Abstract
Objectives
The maternal near-miss case review (NMCR) has been promoted by WHO as an approach to improve quality of care (QoC) at facility level. This systematic review synthesises evidence on the effectiveness of the NMCR on QoC and maternal and perinatal health outcomes in low-income and middle-income countries (LMICs).
Methods
Studies were searched for in six electronic databases (MEDLINE, Index Medicus, Web of Science, the Cochrane library, Embase, LILACS), with no language restrictions. Two authors independently screened papers and selected them for inclusion and independently extracted data. Maternal mortality was the primary outcome. Secondary outcomes included any outcome informing on any of the six dimensions of QoC: efficacy, safety, efficiency, equity, accessibility and timely care, acceptability and patient-centred care.
Results
Out of 24 822 papers retrieved, 17 studies from 11 countries were included. Maternal mortality measured before and after the implementation of the NMCR cycle significantly decreased (OR 0.77, 95% CI 0.61 to 0.98, eight studies, 55 573 043 women; I2=39%). A statistically significant reduction in the incidence of uterine rupture, postpartum haemorrhage and maternal sepsis was observed in three out of six studies. Ten studies reporting on maternal care process all showed some significant improvement when measured against predefined standards. All studies reported that the NMCR resulted in some amelioration of the facility structure (physical structure, staffing, equipment, training, organisation of care). Newborn outcomes were overall poorly reported; four studies showed no significant difference in perinatal mortality. Patient satisfaction and equity were also poorly reported.
Conclusions
Policy makers may consider implementing the maternal NMCR cycle approach among strategies aiming at improving QoC and reducing maternal mortality and morbidity in LMIC. Future studies should better document the effectiveness of the NMCR cycle particularly on outcomes reflecting patient-centred care and cost-effectiveness.
Keywords: quality in health care, clinical audit, maternal medicine
Strengths and limitations of this study.
The maternal near-miss case review (NMCR) approach has been used in different settings; however, so far no systematic review has ever reported on its effectiveness. The present review fills an existing gap in evidence synthesis by reporting latest evidence on the effectiveness of NMCR cycle as a type of criterion base audit in low-income and middle-income countries.
This review collected an appreciable number of studies reporting on the impact of the NMCR cycle from different regions worldwide, including Africa, Central Asia, South East Asia, Latin America and Caribbean and adds as new knowledge that this approach may be effective in reducing maternal mortality and in improving quality of maternal and newborn healthcare at facility level.
Findings of this review are limited by the paucity of existing scientific literature: despite the NMCR approach has been used in many countries, such as China, India, South Africa and the WHO European Region, scientific literature reporting on the NMCR effectiveness is relatively scarce.
Background
Ensuring adequate quality of healthcare is a primary objective of the WHO Global Strategy for Women’s, Children’s and Adolescent’s Health 2016–2030.1 2 Quality in healthcare is recognised by WHO as essential for the health and well-being of the population and as a basic aspect of human rights.2 3
Among different approaches aiming at improving quality of care (QoC) in maternity services, the maternal near-miss cases review (NMCR) approach was promoted by WHO and partners since 2004 within the strategy Beyond the Numbers.4 The facility-based individual NMCR cycle is defined as a type of criterion-based audit seeking to improve maternal and perinatal healthcare and outcomes by conducting a review, at hospital level, of the care provided to maternal near-miss cases.5 A maternal near-miss case is defined as a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or within 6 weeks after pregnancy.5
In the last 20 years, NMCR have been promoted as an alternative way to audit case management, more acceptable for health workers than mortality audits, which have been in use for many years.4 5 As a matter of fact, in low-mortality settings or at the health service level, the number of maternal deaths is usually insufficient or not representative enough to allow reliable policy guidance.4 Moreover, discussing cases of deaths may have legal implications and may be perceived as challenging by hospital staff.4 Near-miss cases occur more frequently than maternal deaths, their review can directly inform on both strengths and weakness in the process of care, and it is usually perceived by staff as easier to perform than mortality audits.5 6
The objective of the NMCR cycle is to identify areas amenable of improving QoC and finding and implementing solutions to the problems identified. Actions for improving QoC are proposed and agreed by hospital staff and subsequently monitored to check their implementation.5 This bottom-up approach aims at ensuring local ownership and facilitating team-building dynamics.5 Besides reviewing clinical management, the NMCR can cover other domains involved with delivery of care, including availability of essential equipment, staffing, training, policies and organisation of services.5 According to the WHO guidance,5 patients’ experience of care should be collected through interviews and taken into account in developing recommendations aiming at improving QoC.
The NMCR approach has been used in different settings;5 however, so far no systematic review has ever reported on its effectiveness. The objective of this review is to systematically evaluate and synthesise the evidence on the effectiveness of the NMCR cycle on the QoC and on maternal and perinatal health outcomes in low-income and middle-income countries (LMICs).
Methods
Search strategy and eligibility criteria
In conducting this review, we followed the guidelines reported in the PRISMA (Preferred Reporting Items for systematic reviews and meta-analyses).7 A protocol including detailed methods of the review was developed before starting the review.
We searched up to September 2017 the following databases: MEDLINE through Pubmed (from 1956); LILACS (no date restrictions); Global Index Medicus (no date restrictions); Science Citation Index Expanded (SCI-EXPANDED) through Web of Science (no date restrictions); Social Sciences Citation Index (SSCI) through Web of Science (no date restrictions); Cochrane library (no date restrictions); Embase through OVID (from 1996). The search strategy is reported in box 1. Manual searches of reference lists were also performed. We did not apply any language restrictions.
Box 1. Search strategy.
PubMed; Date: 15 September 2017; Total retrieved: 5578
“near miss" OR (audit AND (obstetric* OR matern* OR pregnan* OR woman OR women))
Lilacs; Date: 15 September 2017; Total retrieved: 227
(TW:near miss OR MH:near miss) OR ((TW:audit OR MH:audit OR TW:auditoria OR MH:auditoria OR auditoría) AND (gravid$ OR pregnan$ OR enceint$ OR embarazad$ OR obstetr$ OR mulher$ OR mujer$ OR femme$ OR woman OR women OR matern$))
Global Index Medicus; Date: 15 September 2017; Total retrieved: 7806
(TW:near miss OR MH:near miss) OR ((TW:audit OR MH:audit OR TW:auditoria OR MH:auditoria OR auditoría) AND (gravid$ OR pregnan$ OR enceint$ OR embarazad$ OR obstetr$ OR mulher$ OR mujer$ OR femme$ OR woman OR women OR matern$))
Web of Science; Date: 18 September 2017; Total retrieved: 4850
TS= “near miss” OR (TS=audit AND TS=(gravid* OR pregnan* OR obstetr* OR woman OR women OR matern*))
Cochrane Library; Date: 15 September 2017; Total retrieved: 411
“near miss” OR (audit AND (gravid* or pregnan* or obstetr* or woman or women or matern*))
EMBASE; Date: 15 September 2017; Total retrieved: 5927
("near miss" or audit).ab. (34259)
(obstetric* or matern* or pregnan* or woman or women).ab. (1057153)3
1 and 2 (4764)
("near miss" or audit).ti. (13725)
(obstetric* or matern* or pregnan* or woman or women).ti. (325314)
4 and 5 (724)
3 or 6 (4962)
Studies were eligible for inclusion if they reported on the effectiveness (outcome) on maternal and perinatal healthcare (population) of the individual NMCR cycle at facility level (intervention), in a LMIC (setting), defined as for the World Bank definition at the time of the study.8 Given the paucity of randomised controlled trials (RCTs) on the subject, we also opted to include in this review non-randomised controlled clinical trials, controlled before and after studies (CBAs), uncontrolled before and after studies (UCBAs) and intermittent time series (ITSs). Qualitative studies were excluded. Both studies using the WHO definition of a maternal near-miss case published in year 20119 or previous/locally adapted definitions, such as locally developed disease-specific definitions, were included. Studies reporting on interventions where the full audit cycle was implemented (ie, including implementation of changes) were included, while studies only reporting descriptive findings of the case review (ie, identifications of gaps in case management without developing and implementing recommendations) were not eligible. Abstracts and unpublished reports were also not eligible for inclusion.
