Abstract
Avocado (Persea americana Mill. cv Lula) is a climacteric fruit that exhibits a rise in ethylene as the fruit ripens. This rise in ethylene is followed by an increase in abscisic acid (ABA), with the highest level occurring just after the peak in ethylene production. ABA is synthesized from the cleavage of carotenoid precursors. The cleavage of carotenoid precursors produces xanthoxin, which can subsequently be converted into ABA via ABA-aldehyde. Indirect evidence indicates that the cleavage reaction, catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED), is the regulatory step in ABA synthesis. Three genes encoding NCED cleavage-like enzymes were cloned from avocado fruit. Two genes, PaNCED1 and PaNCED3, were strongly induced as the fruit ripened. The other gene, PaNCED2, was constitutively expressed during fruit ripening, as well as in leaves. This gene lacks a predicted chloroplast transit peptide. It is therefore unlikely to be involved in ABA biosynthesis. PaNCED1 was induced by water stress, but expression of PaNCED3 was not detectable in dehydrated leaves. Recombinant PaNCED1 and PaNCED3 were capable of in vitro cleavage of 9-cis-xanthophylls into xanthoxin and C25-apocarotenoids, but PaNCED2 was not. Taken together, the results indicate that ABA biosynthesis in avocado is regulated at the level of carotenoid cleavage.
Fruit ripening involves a complex series of biochemical events in which the tissue undergoes programmed changes in texture, aroma, coloration, flavor, and firmness (Brady, 1987). Climacteric species, such as avocado (Persea americana Mill. cv Lula), are characterized by the autocatalytic production of the ripening hormone ethylene and a ripening-related transient burst in CO2 evolution (Biale and Young, 1981). In avocado the increase in ethylene production is followed by an increase in abscisic acid (ABA) levels (Adato et al., 1976). Although ethylene induces the synthesis of many genes involved in fruit ripening (Brady, 1987), it is not known whether the rise in ethylene is related to the increase in ABA in avocado. Further, the role that ABA plays in the ripening process is also unknown. Ripening avocado fruit produces high levels of ABA and thus provides an ideal system in which to study the regulation of ABA biosynthesis.
ABA plays a role in adaptation to various stresses (e.g. cold and osmotic stress), and also during developmental changes, such as seed germination and embryo development (Zeevaart and Creelman, 1988). The increase in ABA levels in water-stressed leaves can be prevented by transcriptional (Guerrero and Mullet, 1986) and translational inhibitors (Stewart et al., 1986), indicating that RNA and protein synthesis are necessary to mediate the drought-induced increase in ABA levels.
ABA is synthesized from carotenoid precursors that are present in relatively large quantities in most photosynthetic tissues in comparison with ABA (Norman et al., 1990; Parry et al., 1990). Biochemical (Zeevaart and Creelman, 1988) and genetic evidence (Koornneef et al., 1998) has indicated that the cleavage of 9-cis-xanthophylls is likely the key regulatory step in the ABA biosynthetic pathway. The cleavage of 9-cis-xanthophylls produces a C25-apocarotenoid and xanthoxin (Zeevaart, 1999). The xanthoxin can subsequently be converted into ABA via ABA-aldehyde (Fig. 1). The enzymes that carry out these later conversions (xanthoxin into ABA-aldehyde and ABA-aldehyde into ABA) are constitutively expressed in leaves (Sindhu and Walton, 1988) and are therefore not limiting for ABA biosynthesis. Other steps in the ABA biosynthetic pathway, such as the conversion of zeaxanthin into violaxanthin catalyzed by zeaxanthin epoxidase, show little up-regulation during water stress of leaves (Burbidge et al., 1997b). This further supports the notion that another part of the ABA biosynthetic pathway must be regulatory.
Figure 1.
The cleavage reaction in ABA biosynthesis. Both 9-cis-violaxanthin and 9′-cis-neoxanthin can be cleaved to xanthoxin, which can subsequently be converted into ABA.
Confirmation of the regulatory nature of the cleavage reaction was provided by the characterization of a viviparous mutant of maize, vp14, that exhibited a defect in ABA biosynthesis (Tan et al., 1997). The Vp14 gene was cloned and the derived protein sequence of Vp14 is related to lignostilbene dioxygenases, bacterial enzymes that catalyze a double-bond cleavage reaction analogous to the carotenoid cleavage reaction in ABA biosynthesis (Kamoda and Saburi, 1993). Recombinant VP14 protein catalyzes the cleavage of 9-cis-xanthophylls into ABA (Schwartz et al., 1997) in a reaction that requires oxygen, ferrous iron, ascorbate, and a detergent for activity in vitro. Northern analysis of maize leaves showed that Vp14 is induced during wilting in parallel with the increase in ABA levels (Tan et al., 1997).
In the time since the cloning of Vp14, a number of genes with sequence similarity to Vp14 have been reported (Burbidge et al., 1997a; Neill et al., 1998; Watillon et al., 1998), and a number of additional homologous genes are present in the database. Based upon the degree of sequence similarity of these genes with Vp14, it can be inferred that the encoded proteins catalyze reactions in which a double bond is oxidatively cleaved, yielding two products with aldehyde groups at the site of cleavage. Although not all of the homologous genes are necessarily involved in ABA biosynthesis, it seems that at least some are. In particular the notabilis mutant of tomato is impaired in its ability to convert C40-precursors to xanthoxin, and hence notabilis mutant plants have a reduced ABA content (Parry et al., 1988) and exhibit a wilty phenotype. The cloned gene is highly homologous to Vp14 (Burbidge et al., 1999), and message levels of this gene are increased during leaf wilting. The nomenclature now used for designating genes that have homology to Vp14 is 9-cis-epoxycarotenoid dioxygenase genes or NCED.
