high caffeine consumers had 1.15 (95% CI 1.09 to 1.22), 1.30 (95% CI 1.16 to 1.45) and 1.66 (95% CI 1.42 to 1.93) higher odds of excess growth in infancy, compared with children born to low consumers (table 1). Neither exclusion of mothers who smoked during pregnancy or SGA neonates modified the results. The positive association between caffeine intake as a continuous variable and the risk of excess growth in infancy was linear with no apparent threshold (online supplementary figure 1).
Figure 1.
Prevalence of excess growth in infancy and overweight/obesity at ages 3 years, 5 years and 8 years, in the whole population and by maternal caffeine intake during pregnancy.
Table 1.
Maternal caffeine intake in pregnancy and risk of excess growth in infancy (from birth to age 12 months)
| Maternal daily caffeine intake | Risk of excess growth in infancy (from birth to age 12 months)* | |||||
| All children (n=38 338) | After excluding smokers during pregnancy (n=35 672) | After excluding SGA neonates† (n=35 144) | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Low (<50 mg) | 1.00 | 1.00 | 1.00 | |||
| Average (50–199 mg) | 1.15 | 1.09 to 1.22 | 1.15 | 1.08 to 1.22 | 1.14 | 1.07 to 1.22 |
| High (200–299 mg) | 1.30 | 1.16 to 1.45 | 1.32 | 1.17 to 1.49 | 1.25 | 1.11 to 1.41 |
| Very high (≥300 mg) | 1.66 | 1.42 to 1.93 | 1.58 | 1.30 to 1.91 | 1.67 | 1.41 to 1.97 |
All models adjusted for maternal age, parity, parental education, prepregnancy BMI, total energy intake, nausea and/or vomiting during pregnancy, paternal BMI, parental smoking during pregnancy, gestational age and gender.
*Excess growth is defined as a WHO weight-for-age z-score difference >0.67 between birth and age 12 months.
†SGA according to Skjaerven et al. 35
BMI, body mass index; SGA, small for gestational age.
bmjopen-2017-018895supp002.pdf (569.8KB, pdf)
Prenatal caffeine exposure and overweight in childhood
The prevalence of overweight increased by 5% at age 3 years, by 6% at age 5 years and by 3% at age 8 years with increasing prenatal caffeine exposure from low to very high (figure 1). Children born to average, high and very high caffeine consumers had 1.05 (95% CI 0.99 to 1.12), 1.17 (95% CI 1.05 to 1.30) and 1.44 (95% CI 1.24 to 1.67) higher adjusted odds, respectively, for overweight at age 3 years, compared with children born to low caffeine consumers (table 2). Similar ORs were found at age 5 years, but at age 8 years the respective risk was significant only for the highest caffeine consumption category (adjusted OR (aOR) 1.29; 95% CI 1.04 to 1.61). Neither exclusion of mothers who smoked during pregnancy or SGA neonates modified the results. However, adjustment for birth weight slightly increased the odds (online supplementary table 5). We found a linear association between maternal caffeine consumption as a continuous variable and the risk of overweight at ages 3 years and 5 years with, again, higher OR at age 3 years than at age 5 years (online supplementary figure 2). As there was a high degree of concordance between overweight and/or obesity at ages 5 years and 8 years, the plot at age 8 years overlapped with the one at age 5 years and was not included in online supplementary figure 2.
Table 2.