Maternal mortality was predefined as our primary outcome. Secondary outcomes included any outcome informing on any of the six dimensions of QoC,10 namely: efficacy (eg, maternal morbidity), safety (eg, adverse events), efficiency (cost), equity (eg, equitable care), accessibility and timely care (eg, access to care), acceptability and patient-centred care (eg, patient satisfaction). Effectiveness on the QoC is reported according the Donabedian model of quality improvement, which differentiates between: (1) outcomes of care (eg, health outcomes, costs, satisfaction), (2) process of care (eg, diagnosis and treatment); (3) and inputs/structure (eg, physical structure, staffing, equipment and supplies, training, policies and organisation of care).11
Data collection and analysis
Studies were selected for inclusion by two independent authors in two teams (VC and AE, ML and SR). Any disagreement was resolved through discussion. The full text of all eligible citations was examined in detail. Two authors (ML, SR) extracted data from included studies, using a prepiloted data-extraction form. Disagreements were resolved by discussion between the two authors and consensus with a third author.
We extracted information regarding: study setting, design and duration; characteristics of the intervention; type of outcomes evaluated; effectiveness of the NMCR on the outcomes. For the study with ITS design we included in the meta-analysis of maternal mortality the first and the last time point reported. Data on effectiveness were extracted as crude numbers or percentages. Data on maternal mortality were extracted as disease-specific maternal mortality when case reviews focused only on specific diseases and as total maternal mortality when case reviews included all major obstetric emergencies.
When meta-analysis was possible and appropriate, for each outcome factor we generated a pooled OR using the Mantel-Haenszel weighting method.12 Pooled data were presented in forest plots; data that could not be meta-analysed was presented in tables and text. We tested the null hypothesis that all studies evaluate the same true effect by the Cochran’s Q test, with two-sided p<0.05 considered statistically significant. The degree of heterogeneity between studies was assessed by visual inspection of the forest plots and I-squared (I2) statistic with its 95% CI and interpreted according to the Cochrane manual.12
The Cochrane ‘Risk of bias’ tool modified with the Cochrane Effective Practice and Organization of Care Group (EPOC) criteria for ITSs12 was used to assess the risk of bias in included studies. We aimed at performing the following sensitivity analyses: (1) removing the studies with high risk of bias; (2) removing studies including less than 300 cases and less than 30 events (ie, cases of maternal death or perinatal death). We performed a subgroup analysis exploring the effect of NMCR in low-income countries (defined as for the World Bank definition at the time of the study)8 compared with middle-income countries.
Results
Characteristics of the studies
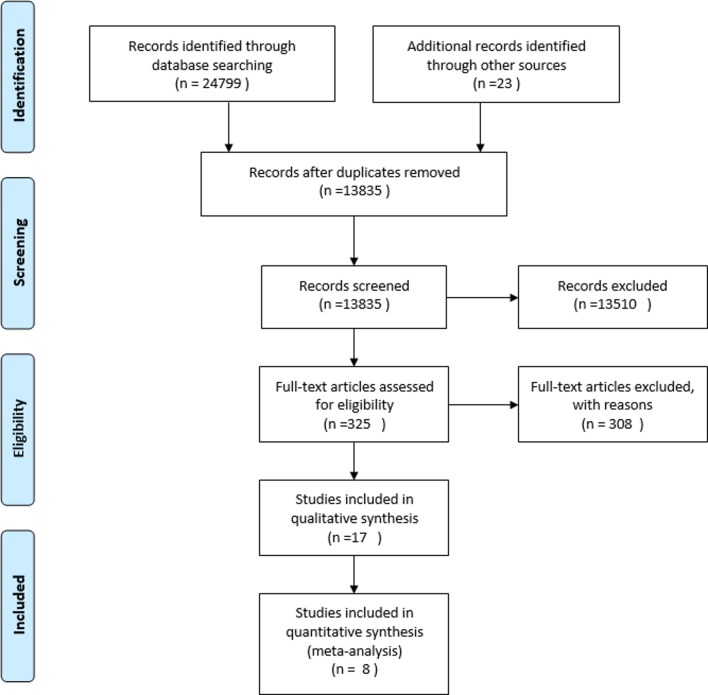
The search yielded overall 24 822 records (figure 1). Overall 17 papers13–29 from Africa (Ghana, Ethiopia Malawi, Nigeria, Tanzania, Uganda), Europe and Central Asia (Kazakhstan, Moldova), South East Asia (Malaysia, Vietnam) and Latin America and Caribbean (Jamaica) met the inclusion criteria.
Figure 1.
Study flow diagram.
Characteristics of the study settings and design are summarised in table 1. All except one study23 were published during the last 15 years. Two papers referred to the same experience;20 21 findings from these studies are jointly reported in the tables, and we used the most recent reference20 to identify them. All studies were UCBAs-, describing the effectiveness of the NMCR cycle with a before and after analysis, except for two studies with ITS design.13 22 Studies duration ranged from a minimum of 6 months27 to a maximum of 26 months.29 Ten studies were held in an urban setting,13–17 19 20 25 28 29 three in a rural setting22 24 27 and three in a mixed setting.18 23 26 One study was multicentred (Ghana and Jamaica).29 Among the 16 experiences reported, nine were of large size: one very large study In Malawi included 73 facilities in three districts;26 another three studies in Malawi enrolled, respectively, 29 and 13 facilities of different level and type,22 27 while one was conducted in one referral hospital plus several (number not further specified) health centres24; a study in Ethiopia involved 10 public hospitals;17 studies in Kazakhstan, Vietnam, Ghana, Jamaica and Moldova involved six, five, four and three hospitals, respectively.18 20 23 29 The remaining seven studies were single-centre studies and took place in one teaching/tertiary level care hospital each.
Table 1.