The regulation of ABA levels in fruit has not previously been investigated. Labeling studies of ABA using 18O2 have shown that the indirect pathway (i.e. synthesis from C40-carotenoids) of ABA biosynthesis in leaves is operational in both avocado and apple fruit (Zeevaart et al., 1989). Carotenoid levels in various fruits appear to be high enough so as not to be limiting for ABA biosynthesis, and therefore the cleavage of xanthophylls is probably the regulatory reaction in fruit. To test this three Vp14 homologs were cloned from ripening avocado fruit, and their expression during the ripening process was monitored. Two of these genes (PaNCED1 and PaNCED3) are induced in parallel as the fruit ripens. A third gene, PaNCED2, exhibits constant expression both during fruit ripening and during the wilting of leaves, suggesting that it has a housekeeping role unrelated to ABA biosynthesis. The tissue-specific differences in expression of the NCED genes, and differences in the activities of the expressed proteins, may have implications for their in vivo physiological role in regulating ABA biosynthesis.
RESULTS
Cloning of NCED Genes
A number of conserved regions are present in NCED genes (Burbidge et al., 1997a). These conserved regions were used for the design of degenerate primers used in the PCR amplification of NCED genes from avocado. Degenerate primers JZ101 and JZ117 (Table I) were used to amplify an approximate 1.1-kb fragment from cDNA of avocado fruit that had been ripened for 8 d. This gene was designated PaNCED1. The full-length gene, obtained using RACE-PCR, contains an open reading frame of 1,710 bp, with a 3′-untranslated region of 377 bp, and a 5′-untranslated region of 66 bp. The predicted molecular mass of the protein is 63.1 kD, slightly smaller than the predicted molecular mass of VP14. The amino terminus is basic with a high content of Ser and Thr residues characteristic of chloroplast transit peptides (Von Heijne et al., 1989), as is also found at the amino terminus of VP14. At the amino acid level, PaNCED1 is approximately 60% identical to VP14.
Table I.
Primers used in the amplification of 9-cis-epoxycarotenoid dioxygenase genes from avocado fruit
| Primer | Sequence | Gene Amplified | Orientation |
|---|---|---|---|
| JZ101 | TTY GAY GGN GAY GGN ATG G | NCED1 | S |
| JZ117 | GCY TTC CAN AGR TCR AAR CA | NCED1 | AS |
| JZ108 | ACR TAN CCR TCR TCY TC | NCED2 | AS |
| JZ110 | ATG ATN CAY GAY TTY GC | NCED2 | S |
| JZ120 | GAG GCG GCA AGT GAT | NCED2 | AS |
| JZ121 | CAG TTA TTG CGA AAT CAT GC | NCED2 | AS |
| JZ147 | AAA TGA GCT GTA TGA AAT GA | NCED2 | S |
| JZ148 | CAG TGG AAT CGT GAA AGA GAA | NCED2 | S |
| JZ153 | AAC GAA TCT GCG CAG GCA TTT CCG | NCED2 | S |
| JZ161 | AAC TTG AAA GCA GTA | NCED2 | S |
| JZ162 | GTT TAC GTA GTT TTA TTT TGC TCG | NCED2 | AS |
| JZ184 | ACG CCG GTT CCG CTG GAG CCG TCG | NCED1 | AS |
| JZ185 | ACA TCG CGA GGA GGT GCC GGT TGA A | NCED1 | AS |
| JZ186 | GAT TCA TGA CTT CGT CAT TAC TGA | NCED1 | S |
| JZ187 | TTC GTC ATA ATT CCA GAC CAG CAG | NCED1 | S |
| JZ200 | CCA ACA ACC CAT TGC TCT TCT | NCED1 | S |
| JZ205 | ATT ATA GAG AAC CAG CTA AGG TAC | NCED3 | AS |
| JZ206 | TTC CAC ACC TAA AAC AAA CAA ATT | NCED3 | AS |
| JZ222 | CCC GGG GAC GCA AGC CTA AT | NCED1 | AS |
| JZ223 | TCG ACT CAC TGA GTC GCA | NCED1 | S |
| JZ224 | TAC AAG CAG TGG AAG AAG GGA AGG | NCED1 | AS |
| JZ225 | GAA CAA GAC CCT GAG ACT GAG | NCED3 | S |
| JZ245 | GTC CAA GGC GAA GGC CAG CAG TCC | NCED3 | AS |
| JZ240 | CAT TCC CTC GTC GGC GTT CAC CA | NCED3 | AS |
| JZ250 | ACT CTT ATG TCA ATG GCT ACT CCT | NCED3 | S |
Y, C/T; N, A/G/C/T; R, A/C; S, Sense; AS, antisense..
Degenerate primers JZ108 and JZ110 (Table I) were used to amplify an approximately 600-bp fragment from cDNA of d 8 avocado fruit. This gene was designated PaNCED2. The full-length cDNA, obtained using 5′ and 3′ RACE, contains an open reading frame of 1,575 bp encoding a protein with a predicted molecular mass of 59.6 kD. The 3′- and 5′-untranslated regions are 226 and 166 bp, respectively. In comparison with VP14 and the tomato homolog, the deduced amino acid sequence of PaNCED2 is truncated at the amino terminus and thus appears to lack a transit peptide for chloroplast targeting. Overall the gene shares approximately 30% identity at the deduced amino acid level with VP14, LeNCED1, and PaNCED1.
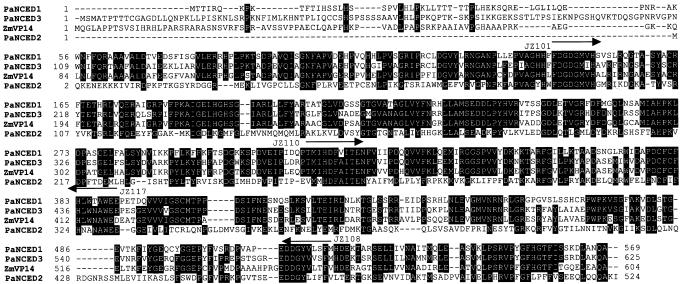
During each of the RACE procedures Southern blotting of the minipreps corresponding to the 3′ and 5′ ends of the gene was performed to ensure that the newly amplified region cross-hybridized with the previously cloned portion. During the cloning of the 3′ end of PaNCED1 it was noticed that the minipreps differed in terms of the strength of the hybridization signal when the fragment corresponding to JZ101/JZ117 was used as probe on Southern blots. These plasmids corresponding to the weaker signal on Southern blots were sequenced and discovered to be a unique NCED gene. This gene, designated PaNCED3, is 60% identical at the amino acid level to Vp14 and LeNCED, and 67% identical to PaNCED1. PaNCED3 contains an open reading frame of 1,878 bp, with 3′- and 5′-untranslated regions of 388 and 44 bp, respectively. The amino terminus of PaNCED3 is similar to those of PaNCED1 and VP14, both in terms of its length relative to the more highly conserved portions of the protein and in the abundance of basic amino acids. An alignment of the deduced protein sequences of the three avocado genes with VP14 is shown in Figure 2. Some properties of the three avocado genes are summarized in Table II.