Maternal caffeine intake in pregnancy and risk of overweight/obesity at age 3 years, 5 years and 8 years
| Risk of overweight and/or obesity* | ||||||
| All children (n=50 943) | ||||||
| Age 3 years | Age 5 years | Age 8 years | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Maternal daily caffeine intake | ||||||
| Low (<50 mg) | 1.00 | 1.00 | 1.00 | |||
| Average (50–199 mg) | 1.05 | 0.99 to 1.12 | 1.00 | 0.95 to 1.06 | 0.95 | 0.86 to 1.04 |
| High (200–299 mg) | 1.17 | 1.05 to 1.30 | 1.12 | 1.02 to 1.23 | 1.11 | 0.94 to 1.31 |
| Very high (≥300 mg) | 1.44 | 1.24 to 1.67 | 1.29 | 1.13 to 1.47 | 1.29 | 1.04 to 1.61 |
| After excluding smokers during pregnancy (n=47 036) | ||||||
| Low (<50 mg) | 1.00 | 1.00 | 1.00 | |||
| Average (50–199 mg) | 1.05 | 0.99 to 1.12 | 1.00 | 0.95 to 1.06 | 0.92 | 0.84 to 1.02 |
| High (200–299 mg) | 1.17 | 1.04 to 1.32 | 1.10 | 1.00 to 1.23 | 1.08 | 0.89 to 1.29 |
| Very high (≥300 mg) | 1.50 | 1.25 to 1.79 | 1.31 | 1.12 to 1.54 | 1.30 | 1.00 to 1.70 |
| After excluding SGA neonates (n=46 718)† | ||||||
| Low (<50 mg) | 1.00 | 1.00 | 1.00 | |||
| Average (50–199 mg) | 1.06 | 0.99 to 1.12 | 1.01 | 0.95 to 1.06 | 0.96 | 0.87 to 1.05 |
| High (200–299 mg) | 1.18 | 1.05 to 1.32 | 1.14 | 1.03 to 1.25 | 1.11 | 0.94 to 1.32 |
| Very high (≥300 mg) | 1.48 | 1.28 to 1.72 | 1.32 | 1.15 to 1.51 | 1.36 | 1.09 to 1.69 |
The same population was included at each age since the outcome was defined using model-derived anthropometrics.
All models adjusted for maternal age, parity, parental education, prepregnancy BMI, total energy intake, nausea and/or vomiting during pregnancy, paternal BMI, parental smoking during pregnancy, gestational age and gender.
*Overweight and/or obesity in children, according to the International Obesity Task Force definition.
†SGA according to Skjaerven et al. 35
BMI, body mass index; SGA, small for gestational age.
Sensitivity analyses
In sensitivity analyses, we found similar results concerning the association of caffeine from different caffeine sources (black coffee, black tea and soda drinks) with infant growth and overweight risk (online supplementary table 6). When excluding very high caffeine consumers and using no coffee drinkers as the reference group, caffeine intake less than 300 mg/day was still significantly associated with increased risk for excess infant growth and overweight (online supplementary table 7). Finally, when growth data from actual measurements were used to assess the relationship between maternal caffeine intake and overweight at these age points, similar trends and associations were observed (online supplementary table 8).
Prenatal caffeine exposure and growth up to 8 years
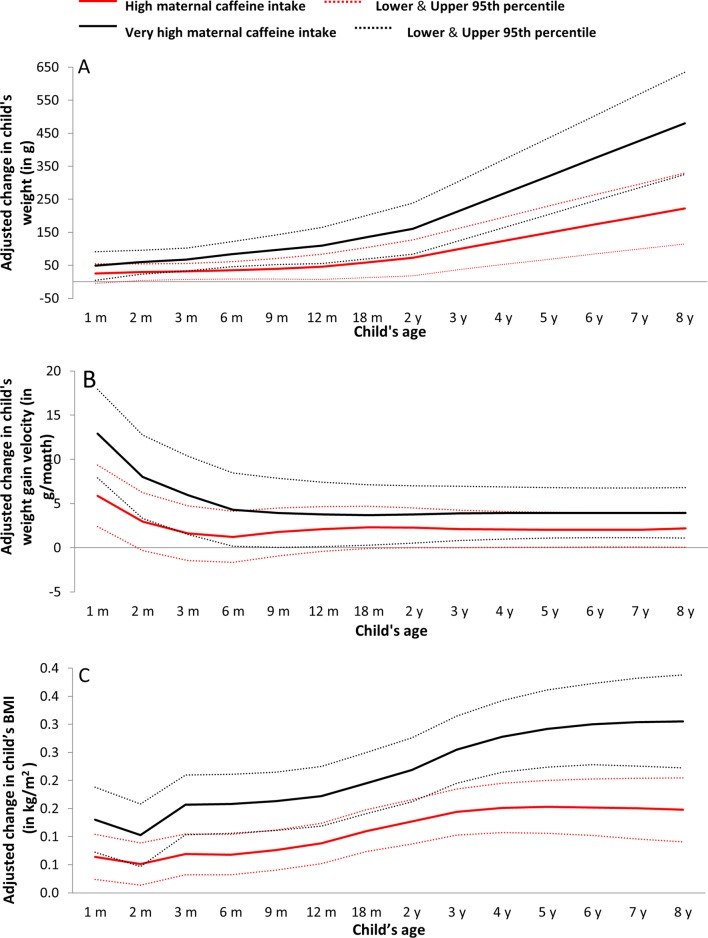
In comparison with low exposure, both high and very high prenatal caffeine exposure were positively associated with a child’s weight, weight gain velocity and BMI from the first month onwards (figure 2). More specifically, children prenatally exposed to very high caffeine levels weighed 67–83 g more in infancy (age 3–12 months), 110–136 g more in toddlerhood (age 12 months to 3 years), 213–320 g more at preschool age (age 3–5 years) and 480 g more at age 8 years, than children exposed to low caffeine levels (table 3). Maternal caffeine intake during pregnancy, at any level above 50 mg/day, was associated with higher BMI from ages 1 month to 8 years. Additional adjustment for birth weight provided better models (P value <0.05 for likelihood ratio test between models with and without birth weight) and the estimates from these models are presented in table 3. Whereas maternal caffeine intake during pregnancy was not associated with child height, it was related to higher height gain velocity up to age 3 months (online supplementary table 9).