Study settings, designs and sample sizes
| Authors | Design | Duration | Country | Setting | Number and type of hospitals |
| Lumala et al, 201713 | ITS | 10 months | Uganda | Urban | One tertiary specialist hospital, catholic funded private non-profit |
| Mgaya et al, 201714 | NCBA | 25 months | Tanzania | Urban | One tertiary specialist hospital |
| Kayiga et al, 201615 | NCBA | 7 months | Uganda | Urban | One tertiary specialist hospital |
| Mohd Azri et al, 201516 | NCBA | 2 years | Malaysia | Urban | One tertiary specialist hospital |
| Gebrehiwot and Tewolde, 201417 | NCBA | 18 months | Ethiopia | Urban | 10 public hospitals |
| Baltag et al, 201218 | NCBA | 13 moths | Moldova | Mixed | Three mixed (referral-level facilities at municipal, national and district levels) |
| Kidanto et al, 201219 | NCBA | 3 years | Tanzania | Urban | One teaching hospital |
| Sukhanberdiyev et al, 201120 and Hodorogea, 201021 |
NCBA | 2 years | Kazakhstan | Urban | Six mixed (national research centre, regional and city hospitals) |
| Van den Akker et al, 201122 | ITS | 2 years | Malawi | Rural | 29 mixed (one referral hospital and 28 government, private and mission smaller facilities) |
| Bailey et al, 201023 | NCBA | 2 years | Vietnam | Mixed | Five mixed (provincial, area and district) |
| Van den Akker et al, 200924 | NCBA | 1 year | Malawi | Rural | One referral hospital+undefined numbers of health centres |
| Hunyinbo et al, 200825 | NCBA | 13 months | Nigeria | Urban | One tertiary specialist hospital |
| Kongnyuy et al, 200826 | NCBA | 2 years | Malawi | Mixed | 73 mixed (hospitals, health centres) |
| Kongnyuy et al, 200827 | NCBA | 6 months | Malawi | Rural | One district hospital, 12 satellite health centres |
| Weeks et al, 200528 | NCBA | 20 months | Uganda | Urban | One teaching hospital |
| Wagaarachchi et al, 200129 | NCBA | 26 months | Ghana and Jamaica | Urban | Four district hospitals |
ITS, intermittent time series; NCBA, non-controlled before and after study.
Characteristics of the intervention are summarised in table 2. In about half of the studies, cases were audited prospectively,15 17 18 20 22 24–26 while in the other studies audits were either conducted retrospectively12 13 27 or retrospectively in a first phase and then prospectively in the second phase.16 19 23 28 29 While in all cases the internal staff within the facility was involved in developing the recommendations, studies differed by who performed the case reviews: in most experiences, audits were conducted by internal staff within the facility/ies, with the exception of four cases where a study investigator/physician audited the cases against predefined criteria and later presented it to hospital staff13 19 25 29 and two cases where this information was not specified.15 16 Type of obstetric complications selected for audit included: severe pre-eclampsia/eclampsia,13 16 19 22 23 25–29 postpartum haemorrhage,13 20 22 23 25–27 29 obstructed labour,14 15 23 26 27 29 uterine rupture,24 25 29 infections,23 25 27 complications of abortion.27 Five studies focused on one complication only14–16 24 28 while in all other studies more than one condition was audited. In three studies, cases of maternal mortality were audited together with cases of near-miss.17 22 26 The criteria for case selection was ‘all cases occurring in the study period’, except in one experience in Malawi where cases of particular educational interest were selected,24 and a study in Moldova where, despite no predefined criteria, it was observed that cases ‘more likely to lead to praises for the maternity team’ were selected.18 The number of total cases audited in each study ranged widely, from 30 cases18 to 2568 cases.17
Table 2.
Characteristics of the interventions
| Authors | Characteristics of the audit | Who performed the audit | Who developed the recommendations | Type of cases audited | Selection criteria | N case audited (before/after) |
Woman interview |
| Lumala et al, 201713 | Two phases, retrospective | Medical doctor | Facility staff | PPH and severe pre-eclampsia, eclampsia | All in-patient cases in the study period, not referred and not receiving hydralazine or magnesium sulfate from the referring unit | 238 (125 before, 133 after) | No |
| Mgaya et al, 201714 | Two phases, retrospective | Trained postnatal ward nurses, (a consultant, a specialist and a midwife were also available for consultation) | Facility staff (AN, L, MO, MW, P) |
Obstructed labour | All cases of obstructed labour with a single fetus in cephalic presentation and no other severe medical conditions or PROM | 510 (260 before, 250 after) | Yes |
| Kayiga et al, 201615 | Two phases, prospective | NR | Facility staff (MO, MW, M) |
Obstructed labour | All cases occurring in the study period | 360 (180 before, 180 after) | Yes |
| Mohd Azri et al, 201516 | First phase retrospective, second regular prospective | NR | Facility staff (members of the obstetric department) |
Eclampsia | All cases occurring in the study period | 51 (42 before, nine after) | No |
| Gebrehiwot et al, 201417 | Prospective | Facility staff (MO, MW and other hospital staff+focal person) | Facility staff | All NM+MD | All cases occurring in the study period | 2568 | No |
| Baltag et al, 201218 | Prospective | Facility staff involved in case management (MO, MD+occasionally L, T, PHC) | Facility staff involved in case management (MO, MD+occasionally L, T, PHC) | NM | Not predefined criteria, cases were chosen by director | 30 approx (one case per month in each hospital) | Yes |
| Kidanto et al, 201219 | First phase retrospective, second prospective | One senior doctor | Facility staff | Eclampsia and pre-eclampsia | All cases occurring in the study period | 477 (389 before, 88 after) | No |
| Sukhanberdiyev et al, 201120 and Hodorogea, 201021 |
Prospective | Facility staff | Facility staff | PPH and severe pre-eclampsia | NR | not more than 10 in each hospital each year | Yes |
| Van den Akker et al, 201122 | Prospective every 2–3 weeks | Facility staff, occasionally external obs gyn | Facility staff | Infection, PPH, uterine rupture, preeclampsia, others)+MD | All cases occurring in the study period | 45 (24 deaths; 21 SOC) | No |
| Bailey et al, 201023 | First phase retrospective, than regular prospective | Facility staff (MO, N, M) |
Facility staff (MO, N, M) |
Severe preeclampsia, postpartum infection, prolonged/obstructed labour, PPH, organisation of emergency service | All cases occurring in the study period | 558 (312 before, 246 after) | No |
| Van den Akker et al, 200924 | Prospective every 2–3 weeks for 3 months | Facility staff (M, MA, MO, MW, N); Two external obstetricians in the second phase |
Facility staff (MO, N, M) |
Uterine rupture | Cases that appeared to be of particular educational value to the PI or any other hospital staff | 35 | No |
| Hunyinbo et al, 200825 | Two phases, prospective | Study investigator/s | Facility staff (M, MA, MO, N, P, L) |
PPH, uterine rupture, eclampsia, obstructed labour, sepsis | All cases occurring in the study period | 130 (65 before, 65 after) | No |
| Kongnyuy et al, 200826 | Two phases, prospective | Facility staff (AN, M, MO, MW, L, T) | Facility staff (quality improvement team) |
PPH, obstructed labour, sepsis, Preeclampsia/eclampsia, neonatal care, CS, women-friendly care+MD | NR | NR | No |
| Kongnyuy et al, 200827 | Two phases, retrospective | District team (N, MW, CO, AN, T) | Hospital staff (quality improvement team) | Pre-eclampsia/eclampsia, PPH, prolonged/obstructed labour, retained placenta, sepsis, complications of abortion, ectopic pregnancy | All cases occurring in the study period | 122 (60 before, 62 after) | No |
| Weeks et al, 200528 | First phase retrospective, second prospective | Facility staff (including low grade staff) |
Facility staff | Severe pre-eclampsia | All cases occurring in the study period | 86 (43 before, 43 after) | No |
| Wagaarachchi et al, 200129 | First phase retrospective, second prospective | Non-medical assistants (10% of cases validated by independent re-review) | Facility staff (M, MO, M+all relevant staff) |
PPH, eclampsia, infection, obstructed labour, uterine rupture | All cases occurring in the study period | 889 (551 before, 338 after) | No |
AN, anaesthetist of anaesthetic technician, CO, clinical officer; CS, caesarean section; L, laboratory staff; M, manager; MA, medical assistant; MD, maternal deaths; MO, medical officer MW, midwife; N, nurse; NM, near miss, NR, not reported; P, pharmacy; PHC, primary healthcare staff; PI, principal investigator; PPH, postpartum haemorrhage; PROM, premature rupture of membranes; SOC, all severe obstetric cases; T, technician.