Figure 2.
Alignment of the deduced amino acid sequences of PaNCED1, PaNCED2, and PaNCED3 from avocado with VP14 from maize. Amino acid residues identical in at least three of the sequences are indicated by black boxes. The arrows indicate the regions that were used in the design of the degenerate primers.
Table II.
Comparison of avocado NCED genes and their encoded proteins
| Properites | PaNCED1 | PaNCED2 | PaNCED3 |
|---|---|---|---|
| GenBank accession no. | AF224672 | AF224670 | AF224671 |
| Total message length (bp) | 2,153 | 1,967 | 2,310 |
| 3′-Untranslated region (bp) | 377 | 226 | 388 |
| 5′-Untranslated region (bp) | 66 | 166 | 44 |
| Total coding bases (bp) | 1,710 | 1,575 | 1,878 |
| No. of amino acids | 569 | 524 | 625 |
| Predicted molecular mass (kD) | 63.1 | 59.6 | 69.7 |
| Percent of identity to VP14a | 63 | 29 | 61 |
| pI | 7.4 | 9.0 | 8.2 |
| Induction during ripening | Yes | No | Yes |
| Induction during wilting | Yes | No | No |
| Predicted chloroplast transit peptideb | Yes | No | Yes |
| In vitro ability to produce xanthoxin | Yes | No | Yes |
At the amino acid level.
Based on the predictions of ChloroP (Emanuelsson et al., 1999).
Because of the high homology of the three genes, particularly PaNCED1 and PaNCED3, gene-specific probes were designed to be used for subsequent northern analysis. Dot blotting of the in vitro transcribed mRNAs of the three genes demonstrated that the three gene-specific probes did not cross-hydridize (Fig. 3). Hence, these probes were used in the northern analysis presented below.
Figure 3.
Dot blot demonstrating the specificity of the PaNCED probes. In vitro transcribed mRNA of PaNCED1, PaNCED2, and PaNCED3 (0.2 ng or 2 ng) were applied in duplicate to the membrane and hybridized with gene-specific probes against PaNCED1, PaNCED2, or PaNCED3.
Monitoring of the Ripening Process
Avocado fruits left at room temperature require on average about 2 weeks to become fully ripe; some variation occurs between varieties (Biale and Young, 1971). Fruits of cv Lula produced little ethylene until 6 d after harvesting, at which time there was a massive increase in ethylene production (Fig. 4A). This autocatalytic ethylene production is typical of climacteric fruit and other senescing tissue (Brady, 1987). By d 10, when ethylene production had declined, the fruit had a soft texture and fruit maturation was complete. In fruit ripened for 6 d, ethylene production had peaked, whereas ABA levels remained at a low level. By d 11, 4 d following the peak in ethylene production, ABA levels had reached 30-fold higher levels compared with the level in unripe fruit. Because the enzymes involved in producing ABA would already be present by d 11, an earlier time point, namely d 8 fruit, was chosen as the RNA source used in reverse transcriptase (RT)-PCR.
Figure 4.
Changes in ABA and ethylene levels, and in PaNCED1, PaNCED2, and PaNCED3 transcript accumulation during the course of avocado fruit ripening. A, Analysis of ABA and ethylene levels plotted as a function of days of ripening. B, Northern analysis of NCED gene expression in the same fruits. Total RNA (30 μg per lane) was isolated from fruit, separated by gel electrophoresis, and blotted onto nylon membranes. The same blot used for analysis of PaNCED1 was stripped and reprobed with probes against PaNCED2 and subsequently PaNCED3 and 17S rDNA. The specific probes used for the PaNCED genes are described in “Materials and Methods.” The 17S rDNA probe was used as a loading control.
Northern-Blot Analysis of the Three PaNCED Genes
In maize there is an increase in transcript level of Vp14 in leaves subjected to wilting (Tan et al., 1997). It was hypothesized that as in water-stressed leaves, the increase in ABA levels during fruit ripening may also be accompanied by an increase in the mRNA levels of the NCED genes. Northern analysis of PaNCED2 showed that it remained fairly constant in expression during the ripening process (Fig. 4B). For analysis of PaNCED1 and PaNCED3 the same blot was stripped and reprobed with gene-specific probes based on the 3′-non-coding region of each of the genes.
Both PaNCED1 and PaNCED3 were barely detectable until 8 d after harvesting. At this time mRNA levels of both PaNCED1 and PaNCED3 increased in a similar fashion, reaching the highest levels at d 10, and falling again as the fruit became very soft (12 d). Since this increase in message levels precedes the increase in ABA levels, both PaNCED1 and PaNCED3 can be viewed as possible cleavage enzyme genes.
To test whether the avocado genes cloned from fruit were up-regulated during wilting of leaves, northern analysis was performed on turgid avocado leaves and on leaves that had been wilted to 95%, 88%, and 80% of their fresh weights. As a result of the dehydration, ABA levels increased approximately 10-fold in leaves that lost 20% of their water content (Fig. 5A). Although PaNCED3 was undetectable under any of these conditions, PaNCED1 increased significantly in response to water loss (Fig. 5B). PaNCED2 remained fairly constant under the same conditions.
Figure 5.
Accumulation of ABA (A), and of PaNCED1, PaNCED2, and PaNCED3 transcripts (B) in response to wilting of avocado leaves. RNA-blot hybridizations were carried out with total RNA (30 μg per lane) isolated from leaves that had been wilted to increasing percentages of their fresh weights. The leaves were wilted using a pressure chamber for approximately 15, 30, and 50 min to achieve water losses of 5%, 12%, and 20%, respectively. After this time, the leaves were incubated in the dark for 4 h, and then frozen in liquid N2. The specific probes used for the NCED genes and the probe (17S rDNA) used as a control are described in “Materials and Methods.”