Figure 2.
Maternal high (red) and very high (black) caffeine intake in pregnancy and adjusted change in children’s (A) weight (in g), (B) weight gain velocity (in g/month) and (C) body mass index (in kg/m2), from age 1 month to 8 years (β coefficients in solid lines and 95% CIs in thin dashed lines). Low caffeine intake is the reference group. m, months; y, years.
Table 3.
Maternal caffeine intake in pregnancy and child’s weight, weight gain velocity and BMI during childhood (n=50 943). Low caffeine intake is the reference group
| Maternal daily caffeine intake | Child’s developmental period | ||||||
| Infancy | Toddlerhood | Preschool age | School age | ||||
| 3 months | 6 months | 12 months | 18 months | 3 years | 5 years | 8 years | |
| β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| Weight (in g) | |||||||
| Average (50–199 mg) | 14.1 (1.6 to 26.6) | 15.1 (1.3 to 28.8) | 14.9 (−5.1 to 34.9) | 14.8 (−9.3 to 38.9) | 16.1 (−16.9 to 49.0) | 18.2 (−24.0 to 60.5) | 21.5 (−35.1 to 78.1) |
| High (200–299 mg) | 31.3 (7.5 to 55.1) | 35.0 (8.8 to 61.1) | 45.4 (7.3 to 83.5) | 59.0 (13.1 to 104.8) | 99.0 (36.3 to 161.7) | 148.9 (68.4 to 229.4) | 222.0 (114.1 to 329.8) |
| Very high (≥300 mg) | 67.0 (32.5 to 101.6) | 83.2 (45.3 to 121.1) | 110.1 (55.2 to 165.0) | 135.5 (69.5 to 201.5) | 213.4 (123.3 to 303.6) | 320.0 (204.4 to 435.6) | 480.3 (325.5 to 635.1) |
| Weight gain velocity (in g/month) | |||||||
| Average (50–199 mg) | 0.8 (−0.8 to 2.4) | 0.4 (−1.2 to 1.9) | 0.2 (−1.2 to 1.5) | 0.3 (−1.0 to 1.5) | 0.3 (−0.7 to 1.4) | 0.3 (−0.7 to 1.4) | 0.3 (−0.7 to 1.4) |
| High (200–299 mg) | 1.(−1.4 to 4.7) | 1.2 (−1.7 to 4.1) | 2. (−0.4 to 4.7) | 2.3 (−0.1 to 4.7) | 2.1 (0 to 4.3) | 2.0 (0.1 to 4.0) | 2.2 (0 to 4.0) |
| Very high (≥300 mg) | 6.0 (1.5 to 10.4) | 4.3 (0.2 to 8.5) | 3.8 (0.1 to 7.4) | 3.7 (0.3 to 7.1) | 3.9 (0.8 to 7.0) | 3.9 (1.1 to 6.8) | 3.9 (1.1 to 6.8) |
| BMI (in kg/m2) | |||||||
| Average (50–199 mg) | 0.03 (0.01 to 0.05) | 0.03 (0.01 to 0.05) | 0.04 (0.02 to 0.06) | 0.04 (0.02 to 0.06) | 0.04 (0.02 to 0.06) | 0.03 (0.01 to 0.06) | 0.02 (−0.01 to 0.05) |
| High (200–299 mg) | 0.07 (0.03 to 0.11) | 0.07 (0.03 to 0.10) | 0.09 (0.05 to 0.12) | 0.11 (0.07 to 0.15) | 0.14 (0.10 to 0.19) | 0.15 (0.11 to 0.20) | 0.15 (0.09 to 0.21) |
| Very high (≥300 mg) | 0.16 (0.10 to 0.21) | 0.16 (0.11 to 0.21) | 0.17 (0.12 to 0.23) | 0.20 (0.14 to 0.25) | 0.26 (0.20 to 0.32) | 0.29 (0.22 to 0.36) | 0.31 (0.22 to 0.39) |
Effect estimates derived from linear mixed-effect models with input of all anthropometric information from age 1 month to 8 years and adjustment for: maternal age, parity, maternal education, prepregnancy BMI, nausea and/or vomiting during pregnancy, maternal smoking during pregnancy, paternal BMI, gestational age and birth weight. The effect estimates are adjusted mean changes of weight, weight gain velocity and BMI.