Only in four experiences, women were interviewed,14 15 18 20 but in one of them this was explicitly merely for recording bureaucratic details,15 rather than for the purpose of collecting women views and perspectives on QoC received. All studies associated the audits with the development or implementation of standards of care (used also in most cases to perform the audits), while few studies also associated additional interventions for the hospital staff, such as development/dissemination of guidelines and training on case management.13 15 23
As reported in online supplementary table S1, types of outcomes evaluated in the studies reported mostly on two dimensions of QoC:10 effectiveness and accessibility and timely care. Outcomes related to the other dimension of QoC, such as patient centrality and acceptability (eg, patient satisfaction), efficiency and equity, safety (eg, rate of adverse events, incident reporting) were not explored, with the exception of one study in Kazakhstan reporting on improved patients satisfaction20 and one in Moldova reporting improved attitude towards patients.18
bmjopen-2017-019787supp001.pdf (214.5KB, pdf)
Effectiveness of the NMCR cycle
Effectiveness on health outcomes
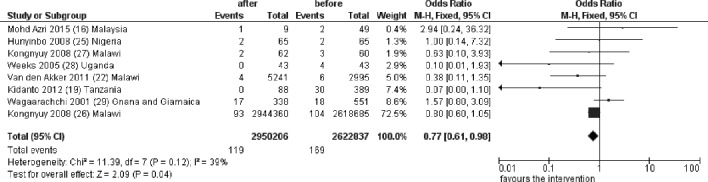
In a meta-analysis including eight studies, maternal mortality, measured before and after the implementation of the NMCR cycle, significantly decreased (OR 0.77, 95% CI 0.61 to 0.98, 55 573 043 women, figure 2), with relatively low heterogeneity between studies (I2=39%). An additional study from Uganda reported to have observed a reduction in maternal mortality, but data were not further made explicit.15
Figure 2.
Pooled effect of the NMCR on maternal mortality. NMCR, near-miss case review.
Three out of six studies reported a statistically significant reduction in the incidence of the following preventable obstetric complications: uterine rupture, major postpartum haemorrhage and maternal sepsis (table 3).
Table 3.
Effectiveness of the NMCR cycle on morbidity and on process outcomes
| Authors | Morbidity and other health outcomes (incidence) | Standards of care (improved standards) | Other process outcomes |
| Lumala et al, 201713 | – | Eclampsia and pre-eclampsia: 7/10 standards PPH: 3/4 standards |
– |
| Mgaya et al, 201714 | SAMM: 9.0% vs 8.8% (p=0.98). Uterine rupture: 1/260 vs 0/250 (p=0.49) Perinatal severe morbidities and deaths and fresh stillbirths: 16% vs 8.8% (p=0.01) |
Obstructed labour: 6/10 standards on diagnosis, 6/10 standards on case management | Significant reduction of time needed from decision to perform a caesarean section to delivery (mean difference: 30 min, p<0.001) |
| Kayiga et al, 201615 | Uterine rupture: 8/180 vs 2/180 (p=0.04) Maternal sepsis: 10/180 vs 2/180 (p=0.02) Postspinal headache: 0/180 vs 13/180 (p<0.001) Baby admitted to intensive care: 27/180 vs 31/180 (p=0.61) |
Obstructed labour: 2/6 standards, 4/13 measures of standards | – |
| Mohd Azri et al, 201516 | Eclampsia: 42/44818 vs 9/10784 (p>0.05) Recurrent eclamptic fits: 8/42 vs 1/9 (p>0.05) Newborn babies with Apgar score (<7) at 5 min after birth: 8/42 vs 3/9 (p>0.05) Birth weight less than 2500 g 22/42 vs 5/9 (p>0.05) |
Improved adherence to 2/2 audit criteria that where substandard in the first phase (all other 10 criteria were already according to standards at baseline) | – |
| Gebrehiwot and Tewolde, 201417 | – | – | Reducing waiting time |
| Baltag et al, 201218 | – | – | Improved medical records Improved attitude towards patients |
| Kidanto et al 201219 | – | Eclampsia and pre-eclampsia: 10/16 standards | Improved records keeping |
| Sukhanberdiyev et al, 201120 and Hodorogea, 201021 |
Improved patient satisfaction (NR) | – | Improved case management and monitoring (eg, weighing of blood losses and documenting systematically) |
| Van den Akker et al, 201122 | SAMM: 33/2295 vs 49/5291 (p=0.08) Major PPH: 17/2295 vs 15/5291 (p=0.006) Uterine rupture: 14/2295 vs 4/5291 (p=0.03) Severe pre-eclampsia: 6/2295 vs 16/5291 (p=0.3) Maternal infections: 10/2295 vs 14/5291 (p=0.6) |
– | Improved patients monitoring |
| Bailey et al, 201023 | – | Eclampsia: 12/18 standards Infections: 11/23 standards Obstructed labour: 1/1 standards PPH: 3/3 standards |
– |
| Van den Akker et al, 200924 | Uterine rupture: 16/833 vs 19/3099 (OR 0.32; 95% CI 0.16 to 0.63) | – | – |
| Hunyinbo et al, 200825 | SAMM: 8/31 standards | – | |
| Kongnyuy et al, 200826 | – | – | Significant increase in the met need for EmOC (15.2% for 2005, 17.0% for 2006 and 18.8% for 2007, p value for trend<0.001). |
| Kongnyuy et al, 200827 | – | SAMM: 4/7 standards (other criteria were already according to standards at baseline) |
– |
| Weeks et al, 200528 | Eclampsia: 5/43 vs 5/43 (p>0.05) | Severe pre-eclampsia: 5/9 standards | – |
| Wagaarachchi et al, 200129 | – | SAMM: 8/31 standards | – |
EmOC, Emergency Obstetric Care; NMCR, near-miss case review; NR, not further specified; PPH, postpartum haemorrhage; SAMM, severe acute maternal morbidity.
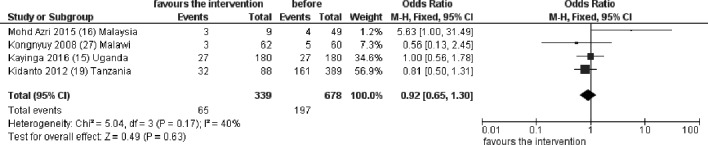
Newborn outcomes were overall poorly reported. Of five studies documenting perinatal mortality, fours could be included in the meta-analysis, showing no significant differences in perinatal deaths in the before and after period (OR 0.92, 95% CI (0.65 to 1.30), figure 3) with low heterogeneity between studies (I2=40%). The fifth study,14 conducted in Uganda, reported a significant reduction in the incidence of a combined outcome including perinatal severe morbidities, deaths and stillbirths (table 3). Only one study reported on number of newborns admitted to intensive care unit, without statistical difference in the before and after NMCR period.15 Another single study reported on Apgar score birth weight, without changes in the before and after period.16
Figure 3.