Assay of Enzymatic Activity of the NCED Protein Products
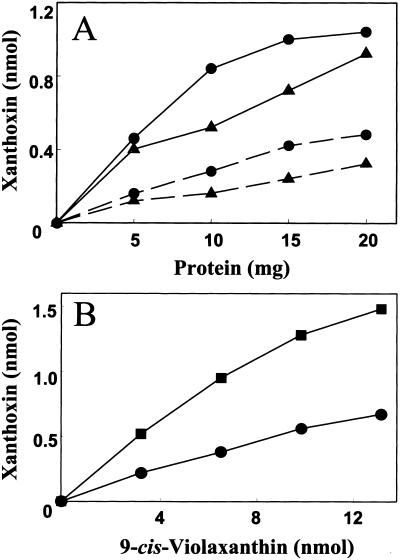
The results of northern analysis and sequence homology to Vp14 supported a role for both PaNCED1 and PaNCED3 in ABA biosynthesis. In contrast, PaNCED2 is constitutively expressed and has less homology to Vp14 than to either PaNCED1 or PaNCED3. Therefore it is unlikely that PaNCED2 is involved in ABA biosynthesis. To test whether the protein products of these genes could catalyze xanthoxin formation in vitro, all three genes were expressed as recombinant proteins fused to glutathione-S-transferase. Although somewhat insoluble, the recombinant proteins were purified to homogeneity and used to assay for carotenoid cleavage. Recombinant PaNCED1 and PaNCED3 cleaved 9-cis-violaxanthin and 9′-cis-neoxanthin to produce xanthoxin and a C25-apocarotenoid (Fig. 6). The reactions exhibited both protein (Fig. 6A) and substrate (Fig. 6B) dependency. Trans-isomers of violaxanthin and neoxanthin were not cleaved, consistent with the results of the VP14 assays, and with the required configuration for cis-ABA synthesis (Schwartz et al., 1997). The identity of xanthoxin and the C25 compounds produced from either neoxanthin or violaxanthin was confirmed by mass spectrometry. Under the same assay conditions used for PaNCED1 and PaNCED3, PaNCED2 did not cleave either the cis- or the trans-isomer of either violaxanthin or neoxanthin.
Figure 6.
Enzyme activities of the NCED1 and NCED3 proteins. A, Increase in xanthoxin formed from either 9′-cis-neoxanthin (▴) or 9-cis-violaxanthin (●) as a function of PaNCED1 (---) or PaNCED3 (—) protein concentrations. Assays contained 6 nmol of substrate. B, Xanthoxin formed by PaNCED1 (●) and PaNCED3 (▪) as a function of 9-cis-violaxanthin concentrations. The xanthoxin and C25-apocarotenoids produced in the in vitro reaction were analyzed by HPLC and identified by mass spectrometry.
Analysis of Carotenoid Composition of Ripening Avocado Fruit
The carotenoid composition of fruit ripened for varying lengths of time was analyzed to determine whether decreases in the levels of specific xanthophylls corresponded to increases in ABA. The carotenoids were identified on the basis of their acid-catalyzed shift in absorption maxima. Lutein and lutein epoxide were the most abundant carotenoids in unripe fruit, with levels remaining high in relation to the other carotenoids in fruit ripened for 10 d (Table III). Between d 1 and 6, there was an increase in lutein epoxide, violaxanthin, neoxanthin, and violaxanthin. As ripening continued (d 9 and 11), levels of these carotenoids decreased. The substantial decrease in neoxanthin that occurred between fruit ripened for 6 and 11 d is consistent with the increase in ABA levels that occurred during that time, but the two quantities cannot be related to one another on a 1:1 stoichiometric basis.
Table III.
Quantification of carotenoids from avocado fruits
| Carotenoid | Days of Ripening
|
|||
|---|---|---|---|---|
| 1 | 6 | 9 | 11 | |
| nmol g−1 fresh wt | ||||
| Lutein | 4.67 ± 0.95 | 5.05 ± 0.65 | 11.43 ± 1.20 | 7.17 ± 0.90 |
| Lutein epoxide | 4.93 ± 0.72 | 4.83 ± 0.70 | 2.88 ± 0.60 | 2.41 ± 0.53 |
| Antheraxanthin | 2.29 ± 0.36 | 2.33 ± 0.48 | 2.48 ± 0.50 | 1.59 ± 0.45 |
| All-trans-violaxanthin | 1.88 ± 0.32 | 2.98 ± 0.52 | 2.05 ± 0.37 | 1.00 ± 0.25 |
| 9-cis-Violaxanthin | 0.93 ± 0.15 | 1.30 ± 0.30 | 0.60 ± 0.12 | 0.45 ± 0.13 |
| 9′-cis-Neoxanthin | 3.98 ± 0.87 | 6.25 ± 1.17 | 1.57 ± 0.40 | 1.23 ± 0.40 |
Carotenoids were extracted from 1 g of fruit ripened for 1, 6, 9, and 11 d and purified using HPLC. The concentration of each carotenoid is expressed on a nmol g−1 fresh wt basis. Data are the mean of four measurements ± se.
DISCUSSION
It is now becoming clear that during developmental (fruit ripening) and physiological changes (wilting), ABA biosynthesis is regulated at the level of cleavage of C40-carotenoid precursors into xanthoxin. The results in this paper demonstrate that two NCED-like genes are up-regulated during fruit ripening, but only one is induced in dehydrated leaves. The data support the circumstantial evidence derived from a variety of studies that implicated the cleavage reaction as the governing step in increasing ABA levels both in development and during wilting.