Infancy is defined as the period from birth to age 12 months, toddlerhood as the period from age 12 months to 3 years, preschool age as the period from age 3 years to 5 years and school age as the period from age 5 years onwards.
β, β coefficients; BMI, body mass index.
Negative control analysis: paternal caffeine intake
We have explored the association between maternal and paternal caffeine intake independently and after adjusting for each other. All models are adjusted for the same confounders as in the main analysis. We have explored the associations with excess infant growth (n=12 289) and overweight at 3 years (n=16 455) (online supplementary figures 3 and 4).
For both the risk of excess infant growth and overweight at 3 years, the association with maternal caffeine intake changed negligibly after adjusting for paternal intake. On the other hand, by using paternal caffeine intake as a negative control, the trend of the association with child’s growth was similar to that of maternal caffeine intake, while the effect estimate was much lower.
Discussion
We found that any maternal caffeine intake during pregnancy was associated with a higher risk of excess growth in infancy and overweight in early childhood. In addition, caffeine intakes in pregnancy above the recommendation (200 mg/day) were associated with modified growth trajectories from very early in life and maintained during childhood. More specifically, children exposed prenatally to caffeine levels above 200 mg/day had persistently higher weight velocity, BMI velocity and weight gain velocity up to 8 years of age.
Strengths and limitations of this study
With the included 50 943 pregnancies, this is, so far, the largest study on the association of prenatal caffeine exposure and childhood growth parameters. It is the first to investigate effects on excess growth in infancy as well as growth velocities rather than just the size of the child, as well as critical age windows of diverging growth. Additional strengths include the prospective data collection, the comprehensive data on possible confounders and the assessment of caffeine intake from different sources. Nevertheless, our findings might be explained by residual confounding of non-accounted factors related to an overall unhealthy lifestyle and high caffeine consumption; though exclusion of smokers and very high caffeine consumers did not modify the results. In an effort to control for the effect of unmeasured familial characteristics, we performed negative control analysis using the father’s caffeine intake. The unchanged effect estimate of maternal caffeine intake after adjustment for paternal intake as well as the weak effect estimate of paternal caffeine intake, indicate minor bias by shared unmeasured confounders.
In addition, the missing body size measurements were handled with the use of a growth model. In sensitivity analyses restricted to the measured data, similar associations were found as with the predicted body size data (online supplementary table 8). This provides some reassurance of the validity of the predicted anthropometrics. However, we still acknowledge the potential for outcome misclassification as only 23% of the cohort had anthropometric information at 8 years (online supplementary table 1). At the time of release of the current data, 53% (27 142 children) of our study population had not reached the age of 8 years, and only 24% of missing anthropometric information at 8 years can be attributed to loss to follow-up. Furthermore, maternal caffeine exposure was not related to loss to follow-up (online supplementary table 1).
The self-report of diet, which can induce misreporting, is a limitation. However, fair agreement between beverage intakes, particularly of coffee and tea, was found in a validation study based on food records and biomarkers.25 39 Observational studies can never establish causality; however, our results fulfil some of the Bradford-Hill’s criteria for causation40 including strong associations, consistent findings for major caffeine sources, a biological gradient with higher caffeine exposure being associated to abnormal growth, consistent findings in animal models and a plausible mechanism, that is, fetal programming.