Pooled effect of the NMCR on perinatal or neonatal mortality. NMCR, near-miss case review.
One study reported increased patient satisfaction after the implementation of the NMCR cycle.20
Effectiveness on process outcomes
The effectiveness of the NMCR on the process of care is synthetised in table 3. Ten studies reported on the process of care when measured quantitatively against predefined standards and all showed some significant improvements.13–16 19 23 25 27–29 Six studies reported other findings, such as improved case documentation, case-referral, use of partograph, monitoring and improved team work.14 17 18 20 22 26
Effectiveness on structure outcomes
Effectiveness on the structure is detailed in table 4. All studies reported some improvements in one or more domains. Overall most frequent changes relate to: purchasing of essential equipment and supplies; additional training, monitoring and supervision; policies and organisation of care including reorganisation of staff and their duties, implementation of systems aiming at standardising case management through dissemination of guidelines, checklists and monitoring forms, better coordination among different services.
Table 4.
Effectiveness of the NMCR cycle on the structure
| Authors | Physical structure | Staffing | Equipment and supplies | Training, monitoring and supervision | Local policies and organisation of services |
| Lumala et al, 201713 | |||||
| Mgaya et al, 201714 | Training on partograph, improved supervision | Improved dissemination and use of guidelines, Improved team work and internal communication among hospital staff | |||
| Kayiga et al, 201615 | Re-engineering hospital Red Alert System: list of responsible person to be contacted during Red Alert activation was put up in all obstetrics facilities; information on the importance of activating the Red Alert in eclampsia cases was disseminated to all staff; hospital telephone operator was informed regarding existence of this system and how it functioned | ||||
| Mohd Azri et al, 201516 | Better specification of roles and responsibilities | Training, improved awareness of standards, improved patient education | Reorganisation of ‘red alert’ system | ||
| Gebrehiwot and Tewolde, 201417 | Some hospitals expanded accommodate more cases | Staff organisation: duties assignment; staff rotation every 12 hours to avoid tiredness | Contribution of resources (stationery, transport) | Provision of training and feedback to health centres | Improved dissemination of protocols, increased use of partograph, Improved documentation and reporting improved coordination with health centres |
| Baltag et al, 201218 | Improved equipment and supplies | Improved dissemination of protocols, organisation of care and management | |||
| Kidanto et al, 201219 | Improved doctor availability 24/24 hours | Additional equipment purchased | Training | Improved dissemination of protocols, monitoring forms, reorganisation of daily routine and setting of priorities, doctors assigned to manage cases of eclampsia | |
| Sukhanberdiyev et al, 201120 and Hodorogea, 201021 |
Rational use of staff by internal redistribution, optimisation of human resources by reducing the working hours, increased role of mid-level staff (midwives and nurses) | Mobile devices for timely alert and warning, drugs and blood components, prostaglandins and uterotonics | Training on protocols and standards, periodic drills, improving time management skills | Developing, diffusing and use new evidenced-based protocols, developing emergency care algorithms and conditions for transportation from remote areas, identifying the responsible person for the readiness of the emergency kit, monitoring forms, weighing of blood losses and documenting systematically | |
| Van den Akker et al, 201122 | Training, regular on job coaching, improved supervision, monitoring of ambulance use | Improved dissemination of protocols and use of partograph, doctors to visits critically ill patients at least once a day | |||
| Bailey et al, 201023 | Purchase of equipment (lab, car for on-call, telephone for emergency), wall flow charts | Training, supervision | Leadership on implementing changes, standardisation of treatment with protocols and checklists, team work record keeping | ||
| Van den Akker et al, 200924 | More ambulances | Training, supervision, follow-up visits in health centres | Improved dissemination of protocols, transport organisation, organise session for theatre staff with the intention to reduce delay in surgical care | ||
| Hunyinbo et al, 200825 | Pharmacy supply including oxytocins, MgSO4, blood and coagulation tests | Improved dissemination of protocols, clinical meetings, observational and fluid balance charts | |||
| Kongnyuy et al, 200826 | The number of comprehensive and basic EmOC facilities did not change | ||||
| Kongnyuy et al, 200827 | Autonomy in decision making in MW-N | Better equipment and set up of service | Training | Reorganisation of emergency care service, including use of ambulances | |
| Weeks et al, 200528 | Staff in the labour room reorganised giving each member a specific role in the management of emergencies; two extra MW | Equipment (urine dipstick, BP machines) | Triage established, leadership (direct of labour appointed), protocol and chart, commitment to improve medical files, departmental meetings, fundraising (a fundraising committee was established to raise funds for the drugs and equipment in recommendations) | ||
| Wagaarachchi et al, 200129 | Record storage, blood cultures, structured patient records | Improved dissemination of protocols, reviewing supervisory responsibilities, organisation of regular clinical meetings |
BP, blood pressure; EmOC, Emergency Obstetric Care; MW, midwives; N, nurses.
Risk of bias and other analyses
All studies were rated as a high risk of bias based on the Cochrane and EPOC criteria (online supplementary table S2), mostly due to the study design (non-CBA or ITS studies).
The sensitivity analysis showed that when studies with a very small sample size were excluded, the effect of the NMCR on maternal mortality becomes stronger than when all studies were included (OR 0.71, 95% CI 0.55 to 0.90, three studies I2=86% online supplementary figure S1). The effect of NMCR on perinatal mortality did not significantly change in the sensitivity analysis (online supplementary figure S2).
bmjopen-2017-019787supp002.pdf (149.1KB, pdf)
Thirteen studies were held in low-income countries,13–15 17 19 22–29 two in upper middle-income countries16 20 and one in a lower middle-income country18 (online supplementary table S3). In the subgroup analysis, the effect of NMCR on maternal mortality was statistically significant in low-income countries (R=0.77, 95% CI 0.60 to 0.98, seven studies), while only one small study could be included in the category of middle-income countries, without statistical significance (online supplementary figure S3). The effect of NMCR on perinatal mortality was not affected by subgroup analysis (online supplementary figure S4).
Funnel plots did not suggest publication bias (online supplementary figures S5 and S6).
Discussion
This review suggests that the facility-based individual maternal NMCR cycle may be an effective strategy for reducing maternal mortality in high-burden countries and for improving overall quality of maternal care in LMIC. Results of a pooled analysis of findings from eight studies showed that the NMCR cycle significantly reduced maternal mortality (OR 0.77, 95% CI 0.61 to 0.98, figure 2), with relatively low heterogeneity of results (I2=39%). Three out of six studies reported a significant reduction in the incidence of preventable obstetric complications such as uterine rupture, major postpartum haemorrhage and maternal sepsis. Out of ten studies reporting on the process of care when measured against predefined standards all showed some statistically significant improvement. Additionally, in all studies, the implementation of the NMCR cycle resulted in some amelioration in the structure of the hospital, such as an increased availability of essential equipment and supplies, additional training, monitoring and supervision and the implementation of new policies and better organisation of services.
Previous systematic reviews had observed a benefit of criterion-base audits in improving the quality of obstetric care.30–32 However, a review on the effectiveness of criterion-base audits in LMIC published some years ago concluded that, despite criterion-base audits being increasingly used, few studies had reported on their effectiveness.33 The present review retrieved all latest evidence on the effectiveness of NMCR cycle as a type of criterion-based audit, synthesised studies from LMIC in different geographical regions—including Africa, Central Asia, South East Asia, Latin America and Caribbean—and adds as new knowledge that this approach may be effective in reducing maternal mortality and in improving quality of healthcare provided.