In maize, Vp14 is part of a multi-gene family (Tan et al., 1997). This is also the case in avocado. PaNCED1 and PaNCED3 are 60% identical at the amino acid level with Vp14 and the tomato homolog, LeNCED1 (Burbidge et al., 1999). Analysis of the sequence similarity of homologous sequences present in the database suggests that a large family of NCED genes exist. The genes can be grouped according to their identity to each other. For example, maize, bean, tomato, and avocado NCED1 and NCED3 share approximately 60% identity at the amino acid level to each other. PaNCED2 is approximately 60% identical to an Arabidopsis sequence called AtNCED1 (Neill et al., 1998), but only 30% identical to the aforementioned sequences. It would seem plausible that genes with 60% identity or greater may have the same function, whereas those with less identity catalyze different reactions. The only proteins with demonstrated functions are the two avocado proteins described here, maize VP14 (Schwartz et al., 1997), the PvNCED1 protein from bean (Qin and Zeevaart, 1999), and lignostilbene dioxygenase (Kamoda and Saburi, 1993). The results of studies of the notabilis mutant (the mutant allele of LeNCED1) suggest that the tomato gene product catalyzes the same reaction (Burbidge et al., 1999). The regions that are conserved among all of the protein sequences are likely involved in substrate and cofactor binding. Site-directed mutagenesis would be useful in determining the function of the conserved residues.
The function of PaNCED2 and similar Vp14 homologs is not known. In Arabidopsis, a PaNCED2 homolog called AtNCED1 (60% amino acid identity) is weakly induced by rapid dehydration of leaves (Neill et al., 1998). We did not find up-regulation of PaNCED2 during dehydration of avocado leaves. In addition, PaNCED2 lacks a chloroplast-targeting signal. The double bond present in both lignostilbene and in violaxanthin and neoxanthin is a common feature found in terpenoids, phytoalexins, and many other natural products. As many of these pathways occur in the cytoplasm, it is possible that protein products of genes such as PaNCED2 catalyze reactions within these pathways. It should be noted however, that as carotenoids are present in the envelope of the chloroplast (Siefermann-Harms et al., 1978), a putative NCED need not be imported into the chloroplast for it to use carotenoids as substrates.
Two avocado genes, PaNCED1 and PaNCED3, encode proteins that are capable of in vitro synthesis of xanthoxin, the precursor of ABA. Evidence for the in vivo role of PaNCED1 and PaNCED3 in ABA biosynthesis is indicated by the correlation of mRNA levels of these genes with endogenous ABA levels. In terms of their in vitro substrate preference, PaNCED1 and PaNCED3 appear to be indistinguishable; both utilize violaxanthin more effectively than neoxanthin. Thus, ABA biosynthesis in fruit appears to be redundant, in the sense that two genes appear to encode proteins with identical functions. However it should be emphasized that in vivo factors such as transport and degradation of ABA are also important in regulating levels (Zeevaart, 1999). In addition, the in vivo accessibility of enzyme to substrate and the isomerization of violaxanthin into neoxanthin may also be determinants of why two enzymes exist. It will be interesting to determine whether the presence of two very similar enzymes in fruit, but only one in water-stressed leaves, is typical of other plants as well.
The carotenoid data (Table III) indicate that there is a substantial decrease in neoxanthin as the fruit ripens. On a molar basis, the amount of ABA at any stage of ripening exceeds the sum of both violaxanthin and neoxanthin. For example, late in ripening (d 11), the sum of the molar amount of violaxanthin and neoxanthin present is 2.7 versus 49.8 nmol of ABA (derived from the data in Fig. 4 and Table III). This indicates that the carotenoid pool must turn over more rapidly than the ABA pool. In tissues such as light-grown leaves and in unripe fruit, the ratio of carotenoids to ABA is very high, making it difficult to demonstrate correlations between decreases in xanthophylls and increases in ABA. In roots (Parry et al., 1992) and in dark-grown, fluridone-treated leaves of Phaseolus vulgaris (Li and Walton 1990; Parry and Horgan, 1991), there is a 1:1 correspondence between xanthophylls cleaved and ABA + ABA catabolites synthesized. The experiments with roots (Parry et al., 1992) and with dark-grown, fluridone-treated tissues (Li and Walton, 1990; Parry and Horgan, 1991) also had the advantage that increases in ABA were measured a few hours following the imposition of stress. In our system of ripening fruit, the developmental increases in ABA occur over a period of days, further complicating derivation of a direct relationship between decreases in carotenoids and increases in ABA. Despite this limitation, the decreases in both violaxanthin and neoxanthin that correlate with increases in ABA levels in fruit are consistent with their proposed role as ABA precursors.
Multi-gene families often encode genes with related functions, but in cases where the function is the same, differential regulation ensures that distinct genes are activated in response to different environmental and other stimuli. A good example of this is the 1-aminocyclopropane-1-carboxylic acid synthase gene family (Zarembinski and Theologis, 1994). The fact that NCED genes are part of a family can perhaps be construed as an indication that sensitive mechanisms are needed to regulate the amount and location of the ABA synthesized. In this regard zeaxanthin epoxidase, an enzyme with both a structural and photoprotective role, does not exist as a gene family (Marin et al., 1996). For ABA to serve its role in drought response, a rapid signaling mechanism must be in place; perhaps the easiest way to achieve this is to have a specific signaling pathway to turn on the appropriate gene.
Differential regulation implies that different signal transduction pathways are activated that allow gene expression in response to the specific stimuli. Ultimately, the distinction between which genes are induced in response to a given stimulus lies in the promoter region. In avocado, two cleavage enzymes are present: both are induced during fruit ripening but only one of the genes is induced by water stress. Analysis of the promoter region of these two genes should reveal whether a dehydration response element as is found in many osmotic-responsive genes (Shinozaki and Yamaguchi-Shinozaki, 1997) is also present in PaNCED1. Promoter elements for genes that are up-regulated during fruit ripening include those that have an ethylene-responsive box (e.g. Itzhaki et al., 1994), those that have ripening-specific elements (e.g. Atkinson et al., 1998; Deikman et al., 1998), and others in which no previously characterized regulatory elements are apparent (e.g. Beaudoin and Rothstein, 1997). It will be of interest to determine whether other developmental processes such as seed germination and embryo development induce the expression of novel NCED genes, and whether overlap exists between the signal transduction pathways that lead to the expression of wilt-related versus developmentally regulated NCED genes.