Our study adds evidence to two previous epidemiological studies19 20 that found an effect of prenatal caffeine exposure on child growth. In the study by Voerman et al, maternal caffeine intake above 360 mg/day was associated with higher weight and BMI from birth to age 6 years, compared with intakes below 180 mg/day.19 In contrast to our findings, they found no association with overweight. Higher caffeine intake (360–540 mg/day) during pregnancy was positively associated with body fat percentage and higher insulin levels in 6-year-olds. The exposure was assessed only by intakes of coffee and tea, which in our study also are the main but not the only caffeine contributors (78% of total caffeine intake). The median intake was double than in our study, resulting in a higher reference level (reference <180 mg/day, median 117 mg/day), providing less contrast between the compared exposure groups and less comparability to our study, as most of these women were not complying with the recommendation. Nevertheless, we found associations with adverse effects on child’s growth even at low caffeine intakes, in the range of the recommendation, that are mostly due to consumption of foods and drinks other than coffee (chocolate, black tea, caffeinated sodas).5 Li et al found likewise that any maternal caffeine intake was associated with an overall increased risk of obesity from ages 2 years to 15 years, with an exposure range similar to the current study.20 We have used similar approaches to study changes in individual growth trajectories, though with shorter follow-up. In addition, we provided age-specific weight and BMI deviations, in order to find sensitive developmental windows when the association with the prenatal caffeine exposure exacerbated. There is no previous report of the association between caffeine intake in pregnancy and excess infant growth.
Potential mechanisms
Since excess growth in infancy, in itself, can be directly related to childhood obesity, adiposity41 and an unfavourable adult cardiometabolic profile,42 the associations between prenatal caffeine exposure with overweight, body fat and insulin, found in this study and the previous reports, might be explained by excess infant growth. Putting together the previous findings in the MoBa Study,5 we have shown that children prenatally exposed to high caffeine levels are smaller at birth, grow faster in infancy and retain a higher weight throughout childhood without significant height differences, thus becoming overweight (online supplementary figure 5). These findings concur with the fetal programming of obesity hypothesis.43 Nevertheless, the effect of prenatal caffeine exposure on postnatal growth and overweight was not dependent on birth weight. Hence, along with a healthy birth weight, it is important to identify the modifiable factors that can independently affect excess growth in infancy, independent of fetal growth. A growing number of studies have shown that other prenatal factors, for example, excess gestational weight,44 high (>3 times/week) fish intake45and postnatal factors, for example, formula feeding and feeding schedule,46 are associated with increased risk of excess growth in infancy. Recent research shows that some perinatal factors can also have a direct effect on postnatal growth, independent of effects on fetal growth, including parental body size, smoking during pregnancy and socioeconomic status.47–49
The biological plausibility supporting our findings is mainly provided by animal studies where, prenatal exposure to caffeine was shown to programme the offspring towards excess growth and cardiometabolic disorders through alterations (1) in the hypothalamic-pituitary-adrenocortical axis that plays a key role in growth and metabolism,50–52 (2) in regulation of adenosine and adenosine antagonists, which are important modulators of development53 54 and (3) in the placental expression and transportation of leptin,55 essential for appetite regulation.
Although most pregnant women reduce their caffeine intake during pregnancy and few have caffeine intakes higher than 200 mg/day (10%), our results show associations between caffeine intakes below 200 mg/day and excess growth. The results add supporting evidence for the current advice to reduce caffeine intake during pregnancy and indicate that complete avoidance might actually be advisable. An absence of a ‘safe intake level’ has been previously reported on the basis of associations between maternal caffeine intake and fetal growth restriction.56 Nevertheless, the authors of a recent systematic review after critically assessing the evidence, concluded that a consumption of up to 300 mg caffeine/day in healthy pregnant women is generally not associated with adverse reproductive and developmental effects.57 Postnatal growth and child’s weight status were not included in this review. Our findings are in agreement with the previous studies assessing a similar hypothesis, with associations reported in caffeine intakes above, but even below, the comparator, indicating that an intake of 300 mg/day might not be a safe level when growth is under study. Given that overweight in childhood is not a rare condition and the number of children highly exposed to caffeine during pregnancy is large, even a small increase in the risk of overweight due to caffeine can result into a large proportion of children becoming overweight, assuming that the effect was causal. Hence, more evidence is needed for the association between prenatal caffeine exposure and postnatal growth and an updated future critical assessment of such studies.
The association between prenatal caffeine exposure and overweight attenuated after 5 years, with only very highly exposed children being at risk for overweight. Residual confounding due to postnatal factors related to overweight in late childhood might explain this attenuation. Nevertheless, adjustment for risk factors of being overweight at 8 years would introduce bias to the association under study. In addition, weight and height are screened from birth to 5 years in scheduled voluntary appointments at the public health centres. Hence, a possible misclassification of outcome from anthropometrics after 5 years, might also explain the attenuation of the association.