Findings of this review are limited by the paucity of existing scientific literature: the NMCR approach has been used in many more countries than could be included in this reviews, such as China,34 India,35 South Africa36 and the WHO European Region,37–41 but scientific literature reporting on the NMCR effectiveness in these countries could not be retrieved. Second, all included studies had an UCBA or ITS design, thus being exposed to a high risk of bias (although most studies checked for potential confounding factors, such as the case mix in the before and after phase). Despite these limitations, this review collected an appreciable number of studies, including also some large studies,17 22 26 27 reporting on the impact of the NMCR cycle from different regions worldwide. Although quantitative findings of the review were to some extent affected by one large study,26 it must be acknowledged that results of most studies were in the same direction (figure 1) and in all studies some significant gains, either in the standards of care or in the process outcomes, were observed. In some studies, a significant benefit in maternal mortality or in standards of care could not be detected because in-hospital maternal mortality was too low18 20 or because standards of care were already good at the baseline.13 23 27 Ideally, it will be advisable to perform large multicentre RCTs to properly document NMCR effectiveness. However, in practice conducting a RCT on criterion-based audit alone may be challenging and may even be perceived as unethical, if no appropriate comparison is chosen. This is because in current practice criterion-based audits are already one of the recommended strategies to improve QoC promoted by many agencies and bodies, such as the National Institute for Clinical Excellence (NICE).42 Notably, the review of ‘near-miss’ cases is already recommended by WHO as a ‘key action to eliminate avoidable maternal and perinatal mortality and morbidity and improve the QoC,43 and as such it is already implemented in several countries.
The audit of maternal near-miss cases is an approach also used in several high-income settings: UK has a well-established programme of confidential enquiries into maternal deaths and a national system for research on maternal near-miss-the UK Obstetric Surveillance System (UKOSS);44 45 New Zealand established a national system for severe maternal morbidity review;46 several countries within the International Network of Obstetric Survey Systems (INOSS) are collecting data on severe maternal morbidities for study purposes,47 while other countries such as Italy (ITOSS) are starting the implementation of near-miss audits.48 49 Although there are some differences in the type of interventions applied (eg, not all of these approaches are facility-based), still the existence of these large networks on maternal NMCRs and the amount of resources devoted to them somehow testify the importance recognised in reviewing near-miss cases.
In the future, rather than investing resources in exploring whether near-miss audits or criterion-based audits in general are overall effective, it will be more interesting to explore which characteristics make them effective and sustainable. Available literature synthesised in this review does not allow for directly comparing the effectiveness of different methodologies on how to perform audits in practice, but at least it does provide some useful starting point for discussion and for future research. First, with regard to the number of cases audited, this varied largely in the included studies from a minimum of less than 10 cases per year18 20 to a maximum of several hundred cases in a few months,14 29 with a third approach consisting in performing a large retrospective review of past cases as the baseline and then collecting fewer new cases prospectively. When many cases were reviewed, this allowed for an in-depth description of the gaps in care. However, the analysis of a large number of cases does not necessarily ensure the development of good recommendations for quality improvement, neither their implementation. Additionally, the sustainability of auditing on a large number of cases, outside a research setting with dedicated human and economic resources, is questionable. Studies included in this review suggest that even the periodic review of few cases may help identifying gaps in routine care, developing SMART recommendations (ie, Specific, Measurable, Achievable, Realistic, Time-bound),50 and improving QoC significantly.18 20 WHO recommends to organise one session of NMCR per month and to review in each session few cases (one or two), but pretends a high quality in the process: each session should start by checking if previous recommendations have been implemented; there should be a in-depth discussion of the underlying causes of the near-miss event (‘why but why’ approach); recommendations should be SMART; regular sessions should be organised; dissemination of results should be ensured and so on.5 At first, few facilities should be selected for pilot implementation and the NMCR approach should be further scaled up only when quality in the process has been ensured.
Second, studies included in this review revealed that most experiences of implementation of NMCR cycles were externally supported, either by the WHO, academia and/or other development partners.15 18 20–24 26–28 This is in line with other existing literature51 52 highlighting that in particular the second part of the audit cycle (ie, developing recommendations, implementing them, checking on progress) is in general problematic and usually less well conducted compared with the first part of the audit cycle. The attitude to openly discuss cases within a multidisciplinary team and agreeing solutions was described as challenging in different settings, especially for mid-level staff (midwives, nurses) who may not be used to voice their views in the presence of doctors and managers.18 20 Hospital staff, managers included, often do not receive any formal training in quality improvement methods or any guidance in correctly performing an audit cycle. The need for ensuring sustained external support, and for establishing a functional quality assurance mechanism, is recognised by WHO as crucial for ensuring an effective NMCR implementation.5
Third, although having a single person appointed to perform the case review—as performed in some studies included in this review10 18 25 29—may increase feasibility, this actually largely reduces ownership of the process, together with minimising occasions for discussion and team building among staff. Studies noted that involvement of all healthcare providers in the audit process promoted successful implementation, ownership and sustainability of the process.14 20 28 The involvement of mid-level staff such as nurses and midwives was reported to result in improved staff autonomy and team work.14 21 27 Some studies observed that participation of the senior management promoted the implementation of recommendations that required allocation of resources and changes in policies and organisation of care.26 28 Currently, the WHO approach5 recommends the NMCR to be performed by the staff who managed the cases, including nurses, midwives and any other staff directly or indirectly involved in case management.
Fourth, the patient experience of care was assessed only in very few of the existing studies and yet not fully taken into account. In the last few years, WHO has given increasing importance to patient experience of care.1 Listening to women’s views may provide important information, as testified by studies in Brazil, Rwanda and the UK53–55 and by a study in Iran where women’s views were successfully used to improve QoC.56 Currently, WHO recommends to always interview women and their families and to use their inputs for improving care.5
Finally, as pointed out by authors of the included studies, interventions aiming at improving QoC without strengthening the health systems and improving community awareness may have minimal success.15 22 A study in Malawi reported that availability of essential supplies, such as blood for transfusions, remained low even after the NMCR, due to health system failures and this clearly was a barrier for improving case management.22 Qualitative findings, collected through focus groups among staff in a study in Uganda,15 pointed out, among issues that may have hampered the effectiveness of NMCR, health facility factors such as: stock-out of essential supplies, shortage of human resources, lack of task allocation, inadequate supervision. However, in most studies, even if the number of staff and available resources remained stable in the before and after phase, as a result of the audit, there was a reorganisation of staff activities, such as better specification of roles and responsibilities, task shifting and improved communication.14 16 17 20 28
Cost of the NMCR approach in improving health outcomes and QoC was not formally evaluated in the retrieved studies. However, several papers stated that the NMCR was an inexpensive and simple intervention, requiring little technology.24 26–28 A study involving 12 health centres in Malawi reported that each audit meeting cost about US$150, including foods and transport of participants to the District Hospital.27 Another study in Uganda stated ‘the audit process had challenged the assumption that all quality improvements need to be externally provided and are expensive’.28 These findings are in line with a systematic review of barriers and facilitators for effective NMCR implementation, reporting that a relatively low budget is needed to facilitate activities.37 In some experiences, the NMCR improved use or availability of existing economic resources: in Malawi, it ‘promoted a wiser allocation of resources for maternity care at the district level’;27 in Uganda, a fundraising committee was established to raise funds for drugs and equipment needed according to the recommendations.28
Conclusion
Implication for policy and research
Among other strategies to reduce maternal mortality and morbidity and for improving the quality of maternal and perinatal care, policy makers may consider the implementation of the maternal NMCR cycle approach.