MATERIALS AND METHODS
Plant Material
Avocado fruits (Persea americana Mill. cv Lula) grown in Homestead, Florida were harvested and shipped overnight to our laboratory. The fruits were kept at room temperature for up to 14 d in a tray moistened with wet paper towels and covered with plastic wrap to prevent desiccation. Each day individual fruits were incubated in sealed containers and after a period of time, 1 mL of the gas phase was removed for ethylene determination by gas chromatography. On various days during the ripening period, one avocado fruit was harvested by removing the rind and cutting it into small pieces that were then frozen in liquid N2 and stored at −80°C to be used for subsequent analysis.
Avocado seedlings were grown from seeds of cv Lula under greenhouse conditions. Mature leaves from the top of the plant were wilted to differing percentages of their fresh weight, up to a maximum of 80%, using a pressure chamber (Boyer, 1995). After removal from the chamber, the leaves were left in a moist plastic bag in the dark for 4 h and then frozen in liquid N2.
Extraction and Purification of ABA and Carotenoids
ABA was extracted with acetone and purified as described by Zeevaart et al. (1989). A small amount of [3H]ABA was added to each sample to determine the percentage recovery. Quantification was by gas chromatography with electron capture detection using endrin as an internal standard.
Extraction and analysis of carotenoids were performed according to Rock and Zeevaart (1991). Carotenoids were quantified by integration of the peak area for A436 with a data module 740 (Waters, Milford, MA). A C25-apocarotenoid, trans-β-apo-8′-carotenal (Fluka, Milwaukee, WI), was added to each sample as a standard so that percentage recovery could be calculated. Corrections were made for differences in extinction coefficients and for the differences in absorption at 436 nm and the maximal absorption for each carotenoid.
RNA Extraction
Total RNA was extracted from avocado mesocarp using a phenol-chloroform method (Vanlerberghe and McIntosh, 1994). Leaf RNA was isolated by a method that is a combination of methods by Callahan et al. (1989) and Ainsworth (1994). Tissue was ground in liquid N2 to a fine powder using a mortar and pestle. The ground tissue was transferred to a tube containing extraction buffer (100 mm sodium acetate, 500 mm NaCl, 50 mm Na2EDTA, 1.4% [w/v] SDS, 2% [w/v] polyvinylpyrrolidone [Mr of 40,000], and 1% [v/v] β-mercaptoethanol) and the homogenate was placed at 65°C for 30 min. Cellular debris was removed by centrifugation (10,000g for 10 min) and the supernatant was extracted with distilled phenol. After centrifugation the aqueous phase was extracted once with phenol/chloroform and once with chloroform. The aqueous phase was placed on ice, 0.1 volume of 3 m Na-acetate (pH 4.8) was added, and the pH was brought to 5 by the addition of glacial acetic acid. After 2 h on ice the RNA was pelleted and washed once with 80% (v/v) cold ethanol. The pellet was resuspended in 2 mL of distilled water and precipitated overnight with 0.25 volume of 10 m LiCl. The RNA was pelleted by centrifugation (10,000g for 10 min) and washed with 80% (v/v) cold ethanol. The pellet was resuspended in deionized, distilled water and RNA quantitated by measuring absorbance at 260 and 280 nm.
RT-PCR
RNA was extracted from fruit as described above. The first-strand cDNAs were synthesized by RT from 4 μg of total RNA isolated from avocado fruit ripened for 8 d using Moloney murine leukemia virus reverse transcriptase (Gibco-BRL, Rockville, MD) and an oligo dT primer. These cDNAs were used as templates for RT-PCR using degenerate primers JZ101 and JZ117 for the amplification of PaNCED1 and degenerate primers JZ108 and JZ110 for the amplification of PaNCED2 (see Table I for a list of primers). These primers were designed based on the conserved regions of the tomato NCED and maize Vp14 genes. Conditions for RT were as follows: 65°C for 5 min, followed by 45°C for 1 h, followed by 75°C for 5 min. PCR amplification was performed as follows: 30 cycles of 94°C for 1 min, 55°C for 1.5 min, and 72°C for 1 min.
Amplification of Full-Length cDNAs by RACE-PCR
To obtain the full-length nucleotide sequences for PaNCED2 and PaNCED1, RACE-PCR was performed using a kit according to the manufacturer's instructions (Gibco-BRL). The 5′ ends of each of the genes were amplified using the following gene-specific primers: JZ184 (nested) and JZ185 (outer) for PaNCED1; JZ121 (outer) and JZ120 (nested) for PaNCED2; and JZ245 and JZ240 for PaNCED3. To amplify the 3′ ends, the following gene-specific primers were used: JZ186 (outer) and JZ185 (inner) for PaNCED1, and JZ148 (outer) and JZ147 (inner) for PaNCED2. Plasmids resulting from cloning of the 3′ end of PaNCED1 were heterogeneous as judged by the differing hybridization signal strengths on Southern blots probed with PCR fragment JZ101/JZ117 of PaNCED1. Sequencing of these plasmids revealed them to be a portion of a new gene that was related to PaNCED1. The new gene was designated PaNCED3, and 5′ RACE (using JZ240 and JZ245) was used to obtain the full-length clone. Primers JZ200 (at the 5′ end) and JZ222 (at the 3′ end) were used in end-to-end PCR to obtain the full-length PaNCED1 gene. Primers JZ162 (at the 3′ end) and JZ153 (at the 5′ end) were used in end-to-end PCR to obtain the full-length PaNCED2 clone. Primers JZ 206 (at the 3′ end) and JZ250 (at the 5′ end) were used to obtain the full-length PaNCED3 gene.
Cloning, DNA Sequencing, and Analysis of DNA Sequences
The PCR products corresponding to either partial fragments or the full-length genes were ligated into pGEMTeasy (Promega, Madison, WI) and then introduced into Escherichia coli DH5α. Plasmids were sequenced using an automated DNA sequencer (model 370A, Applied Biosystems, Foster City, CA) with either the −21M13 or M13 sequencing primers.
Computer analysis of the DNA and amino acid sequences were carried out using the BLAST program at the National Center for Biotechnology Information Services (Bethesda, MD). Alignment of the amino acid sequences was carried out using the Clustal W1.8 program at the Baylor College of Medicine (Houston) Search Launcher and using Boxshade 3.21 for pretty printing of multiple alignment files.