There are two studies showing effects of caffeine intake on body composition and cardiometabolic health,19 58 with discrepant results. In the present study, we did not have any information on body composition. In addition, it is known that several genetic factors can contribute to variation in caffeine metabolism,59 and studies in adults have shown that slower metabolism of caffeine is related to higher risk of cardiovascular disease.60 On the other hand, during pregnancy, maternal caffeine clearance modified the association between maternal caffeine intake and fetal growth restriction, with faster clearance being more detrimental.56 More specifically, a genotype of rapid caffeine metabolism was associated with reduced birth weight, while in women with a different polymorphism on the gene CYP1A2 C164A, no effect was found.61 Thus, there is a need to investigate the programming effect of prenatal caffeine exposure on child and adult body composition and cardiometabolic health, taking into account the genetic variation of maternal caffeine metabolism.
Conclusions
We found that the risk of excess infant growth and overweight in childhood—important risk factors for later cardiometabolic disease—is increasing with maternal caffeine intake, with no apparent threshold. Maternal caffeine intake >200 mg/day during pregnancy was associated with high weight gain velocity beginning from the first months of life and higher BMI throughout childhood. Our findings support the recommendation to limit caffeine intake during pregnancy (<200 mg/day).
Supplementary Material
Acknowledgments
The authors thank all the families in Norway who have participated in this ongoing cohort study.
Footnotes
Contributors: EP contributed to study design, data analysis and interpretation of the results and had the main responsibility of writing the paper. JB contributed to the statistical analysis plan and database preparation and interpretation of the results. A-LB contributed to study design, interpretation of the results and revising the paper. MH, JA, HMM contributed to the design of data collection tools, the study design and interpretation of the results. JB contributed to the statistical analysis plan and database preparation. AE contributed to interpretation of the results. BJ initiated this collaborative project, contributed to the study design and the interpretation of the results. VS defined the research question, contributed to the study design, database preparation and interpretation of the results. She is guarantor and had final responsibility for the decision to submit for publication. All authors read, revised and approved the final version of the paper.
Funding: The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no. 1 UO1 NS 047537- 01 and grant no. 2 UO1 NS 047537-06A1). VS has received grants from Stiftelsen Sigurd och Elsa Goljes Minnesfond (LA2013-0241 “Koffeinintag, födelsevikt och barnutfall”), Stiftelsen Fru Mary von Sydows, född Wijk, donationsfond (2014 “Koffeinintag, födelsevikt och barnutfall”) and Wilhelm och Martina Lundgrens Vetenskapsfond (1 vet1-119/2014: “Koffeinintag, födelsevikt och barnutfall”).
Disclaimer: EP as the lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The data collection in MoBa and this study was approved by the Regional Committee for Medical Research Ethics in South-Eastern Norway (2010/2683/REK Sør-øst A).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available. All data from the MoBa study are available to all qualified researchers/research groups in Norway and to international researchers who are collaborating with a Norwegian researcher.
References
- 1. EFSA EFSA-PoDP Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA Journal 2015;13:4102. [Google Scholar]
- 2. Mort JR, Kruse HR. Timing of blood pressure measurement related to caffeine consumption. Ann Pharmacother 2008;42:105–10. 10.1345/aph.1K337 [DOI] [PubMed] [Google Scholar]
- 3. Tomimatsu T, Lee SJ, Peña JP, et al. . Maternal caffeine administration and cerebral oxygenation in near-term fetal sheep. Reprod Sci 2007;14:588–94. 10.1177/1933719107307717 [DOI] [PubMed] [Google Scholar]
- 4. VKM. Risk assessment of "other substances"– Caffeine. Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics of the Norwegian Scientific Committee for Food Safety. Oslo, Norway: VKM, 2015. [Google Scholar]