Researchers should aim at generating more evidence on how to effectively implement the NMCR cycle, how to improve its impact on newborn outcomes and on outcomes reflecting patients’ centrality (such as patient satisfaction and/or perception of QoC received), together with documenting the cost effectiveness of the NMCR approach.
Supplementary Material
Footnotes
Contributors: ML conceived the papers, screened the study, extracted data, drafted the paper and finalised the paper. SR, VC and AE screened the study, extracted data and revised the first draft.
Funding: This review was funded by a grant from the GREAT Network, Canadian Institutes of Health Research, St. Michael’s Hospital, Toronto.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All details of the analyses conducted are provided within the manuscript.
References
- 1. Tunçalp Ӧ, Were WM, MacLennan C, et al. Quality of care for pregnant women and newborns-the WHO vision. BJOG 2015;122:1045–9. 10.1111/1471-0528.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Global Strategy for Women’s, Children’s and Adolescent’s Health 2016-2030. http://www.who.int/life-course/partners/global-strategy/global-strategy-2016-2030/en/ (accessed 15 Sep 2017). [DOI] [PMC free article] [PubMed]
- 3. World Health Organization (WHO). The prevention and elimination of disrespect and abuse during facility-based childbirth. Geneva: World Health Organization, 2014. (accessed 15 Sep 2017). [Google Scholar]
- 4. World Health Organization. Beyond the numbers: Reviewing maternal deaths and complications to make pregnancy safer. Geneva: World Health Organization, 2004. (accessed 15 Sep 2017). [Google Scholar]
- 5. World Health Organization. Regional Office for Europe. Conducting a maternal near-miss case review cycle at the hospital level” manual with practical tools. 2016. (accessed 15 Sep 2017).
- 6. Tunçalp O, Hindin MJ, Souza JP, et al. The prevalence of maternal near miss: a systematic review. BJOG 2012;119:653–61. 10.1111/j.1471-0528.2012.03294.x [DOI] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The World Bank, Country and Lending Groups. Historical classification. 2014. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 15 Sept 2017).
- 9. World Health Organization 2011. Evaluating the quality of care for severe pregnancy complications: the WHO near-miss approach for maternal health. Geneva: World Health Organization, 2011. [Google Scholar]
- 10. Institute of Medicine. Crossing the quality chasm. Washington, DC: National Academy Press, 2001. (accessed on 15 Sep 2017). [Google Scholar]
- 11. Donabedian A. The quality of care. how can it be assessed? JAMA 1988;260:1743–8. [DOI] [PubMed] [Google Scholar]
- 12. : Higgins JPT, Green S, Cochrane Handbook for systematic reviews of interventions version 5.1.0: The Cochrane Collaboration, 2011. (accessed 15 Sep 2017). [Google Scholar]
- 13. Lumala A, Sekweyama P, Abaasa A, et al. Assessment of quality of care among in-patients with postpartum haemorrhage and severe pre-eclampsia at st. Francis hospital nsambya: a criteria-based audit. BMC Pregnancy Childbirth 2017;17:29 10.1186/s12884-016-1219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mgaya AH, Kidanto HL, Nystrom L, et al. Improving standards of care in obstructed labour: a criteria-based audit at a referral hospital in a low-resource setting in Tanzania. PLoS One 2016;11:e0166619 10.1371/journal.pone.0166619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kayiga H, Ajeani J, Kiondo P, et al. Improving the quality of obstetric care for women with obstructed labour in the national referral hospital in Uganda: lessons learnt from criteria based audit. BMC Pregnancy Childbirth 2016;16:152 10.1186/s12884-016-0949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohd Azri MS, Edahayati AT, Kunasegaran K. Audit on management of eclampsia at Sultan Abdul Halim Hospital. Med J Malaysia 2015;70:142–7. [PubMed] [Google Scholar]
- 17. Gebrehiwot Y, Tewolde BT. Improving maternity care in Ethiopia through facility based review of maternal deaths and near misses. Int J Gynaecol Obstet 2014;127 (Suppl 1):S29–34. 10.1016/j.ijgo.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 18. Baltag V, Filippi V, Bacci A. Putting theory into practice: the introduction of obstetric near-miss case reviews in the Republic of Moldova. Int J Qual Health Care 2012;24:182–8. 10.1093/intqhc/mzr079 [DOI] [PubMed] [Google Scholar]
- 19. Kidanto HL, Wangwe P, Kilewo CD, et al. Improved quality of management of eclampsia patients through criteria based audit at Muhimbili National Hospital, Dar es Salaam, Tanzania. Bridging the quality gap. BMC Pregnancy Childbirth 2012;12:134 10.1186/1471-2393-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sukhanberdiyev K, Ayazbekov A, Issina A, et al. Initial experience of Near Miss Case Review: improving the management of haemorrhage. Entre Nous 2011;74:18–19. [Google Scholar]
- 21. Hodorogea S. Piloting near miss case reviews in Kazakhstan: improving quality of maternal care. Entre Nous 2010;70:28–9. [Google Scholar]
- 22. van den Akker T, van Rhenen J, Mwagomba B, et al. Reduction of severe acute maternal morbidity and maternal mortality in Thyolo District, Malawi: the impact of obstetric audit. PLoS One 2011;6:e20776 10.1371/journal.pone.0020776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailey PE, Binh HT, Bang HT. Promoting accountability in obstetric care: use of criteria-based audit in Viet Nam. Glob Public Health 2010;5:62–74. 10.1080/17441690802190776 [DOI] [PubMed] [Google Scholar]
- 24. van den Akker T, Mwagomba B, Irlam J, et al. Using audits to reduce the incidence of uterine rupture in a Malawian district hospital. Int J Gynaecol Obstet 2009;107:289–94. 10.1016/j.ijgo.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 25. Hunyinbo KI, Fawole AO, Sotiloye OS, et al. Evaluation of criteria-based clinical audit in improving quality of obstetric care in a developing country hospital. Afr J Reprod Health 2008;12:59–70. [PubMed] [Google Scholar]
- 26. Kongnyuy EJ, Leigh B, van den Broek N. Effect of audit and feedback on the availability, utilisation and quality of emergency obstetric care in three districts in Malawi. Women Birth 2008;21:149–55. 10.1016/j.wombi.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 27. Kongnyuy EJ, Mlava G, van den Broek N. Criteria-based audit to improve a district referral system in Malawi: a pilot study. BMC Health Serv Res 2008;8:190 10.1186/1472-6963-8-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weeks AD, Alia G, Ononge S, et al. A criteria-based audit of the management of severe pre-eclampsia in Kampala, Uganda. Int J Gynaecol Obstet 2005;91:292–7. Discussion 283-4 10.1016/j.ijgo.2005.07.022 [DOI] [PubMed] [Google Scholar]
- 29. Wagaarachchi PT, Graham WJ, Penney GC, et al. Holding up a mirror: changing obstetric practice through criterion-based clinical audit in developing countries. Int J Gynaecol Obstet 2001;74:119–30. 10.1016/S0020-7292(01)00427-1 [DOI] [PubMed] [Google Scholar]