Probe Synthesis and Labeling
For gene-specific probes, JZ205 and JZ225 were used to amplify a fragment corresponding to the 3′-untranslated region of PaNCED3. The region amplified using these primers extended from bp 1,939 to 2,239, resulting in a PCR product of 300 bp. Gene-specific primers JZ223 and JZ224 (corresponding to bp 1,777 and 2,112, respectively) were used to amplify a 335-bp gene-specific fragment from PaNCED1. For PaNCED2, a 200-bp gene-specific fragment was amplified using primers JZ161 (bp 1,742) and JZ162 (bp 1,942). The PCR products were analyzed by electrophoresis in agarose gels and purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Purified probes were labeled by random-prime labeling (Gibco-BRL) using [α-32P]dCTP (DuPont-New England Nuclear, Boston). Nonincorporated nucleotides were removed by spin chromatography.
Northern- and Dot-Blot Analyses
Northern analysis was carried out by electrophoresis of 30 μg of total RNA in 1.2% (w/v) agarose gels containing 2.2% (v/v) formaldehyde according to Maniatis et al. (1982) and transferred onto nylon membranes (Hybond-N+, Amersham, Buckinghamshire, UK). Hybridization was performed at 65°C using Church-Gilbert hybridization buffer (Church and Gilbert, 1984). Blots were washed first at low stringency (2× SSC and 0.1% [w/v] SDS, twice at room temperature for 15 min each, followed by twice at 15 min each at 65°C), then at high stringency (additional wash 0.2× SSC and 0.1% [w/v] SDS for 30 min at 65°C). Following hybridization and development of the autoradiograms, blots were stripped in 0.2% (w/v) hot SDS and reprobed. As a loading control, the HindIII-EcoRI fragment of the rice gene for 17S rDNA was used.
For dot-blot analysis, in vitro transcribed mRNA of PaNCED1, PaNCED2, and PaNCED3 (0.2 or 2 ng) was applied to nylon membranes. Blots were hybridized with the appropriate gene-specific probe generated by random-prime labeling.
Protein Expression and Purification
PaNCED1 in pGEMTeasy was digested with NotI to clone into the NotI site of pGEX5-2 (Pharmacia Biotech, Piscataway, NJ). PaNCED3 was excised from pGEMTeasy using NotI and cloned into pGEX5-1. PaNCED2 was cloned into the EcoRI site of pGEX5-2. The plasmids were transformed into BL21 cells. A flask with 200 mL of 2YT medium (bacto-tryptone, bacto-yeast extract, and NaCl) was inoculated with a 4-mL overnight culture and grown at 37°C until the optical density reached 0.7. At that time, 200 mm isopropylthio-β-d-galactoside was added, and the cultures were transferred to a shaker at 25°C in the case of PaNCED2 and PaNCED3, or to 18°C in the case of PaNCED1. After 5 h for PaNCED2 and PaNCED3, or 16 h for PaNCED1, cells were harvested by centrifugation at 12,000g for 10 min, washed once with Tris [tris(hydroxymethyl)aminomethane]-buffered saline (TBS), pH 7, centrifuged at 12,000g for 10 min, and resuspended in 10 mL of TBS. One milliliter of 100 mg/mL lysozyme was added and the suspension was left on ice 30 min before being frozen overnight at −20°C. The extract was thawed the next morning, 0.1 m dithiothreitol was added, and it was sonicated using a probe sonicator (model 450, Branson Ultrasonics, Danbury, CT) in 15-s pulses for approximately 6 min total. The extract was separated into soluble and insoluble fractions by centrifugation (10,000g, 10 min). The soluble fraction was added to a 1-mL 50% slurry of Glutathione Sephadex (Pharmacia), and incubated 2 h at 8°C. At this time the mixture was centrifuged in a tabletop centrifuge and the beads washed three times with 1× TBS, and once with Factor Xa buffer. One-half of one milliliter of Factor Xa buffer and 25 units of Factor Xa (Pharmacia) were added and the beads were shaken for 3 h at room temperature. At this time 5 μL of 20% (w/v) Triton X-100 was added, and incubation was continued for one additional h. The beads were pelleted by centrifugation in a tabletop centrifuge and the supernatant containing the eluted protein was collected and frozen at −80°C.
Assay of Enzymatic Activity of NCED
Assay of the enzymatic activity of PaNCED1, PaNCED2, and PaNCED3 was performed as described by Schwartz et al. (1997). The cleavage reaction products were analyzed by HPLC on a μPorasil column (Waters) equilibrated with 90% (v/v) hexane and 10% (w/v) ethyl acetate. The column was eluted with a linear gradient to 20% (v/v) hexane and 80% (v/v) ethyl acetate over 17 min. The xanthoxin and C25 products from the cleavage of 9′-cis-neoxanthin and 9-cis-violaxanthin were collected and identified by mass spectrometry according to Schwartz et al. (1997). A standard curve of xanthoxin was constructed by injecting known quantities and integrating the peak areas.
ACKNOWLEDGMENTS
We are grateful to Dr. Tom Davenport (Homestead, FL) for supplying the avocado fruits. We thank the laboratory of Dr. Hans Kende for providing the rice 17S rRNA gene.
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–9723408) and by the U.S. Department of Energy (grant no. DE–FG02–91ER20021).