- 5. Sengpiel V, Elind E, Bacelis J, et al. . Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med 2013;11:42 10.1186/1741-7015-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee J, Kim R, Kim Y, et al. . Maternal Caffeine Consumption during Pregnancy and Risk of Low Birth Weight: A Dose-Response Meta-Analysis of Observational Studies. PLoS One 2015;10:e0132334 10.1371/journal.pone.0132334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whincup PH, Kaye SJ, Owen CG, et al. . Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–97. 10.1001/jama.2008.886 [DOI] [PubMed] [Google Scholar]
- 8. Lawlor DA, Ronalds G, Clark H, et al. . Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation 2005;112:1414–8. 10.1161/CIRCULATIONAHA.104.528356 [DOI] [PubMed] [Google Scholar]
- 9. Monasta L, Batty GD, Cattaneo A, et al. . Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev 2010;11:695–708. 10.1111/j.1467-789X.2010.00735.x [DOI] [PubMed] [Google Scholar]
- 10. Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006;95:904–8. 10.1080/08035250600719754 [DOI] [PubMed] [Google Scholar]
- 11. Baird J, Fisher D, Lucas P, et al. . Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929 10.1136/bmj.38586.411273.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones-Smith JC, Neufeld LM, Laraia B, et al. . Early life growth trajectories and future risk for overweight. Nutr Diabetes 2013;3:e60 10.1038/nutd.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Botton J, Heude B, Maccario J, et al. . Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr 2008;87:1760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perng W, Hajj H, Belfort MB, et al. . Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J Pediatr 2016;173:122–30. 10.1016/j.jpeds.2016.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ekelund U, Ong KK, Linné Y, et al. . Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 2007;92:98–103. 10.1210/jc.2006-1071 [DOI] [PubMed] [Google Scholar]
- 16. Di Cesare M, Bentham J. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woo Baidal JA, Locks LM, Cheng ER, et al. . Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med 2016;50:761–79. 10.1016/j.amepre.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 18. Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr 2011;94(6 Suppl):1754S–8. 10.3945/ajcn.110.001206 [DOI] [PubMed] [Google Scholar]
- 19. Voerman E, Jaddoe VW, Gishti O, et al. . Maternal caffeine intake during pregnancy, early growth, and body fat distribution at school age. Obesity 2016;24:1170–7. 10.1002/oby.21466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li DK, Ferber JR, Odouli R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: a prospective cohort study. Int J Obes 2015;39:658–64. 10.1038/ijo.2014.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barker DJ, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol 2005;16:2537–44. 10.1681/ASN.2005020160 [DOI] [PubMed] [Google Scholar]
- 22. Gluckman PD, Cutfield W, Hofman P, et al. . The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev 2005;81:51–9. 10.1016/j.earlhumdev.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 23. Barker DJ. In utero programming of chronic disease. Clin Sci 1998;95:115–28. 10.1042/cs0950115 [DOI] [PubMed] [Google Scholar]
- 24. Magnus P, Birke C, Vejrup K, et al. . Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–8. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 25. Brantsaeter AL, Haugen M, Alexander J, et al. . Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:28–43. 10.1111/j.1740-8709.2007.00103.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WHO MGRSG. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, 2006. [Google Scholar]
- 27. Yang S, Hutcheon JA. Identifying outliers and implausible values in growth trajectory data. Ann Epidemiol 2016;26:77–80. 10.1016/j.annepidem.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ong KK, Ahmed ML, Emmett PM, et al. . Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 2000;320:967–71. 10.1136/bmj.320.7240.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev 2005;6:143–54. 10.1111/j.1467-789X.2005.00183.x [DOI] [PubMed] [Google Scholar]
- 30. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012;7:284–94. 10.1111/j.2047-6310.2012.00064.x [DOI] [PubMed] [Google Scholar]
- 31. Jenss RM, Bayley N. A mathematical method for studying the growth of a child. Human Biology 1937;9:556–63. [Google Scholar]
- 32. Berkey CS. Comparison of two longitudinal growth models for preschool children. Biometrics 1982;38:221–34. 10.2307/2530305 [DOI] [PubMed] [Google Scholar]
- 33. Comets E, Lavenu A, Lavielle M. saemix: Stochastic Approximation Expectation Maximization (SAEM) algorithm. 2014. https://cran.r-project.org/web/packages/saemix/index.html.