- 30. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6:CD000259 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pantoja T, Opiyo N, Lewin S, et al. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev 2017;9:CD011086 10.1002/14651858.CD011086.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kongnyuy EJ, Uthman OA. Use of criterion-based clinical audit to improve the quality of obstetric care: a systematic review. Acta Obstet Gynecol Scand 2009;88:873–81. 10.1080/00016340903093542 [DOI] [PubMed] [Google Scholar]
- 33. Pirkle CM, Dumont A, Zunzunegui MV. Criterion-based clinical audit to assess quality of obstetrical care in low- and middle-income countries: a systematic review. Int J Qual Health Care 2011;23:456–63. 10.1093/intqhc/mzr033 [DOI] [PubMed] [Google Scholar]
- 34. Wu J, Song B, et al. Evaluating the effectiveness of maternal near-miss audit in China. International Journal of Gynecology and Obstetrics 2015 Conference: 21st FIGO World Congress of Gynecology and Obstetrics. Vancouver, BC Canada:131 Conference Start: 20151004. Conference End: 20151009. Conference Publication: (var.pagings). [Google Scholar]
- 35. Lewis G. Emerging lessons from the FIGO LOGIC initiative on maternal death and near-miss reviews. Int J Gynaecol Obstet 2014;127 (Suppl 1):S17–20. 10.1016/j.ijgo.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 36. Heitkamp A, Vollmer L, Van Den Akker T, et al. Severe acute maternal morbidity (SAMM) in metro east, western cape, South Africa: "Every human being has the right to live, every child needs a mother, mothers should not die because of their pregnancy."How can we improve the quality of care in the existing health system? International Journal of Gynecology and Obstetrics 2015. Conference: 21st FIGO World Congress of Gynecology and Obstetrics. Vancouver, BC Canada, 2015:131 Conference Start: 20151004. Conference End: 20151009. Conference Publication: (var.pagings). [Google Scholar]
- 37. Lazzerini M, Ciuch M, Covi B, et al. Facilitators and barriers to the effective implementation of the maternal near-miss case reviews in low and middle income countries: systematic review (submitted for publication). [DOI] [PMC free article] [PubMed]
- 38. Bacci A, Lewis G, Baltag V, et al. The introduction of confidential enquiries into maternal deaths and near-miss case reviews in the WHO European Region. Reprod Health Matters 2007;15:145–52. 10.1016/S0968-8080(07)30334-0 [DOI] [PubMed] [Google Scholar]
- 39. WHO Regional Office for Europe. Multi-Country review meeting on maternal mortality and morbidity audit “Beyond the Numbers”, Report of a WHO meeting, Charvak, Uzbekistan 14–17 June 2010. Copenhagen: WHO Regional Office for Europe, 2010. (accessed 8 Sep 2017). [Google Scholar]
- 40. WHO Regional Office for Europe. The impact of implementation of ’Beyond the numbers' approach in improving maternal and perinatal health. Bishkek, Kyrgyzstan: WHO Regional Office for Europe, 2014. [Google Scholar]
- 41. WHO Regional Office for Europe Making Pregnancy Safer in Uzbekistan. Maternal mortality and morbidity audit Activities Report 2002-2008. 2008. http://www.euro.who.int/__data/assets/pdf_file/0004/98797/MPS_UZB.pdf (accessed 8 Sep 2017).
- 42. National Institute for Clinical Excellence (NICE). Principles for best practice in clinical audit. Abingdon, Berks: Radcliffe Medical Press, 2002. (accessed 22 Sep 2017). [Google Scholar]
- 43. World Health Organization Regional Office for Europe. Action plan for sexual and reproductive health: towards achieving the 2030 Agenda for Sustainable Development in Europe – leaving no one behind. Copenhagen: World Health Organization Regional Office for Europe, 2016. (accessed 22 Sep 2017). [Google Scholar]
- 44. Knight M, Lewis G, Acosta CD, et al. Maternal near-miss case reviews: the UK approach. BJOG 2014;121 Suppl 4:112–6. 10.1111/1471-0528.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knight M, Acosta C, Brocklehurst P, et al. Beyond maternal death: improving the quality of maternal care through national studies of ‘near-miss’ maternal morbidity. Southampton (UK: NIHR Journals Library, 2016. [PubMed] [Google Scholar]
- 46. MacDonald EJ, Geller SE, Lawton B. Establishment of a national severe maternal morbidity preventability review in New Zealand. Int J Gynaecol Obstet 2016;135:120–3. 10.1016/j.ijgo.2016.03.034 [DOI] [PubMed] [Google Scholar]
- 47. Knight M; INOSS. The International Network of Obstetric Survey Systems (INOSS): benefits of multi-country studies of severe and uncommon maternal morbidities. Acta Obstet Gynecol Scand 2014;93:127–31. 10.1111/aogs.12316 [DOI] [PubMed] [Google Scholar]
- 48. Donati S, Maraschini A, Buoncristinao M, et al. Grave morbosità materna da emorragia del post partum: aspetti metodologici del progetto coordinato dall’Italia Obstetrics Surveillance System. Rapporto Osservasalute 2014. Stato di salute e qualità dell’assistenza nelle regioni italiane. Milano: Prex 2014:260–1. [Google Scholar]
- 49. Donati S, Maraschini A, Buoncristiano M, et al. Attività della sorveglianza ostetrica: l’Istituto Superiore di Sanità-Regioni per la gestione della grave morbosità materna da emorragia del post partum. Rapporto Osservasalute 2015. Stato di salute e qualità dell’assistenza nelle regioni italiane. Milano: Prex 2016:264–6. [Google Scholar]
- 50. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Management Review 1981;70:35–6. [Google Scholar]
- 51. Borchert M, Goufodji S, Alihonou E, et al. Can hospital audit teams identify case management problems, analyse their causes, identify and implement improvements? A cross-sectional process evaluation of obstetric near-miss case reviews in Benin. BMC Pregnancy Childbirth 2012;12:109 10.1186/1471-2393-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bacci A, Hodorogea S, Khachatryan H, et al. What is the quality of the maternal near-miss case reviews in WHO European Region: Cross-sectional study in Armenia, Georgia, Latvia, Republic of Moldova, and Uzbekistan. BMJ Open 2018. 10.1136/bmjopen-2017-017696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aguiar Cde A, Tanaka AC. Collective memories of women who have experienced maternal near miss: health needs and human rights. Cad Saude Publica 2016;32:e00161215. [DOI] [PubMed] [Google Scholar]
- 54. Påfs J, Musafili A, Binder-Finnema P, et al. Beyond the numbers of maternal near-miss in Rwanda – a qualitative study on women’s perspectives on access and experiences of care in early and late stage of pregnancy. BMC Pregnancy Childbirth 2016;16:257 10.1186/s12884-016-1051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hinton L, Locock L, Knight M. Experiences of the quality of care of women with near-miss maternal morbidities in the UK. BJOG 2014;121 (Suppl 4):20–3. 10.1111/1471-0528.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aghlmand S, Akbari F, Lameei A, et al. Developing evidence-based maternity care in Iran: a quality improvement study. BMC Pregnancy Childbirth 2008;8:20 10.1186/1471-2393-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019787supp001.pdf (214.5KB, pdf)
bmjopen-2017-019787supp002.pdf (149.1KB, pdf)