LITERATURE CITED
- Adato I, Gazit S, Blumenfeld A. Relationship between changes in abscisic acid and ethylene production during ripening of avocado fruits. Aust J Plant Physiol. 1976;3:555–558. [Google Scholar]
- Ainsworth C. Isolation of RNA from floral tissue of Rumex acetosa (Sorrel) Plant Mol Biol Rep. 1994;12:199–203. [Google Scholar]
- Atkinson RG, Bolitho KM, Wright MA, Iturriagagoitia-Bueno T, Reid SJ, Ross GS. Apple ACC-oxidase and polygalacturonase: ripening-specific gene expression and promoter analysis in transgenic tomato. Plant Mol Biol. 1998;38:449–460. doi: 10.1023/a:1006065926397. [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Rothstein SJ. Developmental regulation of two tomato lipoxygenase promoters in transgenic tobacco and tomato. Plant Mol Biol. 1997;33:835–846. doi: 10.1023/a:1005773722657. [DOI] [PubMed] [Google Scholar]
- Biale JB, Young RE. The avocado pear. In: Hulme AC, editor. The Biochemistry of Fruits and Their Products. Vol. 2. London: Academic Press; 1971. pp. 1–63. [Google Scholar]
- Biale JB, Young RE. Respiration and ripening in fruit: retrospect and prospect. In: Friend J, Rhodes MJC, editors. Recent Advances in the Biochemistry of Fruits and Vegetables. London: Academic Press; 1981. pp. 1–39. [Google Scholar]
- Boyer JS. Measuring the Water Status of Plants and Soils. San Diego: Academic Press; 1995. [Google Scholar]
- Brady CJ. Fruit ripening. Annu Rev Plant Physiol. 1987;38:155–178. [Google Scholar]
- Burbidge A, Grieve T, Jackson A, Thompson A, Taylor I. Structure and expression of a cDNA encoding a putative neoxanthin cleavage enzyme (NCE), isolated from a wilt-related tomato (Lycopersicon esculentum Mill.) library. J Exp Bot. 1997a;47:2111–2112. [Google Scholar]
- Burbidge A, Grieve T, Terry C, Corlett J, Thompson A, Taylor I. Structure and expression of a cDNA encoding zeaxanthin epoxidase, isolated from a wilt-related tomato (Lycopersicon esculentum Mill.) library. J Exp Bot. 1997b;48:1749–1750. [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J. 1999;17:427–431. doi: 10.1046/j.1365-313x.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- Callahan A, Morgens P, Walton E. Isolation and in vitro translation of RNAs from developing peach fruit. Hort Sci. 1989;24:356–358. [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J, Xu R, Kneissl ML, Ciardi JA, Kim K-N, Pelah D. Separation of cis elements responsive to ethylene, fruit development, and ripening in the 5′-flanking region of the ripening-related E8 gene. Plant Mol Biol. 1998;37:1001–1011. doi: 10.1023/a:1006091928367. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Von Heijne G. ChloroP, a neural network-based method for predicting transit peptides and their cleavage sites. Protein Sci. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero F, Mullet J. Increased abscisic acid biosynthesis during dehydration requires transcription. Plant Physiol. 1986;80:588–591. doi: 10.1104/pp.80.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki H, Maxson JM, Woodson WR. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc Natl Acad Sci USA. 1994;91:8925–8929. doi: 10.1073/pnas.91.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoda S, Saburi Y. Cloning, expression, and sequence-analysis of a lignostilbene-α-β-dioxygenase gene from Pseudomonas paucimobilis TMY 1009. Biosci Biotech Biochem. 1993;57:926–930. doi: 10.1271/bbb.57.926. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Léon-Kloosterziel KM, Schwartz SH, Zeevaart JAD. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem. 1998;36:83–89. [Google Scholar]
- Li Y, Walton DC. Violaxanthin is an abscisic acid precursor in water-stressed dark-grown bean leaves. Plant Physiol. 1990;92:551–559. doi: 10.1104/pp.92.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996;15:2331–2342. [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Burnett EC, Desikan R, Hancock JT. Cloning of a wilt-responsive cDNA from an Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot. 1998;49:1893–1894. [Google Scholar]
- Norman SM, Maier VP, Pon DL. Abscisic acid accumulation and carotenoid and chlorophyll content in relation to water-stress and leaf age of different types of citrus. J Agric Food Chem. 1990;38:1326–1334. [Google Scholar]
- Parry AD, Babiano MJ, Horgan R. The role of cis-carotenoids in abscisic acid biosynthesis. Planta. 1990;182:118–128. doi: 10.1007/BF00239993. [DOI] [PubMed] [Google Scholar]
- Parry AD, Griffiths A, Horgan R. Abscisic acid biosynthesis in roots: II. The effects of water-stress in wild-type and abscisic-acid-deficient mutant (notabilis) plants of Lycopersicon esculentum Mill. Planta. 1992;187:192–197. doi: 10.1007/BF00201937. [DOI] [PubMed] [Google Scholar]
- Parry AD, Horgan R. Carotenoid metabolism and the biosynthesis of abscisic acid. Phytochemistry. 1991;30:815–821. [Google Scholar]
- Parry AD, Neill SJ, Horgan R. Xanthoxin levels and metabolism in the wild-type and wilty mutants of tomato. Planta. 1988;173:397–404. doi: 10.1007/BF00401027. [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Zeevaart JAD. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA. 1991;88:7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefermann-Harms D, Joyard J, Douce R. Light-induced changes of the carotenoid levels in the chloroplast envelopes. Plant Physiol. 1978;61:530–533. doi: 10.1104/pp.61.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu RK, Walton DC. Xanthoxin metabolism in cell-free preparations from wild type and wilty mutants of tomato. Plant Physiol. 1988;88:178–182. doi: 10.1104/pp.88.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Voetberg G, Rayapati PJ. The effects of benzyladenine, cycloheximide, and cordycepin on wilting-induced abscisic acid and proline accumulations and abscisic acid- and salt-induced proline accumulation in barley leaves. Plant Physiol. 1986;82:703–707. doi: 10.1104/pp.82.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR. Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA. 1997;94:12235–12240. doi: 10.1073/pnas.94.22.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression: studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Heijne G, Steppuhn J, Herrmann RG. Domain-structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Watillon B, Kettmann R, Arredouani A, Hecquet JF, Boxus P, Burny A. Apple messenger RNAs related to bacterial lignostilbene dioxygenase and plant SAUR genes are preferentially expressed in flowers. Plant Mol Biol. 1998;36:909–915. doi: 10.1023/a:1005914506110. [DOI] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case in conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. Abscisic acid metabolism and its regulation. In: Hooykaas PJJ, Hall MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. Amsterdam: Elsevier Science; 1999. pp. 189–207. [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]
- Zeevaart JAD, Heath TG, Gage DA. Evidence for a universal pathway of abscisic acid biosynthesis from 18O incorporation patterns. Plant Physiol. 1989;91:1594–1601. doi: 10.1104/pp.91.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]