- 34. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 1 ed. New York: Springer-Verlag New York, 2001. [Google Scholar]
- 35. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 2000;79:440–9. 10.1080/j.1600-0412.2000.079006440.x [DOI] [PubMed] [Google Scholar]
- 36. Richmond RC, Al-Amin A, Smith GD, et al. . Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Hum Dev 2014;90:769–80. 10.1016/j.earlhumdev.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brew BK, Gong T, Williams DM, et al. . Using fathers as a negative control exposure to test the Developmental Origins of Health and Disease Hypothesis: A case study on maternal distress and offspring asthma using Swedish register data. Scand J Public Health 2017;45(17_suppl):36–40. 10.1177/1403494817702324 [DOI] [PubMed] [Google Scholar]
- 38. R Development Core Team. R: A language and environment for statistical computing [program]. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 39. Brantsaeter AL, Haugen M, Rasmussen SE, et al. . Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr 2007;10:838–47. 10.1017/S1368980007339037 [DOI] [PubMed] [Google Scholar]
- 40. Hill AB. The environment and disease: Association or causation? Proc R Soc Med 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 41. Karaolis-Danckert N, Buyken AE, Bolzenius K, et al. . Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr 2006;84:1449–55. [DOI] [PubMed] [Google Scholar]
- 42. Leunissen RW, Kerkhof GF, Stijnen T, et al. . Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009;301:2234–42. 10.1001/jama.2009.761 [DOI] [PubMed] [Google Scholar]
- 43. Barker DJ. Fetal origins of coronary heart disease. BMJ 1995;311:171–4. 10.1136/bmj.311.6998.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Subhan FB, Colman I, McCargar L, et al. . Higher Pre-pregnancy BMI and Excessive Gestational Weight Gain are Risk Factors for Rapid Weight Gain in Infants. Matern Child Health J 2017;21:1396–407. 10.1007/s10995-016-2246-z [DOI] [PubMed] [Google Scholar]
- 45. Stratakis N, Roumeliotaki T, Oken E, et al. . Fish Intake in Pregnancy and Child Growth: A Pooled Analysis of 15 European and US Birth Cohorts. JAMA Pediatr 2016;170:381–90. 10.1001/jamapediatrics.2015.4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mihrshahi S, Battistutta D, Magarey A, et al. . Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr 2011;11:99 10.1186/1471-2431-11-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu JX, Xu X, Liu JH, et al. . Association of maternal gestational weight gain with their offspring’s anthropometric outcomes at late infancy and 6 years old: mediating roles of birth weight and breastfeeding duration. Int J Obes 2018;42:8–14. 10.1038/ijo.2017.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hindmarsh PC, Geary MP, Rodeck CH, et al. . Factors predicting ante- and postnatal growth. Pediatr Res 2008;63:99–102. 10.1203/PDR.0b013e31815b8e8f [DOI] [PubMed] [Google Scholar]
- 49. Morgen CS, Ängquist L, Baker JL, et al. . Prenatal risk factors influencing childhood BMI and overweight independent of birth weight and infancy BMI: a path analysis within the Danish National Birth Cohort. Int J Obes 2017:10.1038/ijo.2017.217 [Epub ahead of print 8 Sep 2017]. 10.1038/ijo.2017.217 [DOI] [PubMed] [Google Scholar]
- 50. Xu D, Zhang B, Liang G, et al. . Caffeine-induced activated glucocorticoid metabolism in the hippocampus causes hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS One 2012;7:e44497 10.1371/journal.pone.0044497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Luo H, Wu Y, et al. . Gender-specific increase in susceptibility to metabolic syndrome of offspring rats after prenatal caffeine exposure with post-weaning high-fat diet. Toxicol Appl Pharmacol 2015;284:345–53. 10.1016/j.taap.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 52. Xu D, Wu Y, Liu F, et al. . A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion. Toxicol Appl Pharmacol 2012;264:395–403. 10.1016/j.taap.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 53. Buscariollo DL, Fang X, Greenwood V, et al. . Embryonic caffeine exposure acts via A1 adenosine receptors to alter adult cardiac function and DNA methylation in mice. PLoS One 2014;9:e87547 10.1371/journal.pone.0087547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rivkees SA, Wendler CC. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr Res 2011;69:271–8. 10.1203/PDR.0b013e31820efbcf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu YM, Luo HW, Kou H, et al. . Prenatal caffeine exposure induced a lower level of fetal blood leptin mainly via placental mechanism. Toxicol Appl Pharmacol 2015;289:109–16. 10.1016/j.taap.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 56. CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 2008;337:a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wikoff D, Welsh BT, Henderson R, et al. . Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol 2017;109(Pt 1):585–648. 10.1016/j.fct.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 58. de Medeiros TS, Bernardi JR, de Brito ML, et al. . Caffeine Intake During Pregnancy in Different Intrauterine Environments and its Association with Infant Anthropometric Measurements at 3 and 6 Months of Age. Matern Child Health J 2017;21:1297–307. 10.1007/s10995-016-2230-7 [DOI] [PubMed] [Google Scholar]
- 59. Cornelis MC, Kacprowski T, Menni C, et al. . Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet 2016;25:ddw334–82. 10.1093/hmg/ddw334 [DOI] [PubMed] [Google Scholar]
- 60. Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology 2010;211:245–57. 10.1007/s00213-010-1900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Norwegian Institute of Public Health. Norwegian Mother and Child Study. http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5895:0:15,3046:1:0:0:::0:02010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018895supp001.pdf (403.3KB, pdf)
bmjopen-2017-018895supp002.pdf (569.8KB, pdf)